Nitrogen Immobilization by Wood Fiber Substrates Strongly Affects the Photosynthetic Performance of Lettuce
Abstract
1. Introduction
2. Results
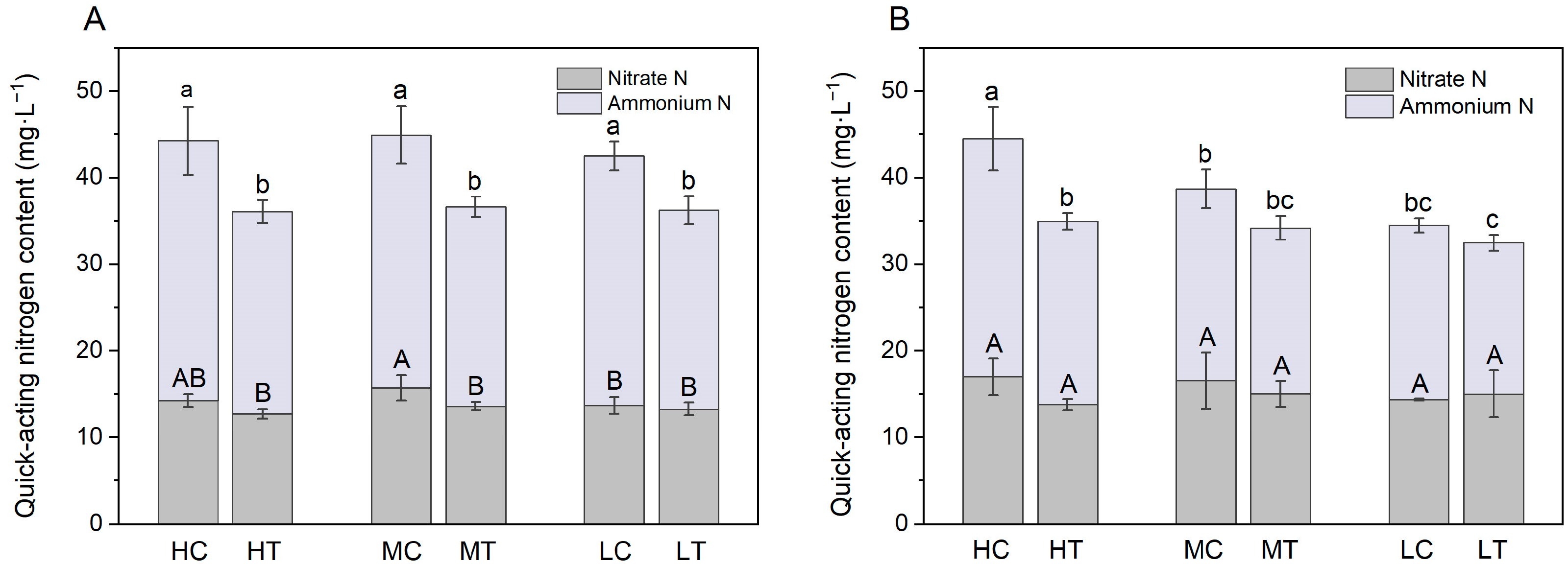
2.1. Fast-Acting Nitrogen Supply in the Substrate
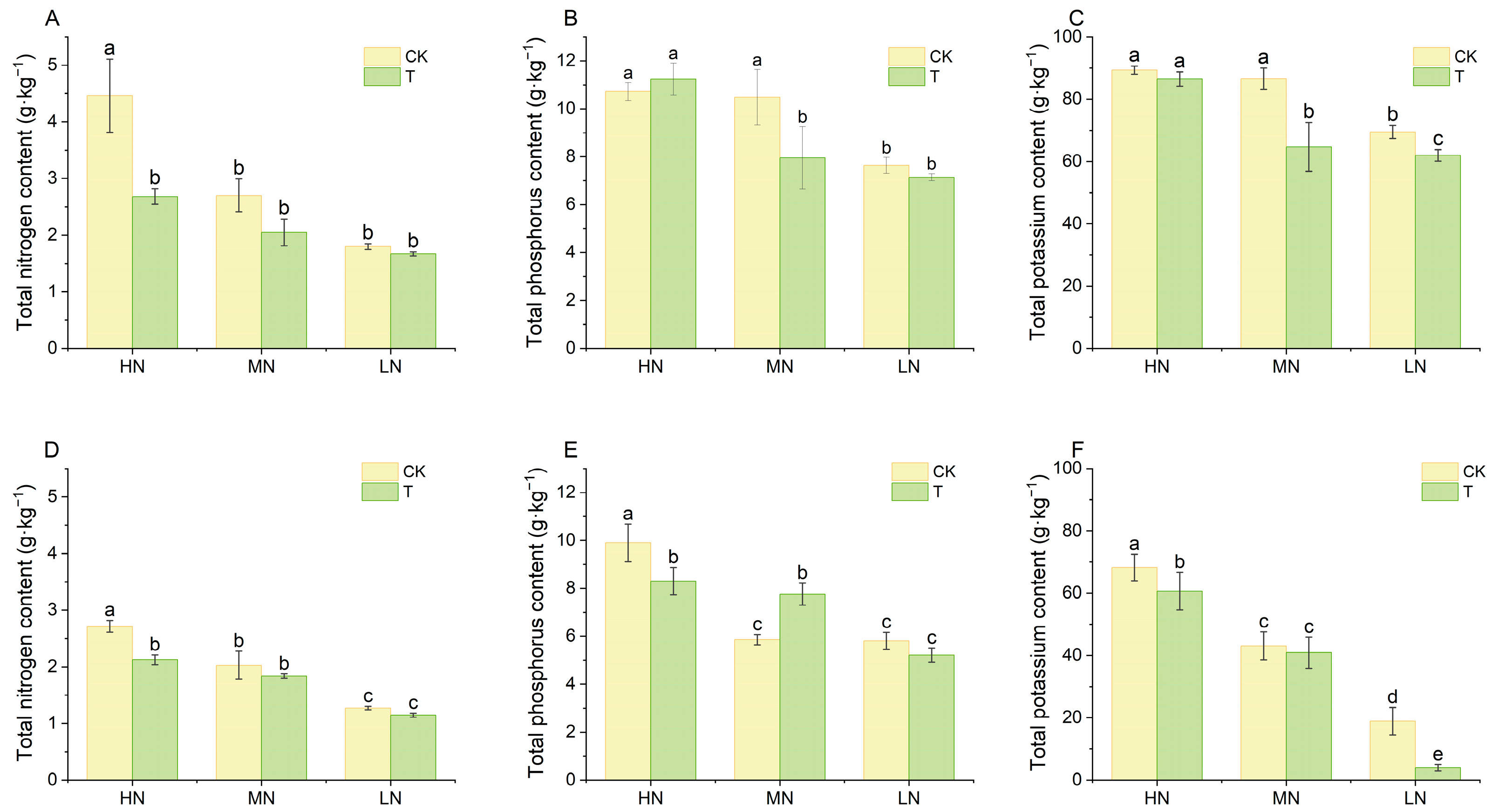
2.2. Lettuce Growth Performance and Nutrient Accumulation
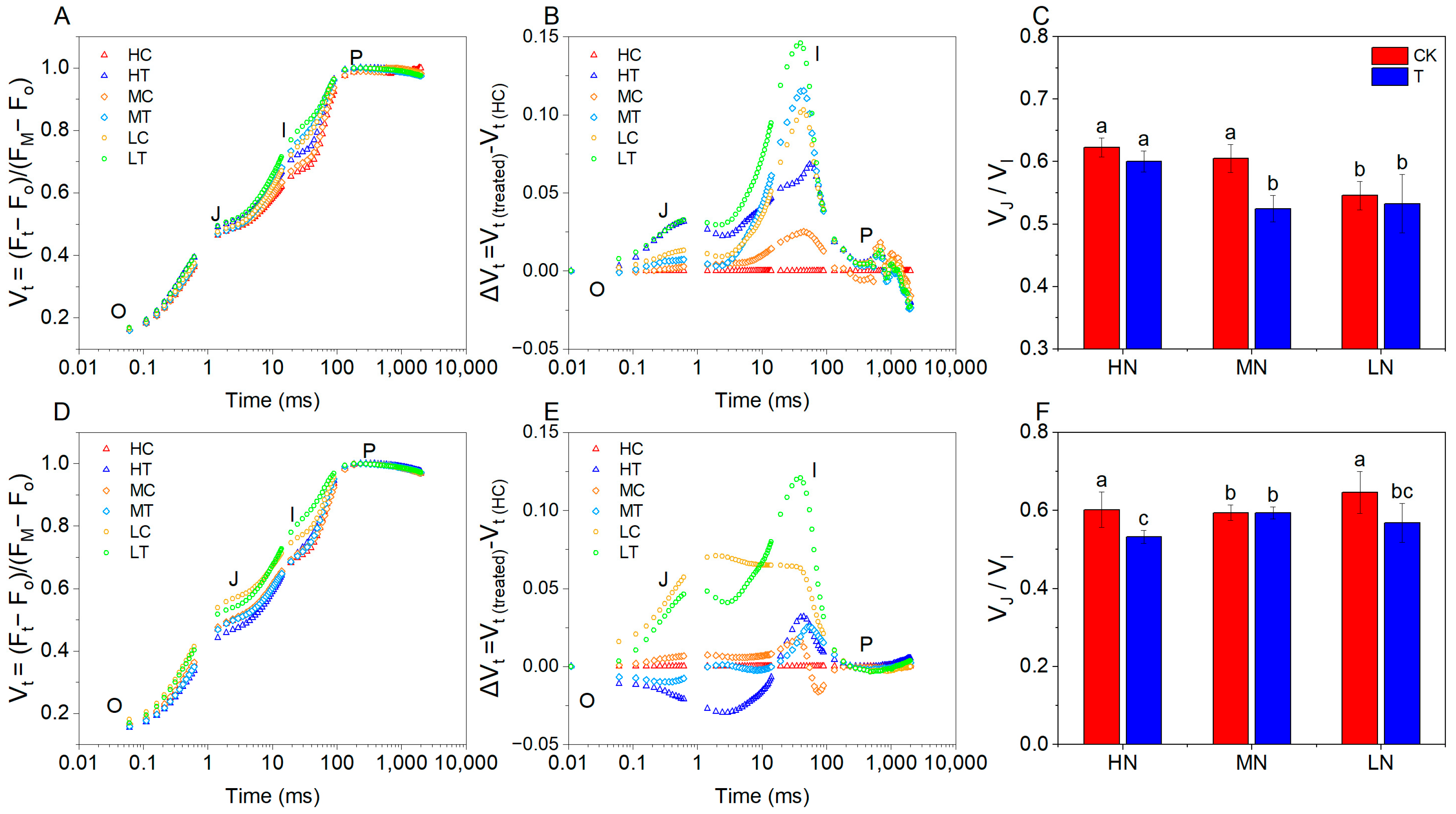
2.3. Chlorophyll a Fluorescence Properties
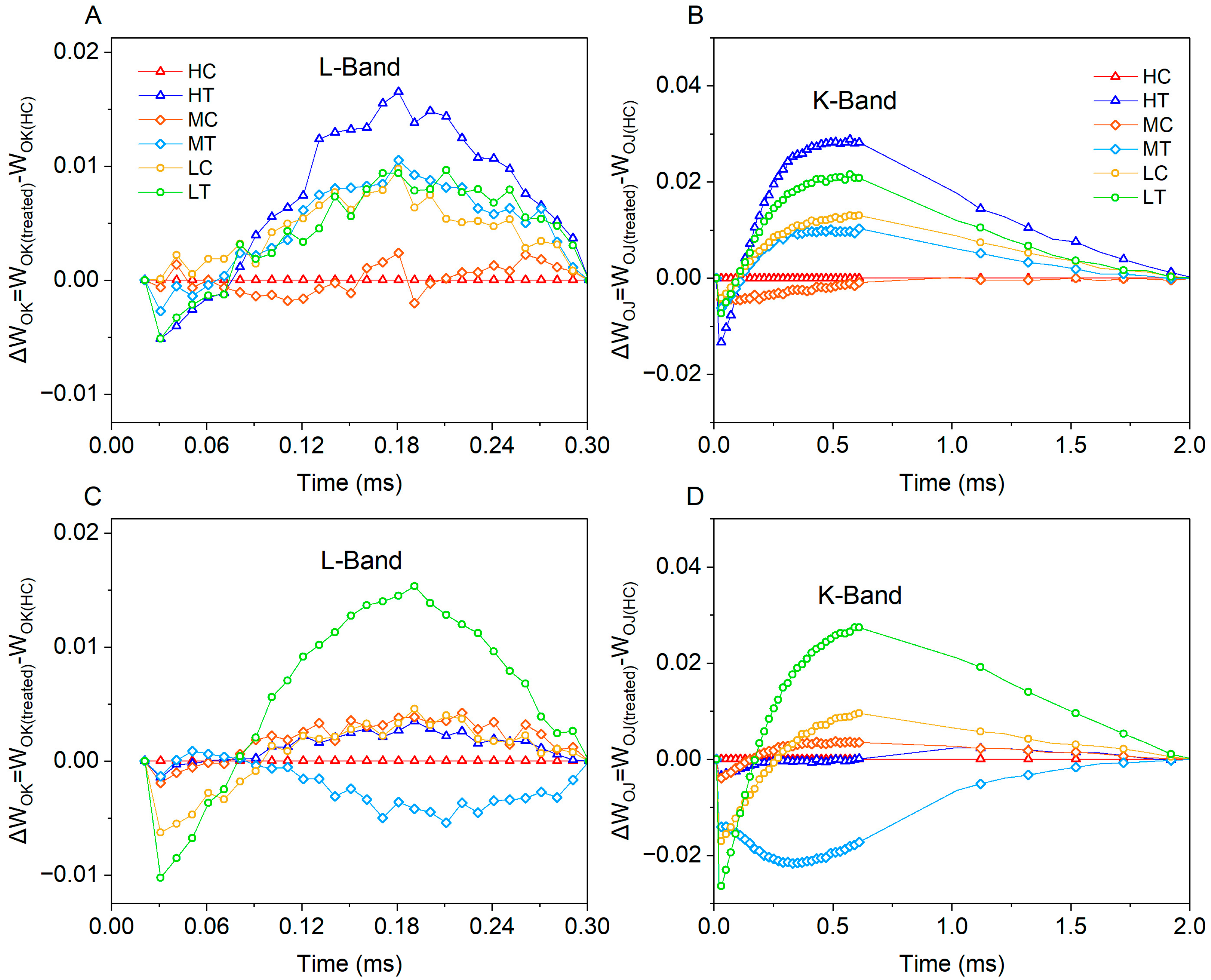
2.3.1. OJIP Chlorophyll Fluorescence Kinetics
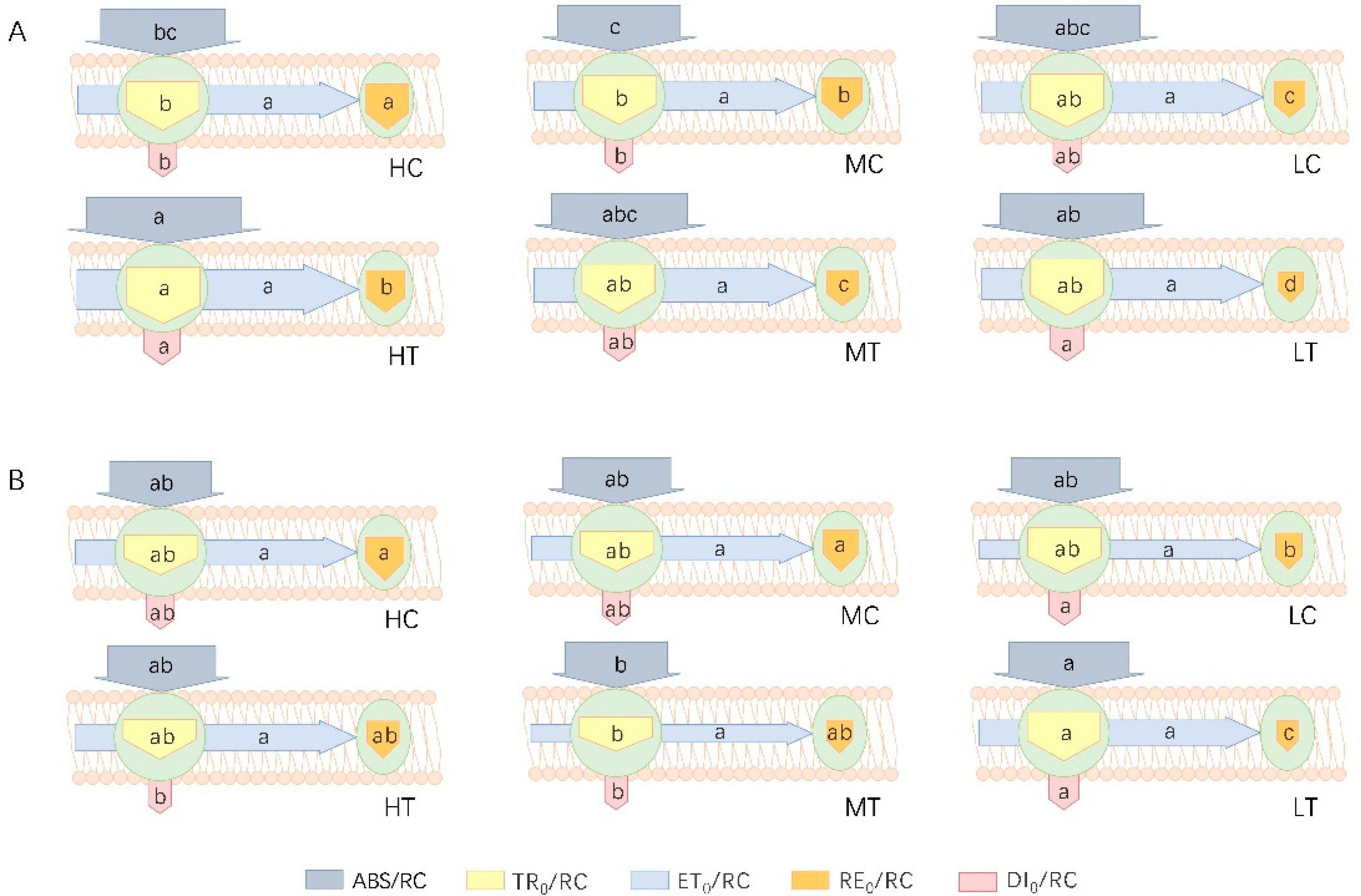
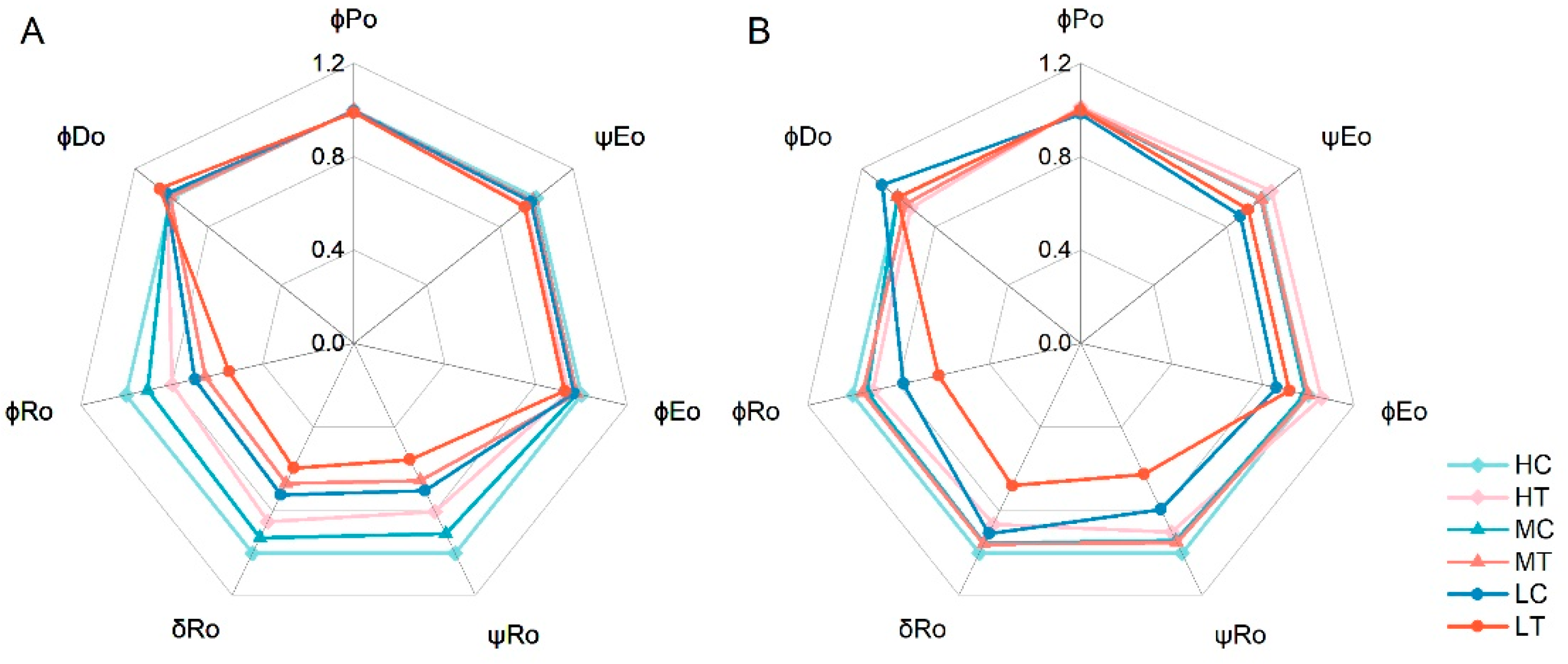
2.3.2. Photosynthetic Electron Transfer
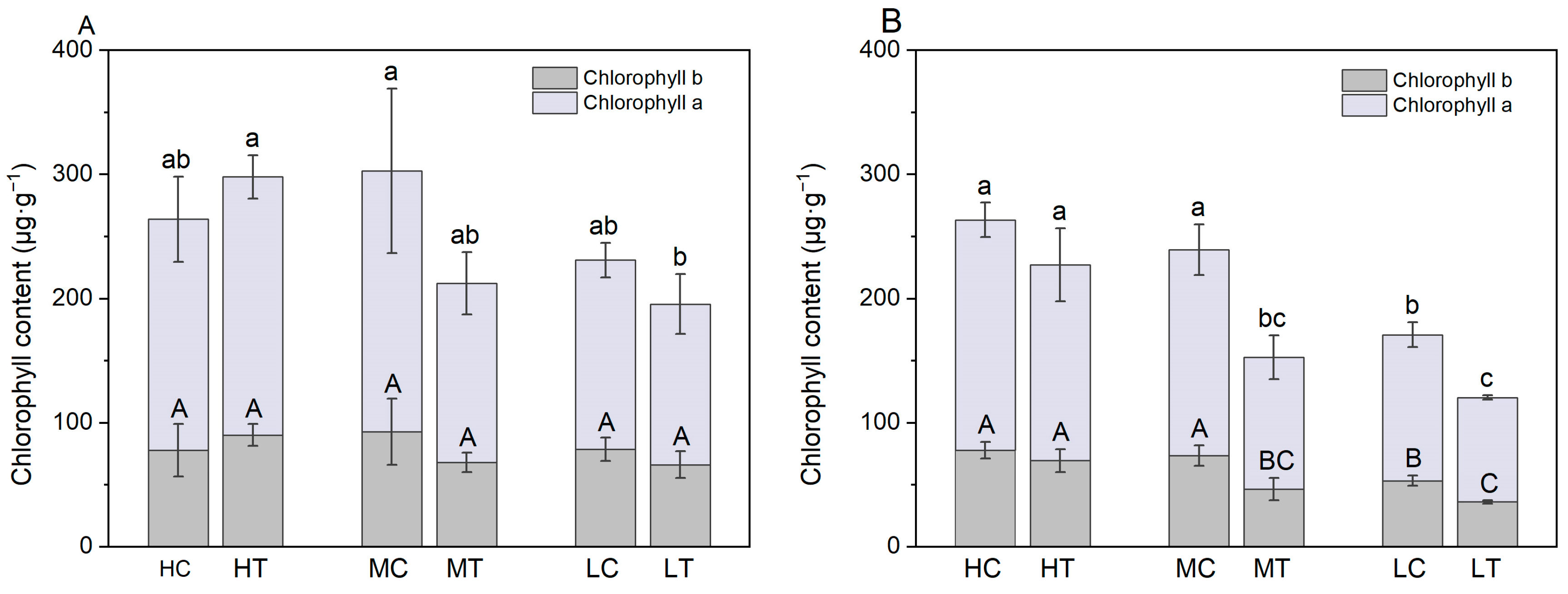
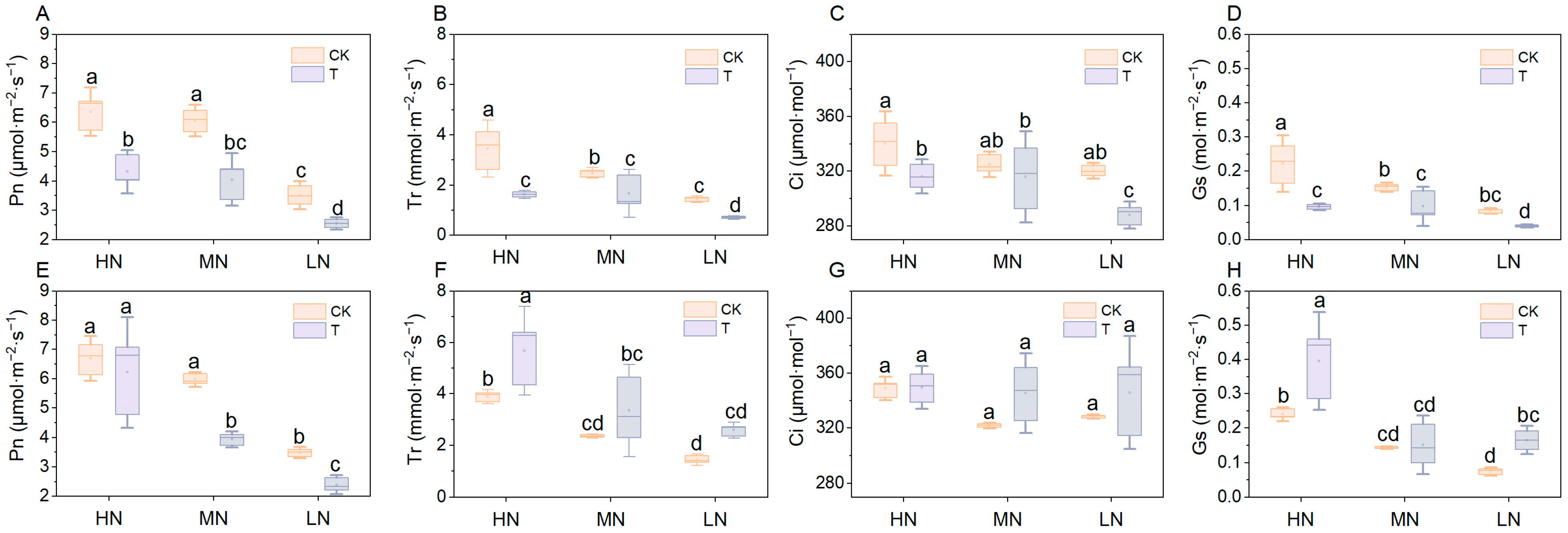
2.4. Lettuce Chlorophyll Content and Gas Exchange Parameters
3. Discussion
3.1. Effect of Wood Fiber Substrate on Nutrient Supply and Growth and Development of Lettuce
3.2. Effect of Wood Fiber Substrate on the Structure and Function of PSII and PSI in Lettuce
3.3. Effect of Wood Fiber Substrates on Photosynthesis
4. Materials and Methods
4.1. Experimental Materials and Treatment Setup
4.2. Plant Cultivation and Sampling
4.3. Determination of Physico-Chemical Properties of Cultivated Substrates
4.3.1. Determination of Physical and Chemical Properties of Substrates
4.3.2. Determination of Substrate Nitrogen Immobilization
4.3.3. Determination of Ammonia and Nitrate Nitrogen in Substrates
4.4. Determination of Physiological Indicators in Lettuce
4.4.1. Determination of Chlorophyll Content and Plant Nutrients in Lettuce
4.4.2. Determination of Chlorophyll a Fluorescence Parameters in Lettuce
4.4.3. Determination of Gas Exchange Data for Lettuce
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| PSII | Photosystem system II |
| PSI | Photosystem system I |
| OEC | Oxygen evolving complex |
| Pn | Photosynthesis rate |
| Ci | Inter-cellular CO2 concentration |
| Tr | Transpiration rate |
| Gs | Stomatal conductance |
| Ls | Stomatal limiting values |
| CK | Control substrate |
| T | Wood fiber substrate |
| L | Low nitrogen |
| M | Medium nitrogen |
| H | High nitrogen |
Appendix A
| Formulation Name | Elemental (mg·L−1) | Nitrogen Level | Concentration of Each Element (mg·L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca(NO3)2·4H2O | KNO3 | NH4H2PO4 | MgSO4·7H2O | N | P | K | Ca | Mg | |||
| Hogeland and Anon Universal Nutrient Solution (modified) | HN | 945 | 607 | 115 | 493 | 100% | 210.3 | 31.0 | 234.4 | 160.2 | 48.1 |
| MN | 240 | 607 | 115 | 493 | 60% | 126.6 | 31.0 | 234.4 | 40.7 | 48.1 | |
| LN | 0 | 350 | 115 | 493 | 30% | 62.5 | 31.0 | 135.1 | 0.0 | 48.1 | |
| Terms and Formulas | Illustrations |
|---|---|
| FO = F (20 μs) | FO (Minimum fluorescence): the fluorescence value in the dark-adapted state when all reaction centres are open, i.e., when QA− , the primary electron acceptor of the reaction centre, is in the oxidation state |
| FL = F (150 μs) | Fluorescence value at 150 μs |
| FK = F (300 μs) | Fluorescence value at 300 μs |
| FJ = F (3 ms) | Fluorescence value at 3 ms (J level of OJIP) |
| FI = F (30 ms) | Fluorescence value at 30 ms (I level of OJIP) |
| FM = FP = F (t)max | FM (maximum fluorescence): the fluorescence value when all reaction centres are switched off in the dark-adapted state |
| Fv = FM − FO | Maximum variable fluorescence |
| VL = (FL − FO)/ (FM − FO) | Relative variable fluorescence in the L phase of the fluorescence induction curve |
| VK = (FK − FO)/ (FM − FO) | Relative variable fluorescence in the K phase of the fluorescence induction curve |
| VJ = (FJ − FO)/ (FM − FO) | Relative variable fluorescence in the J phase of the fluorescence induction curve |
| VI = (FI − FO)/ (FM − FO) | Relative variable fluorescence in stage I of the fluorescence induction curve |
| M0 = (ΔV/Δt)0 = 4 (F300μs − FO)/ (FM − FO) = TR0/RC − ET0/RC | Approximate initial slope (per millisecond) of transient fluorescence V = f(t).This parameter reflects the rate of closure of PS II active reaction centres (RCs). |
| ABS/RC = [4 (F300μs − FO)/ (F2ms − FO)] (FM/FV) = M0 (1/VJ) (1/ϕPo) | Energy flux absorbed per unit PS II active reaction centre |
| TR0/RC = ϕ Po (ABS/RC) | Energy flux captured per unit PS II active reaction centre at t = 0 |
| ET0/RC = ϕEo (ABS/RC) | Energy flux transferred per unit PS II active reaction centre at t = 0 |
| RE0/RC = ϕRo (ABS/RC) = (1 − VI)× (M0/VJ) | Specific electron flux per unit of PS II active reaction centre reducing PS I terminal electron acceptor at t = 0 |
| DI0/RC = (ABS/RC) − (TR0/RC) | Energy flux per unit PS II active reaction centre heat dissipation at t = 0 |
| ϕPo = TR0/ABS = FV/FM = 1 − FO/FM | The maximum quantum yield of the primary photochemical reaction at t = 0 refers to the quantum yield of photons captured by the PSII reaction centre and also indicates the maximum photochemical efficiency of the PSII reaction centre in the dark-adapted state |
| ψEo = ET0/TR0 = (1 − VJ) | Ratio of electrons captured by the active reaction centre of PS II being transferred at t = 0 |
| ϕEo = ET0/ABS = (1 − FO/FM) ψEo = ϕPoψEo | Quantum efficiency of electron transfer from QA− to electron transfer chain at t = 0 |
| ψRo = RE0/TR0 = 1 − VI | Ratio of electrons captured by the active reaction centre of PSII being transferred to PSI at t = 0 |
| δ Ro = RE0/ET0 = (1 − VI)/ (1 − VJ) | Ratio of electrons in the reduced PSI receptor measuring terminal electron acceptor to electrons in the electron transport chain |
| ϕRo = RE0/ABS = ϕPoψRo = ϕPoψEoδRo | Quantum yields of electron acceptors at the lateral end of reduced PSI receptors |
| ϕDo = FO/FM = 1 − ϕPo | Thermal dissipation quantum yield at t = 0, reflecting the proportion of absorbed light energy dissipated by non-photochemical quenching processes in PSII |
References
- Schmilewski, G. Growing medium constituents used in the eu. Acta Hortic. 2009, 819, 33–46. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Barnes, M.C. Pine Tree Substrate, Nitrogen Rate, Particle Size, and Peat Amendment Affect Poinsettia Growth and Substrate Physical Properties. HortScience 2008, 43, 2155–2161. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Gruda, N.S. Current and Future Perspective of Growing Media in Europe. 2011. Available online: https://www.actahort.org/books/960/960_3.htm (accessed on 24 January 2025).
- Agarwal, P.; Saha, S.; Hariprasad, P. Agro-industrial-residues as potting media: Physicochemical and biological characters and their influence on plant growth. Biomass Convers. Biorefinery 2023, 13, 9601–9624. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, M.; Panettieri, M.; García de Castro Barragán, J.M.; Mastrolonardo, G.; Knicker, H. Recycling pyrolyzed organic waste from plant nurseries, rice production and shrimp industry as peat substitute in potting substrates. J. Environ. Manag. 2021, 277, 111436. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrate for production of vegetable transplants. Sci. Hortic. 2004, 100, 309–322. [Google Scholar] [CrossRef]
- Schmilewski, G. The role of peat in assuring the quality of growing media. Mires Peat 2008, 3, 1–8. [Google Scholar]
- Dickson, R.W.; Helms, K.M.; Jackson, B.E.; Machesney, L.M.; Lee, J.A. Evaluation of Peat Blended with Pine Wood Components for Effects on Substrate Physical Properties, Nitrogen Immobilization, and Growth of Petunia (Petunia ×hybrida Vilm.-Andr.). HortScience 2022, 57, 304–311. [Google Scholar] [CrossRef]
- Handreck, K. Use of the nitrogen drawdown index to predict fertilizer nitrogen requirements in soilless potting media. Commun. Soil Sci. Plant Anal. Commun. Soil Sci. Plant Anal. 1993, 24, 2137–2151. [Google Scholar] [CrossRef]
- Wright, R.D.; Jackson, B.E.; Browder, J.F.; Latimer, J.G. Growth of Chrysanthemum in a Pine Tree Substrate Requires Additional Fertilizer. HortTechnology 2008, 18, 111–115. [Google Scholar] [CrossRef]
- Bunt, A.C. Media and Mixes for Container-Grown Plants; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- Prasad, M. Physical, chemical and biological properties of coir dust. Acta Hortic. 1997, 450, 21–30. [Google Scholar] [CrossRef]
- Handreck, K.A. Rapid assessment of the rate of nitrogen immobilisation in organic components of potting media: II. Nitrogen drawdown index and plant growth. Commun. Soil Sci. Plant Anal. 1992, 23, 217–230. [Google Scholar] [CrossRef]
- Maher, M.; Prasad, M.; Raviv, M. Organic Soilless Media Components. Soil. Cult. 2019, 6, 303–378. [Google Scholar]
- Owen, W.; Jackson, B.; Whipker, B.; Fonteno, W. Pine Wood Chips as an Alternative to Perlite in Greenhouse Substrates: Nitrogen Requirements. HortTechnology 2016, 26, 199–205. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Viaene, J.; Debode, J.; Muylle, H.; Perneel, M. Selecting raw materials for sustainable growing media: A protocol to assess the risk for N immobilization. In Proceedings of the International Society for Horticultural Science (ISHS), Leuven, Belgium, 2017; pp. 149–160. Available online: https://www.actahort.org/books/1168/1168_20.htm (accessed on 24 January 2025).
- Gruda, N.; Tucher, S.; Schnitzler, W. N-Immobilization of wood fiber substrates in the production of tomato transplants (Lycopersicon lycopersicum (L.) Karst. Ex Farw.). J. Appl. Bot. Food Qual. 2000, 74, 32–37. [Google Scholar]
- Redillas, M.C.F.R.; Jeong, J.S.; Strasser, R.J.; Kim, Y.S.; Kim, J.-K. JIP analysis on rice (Oryza sativa cv Nipponbare) grown under limited nitrogen conditions. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 827–832. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J.; et al. The Use of Chlorophyll Fluorescence Kinetics Analysis to Study the Performance of Photosynthetic Machinery in Plants. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: New York, NY, USA, 2014; pp. 347–384. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Cetner, M.D.; Kalaji, H.M.; Goltsev, V.; Aleksandrov, V.; Kowalczyk, K.; Borucki, W.; Jajoo, A. Effects of nitrogen-deficiency on efficiency of light-harvesting apparatus in radish. Plant Physiol. Biochem. 2017, 119, 81–92. [Google Scholar] [CrossRef]
- Guo, P.; Ren, J.; Shi, X.; Xu, A.; Zhang, P.; Guo, F.; Feng, Y.; Zhao, X.; Yu, H.; Jiang, C. Optimized nitrogen application ameliorates the photosynthetic performance and yield potential in peanuts as revealed by OJIP chlorophyll fluorescence kinetics. BMC Plant Biol. 2024, 24, 774. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of nitrogen application rate on the photosynthetic pigment, leaf fluorescence characteristics, and yield of indica hybrid rice and their interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Gao, G.; Feng, Q.; Liu, X. Stomatal and no-stomatal limitations to photosynthesis of Populus euphratica leaves under natural conditions. J. Arid Land Resour. Environ. 2020, 34, 182–188. [Google Scholar]
- Debode, J.; De Tender, C.; Cremelie, P.; Lee, A.S.; Kyndt, T.; Muylle, H.; De Swaef, T.; Vandecasteele, B. Trichoderma-Inoculated Miscanthus Straw Can Replace Peat in Strawberry Cultivation, with Beneficial Effects on Disease Control. Front. Plant Sci. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.E.; Wright, R.D.; Alley, M.M. Comparison of Fertilizer Nitrogen Availability, Nitrogen Immobilization, Substrate Carbon Dioxide Efflux, and Nutrient Leaching in Peat-lite, Pine Bark, and Pine Tree Substrates. HortScience Horts 2009, 44, 781–790. [Google Scholar] [CrossRef]
- Harris, C.N.; Dickson, R.W.; Fisher, P.R.; Jackson, B.E.; Poleatewich, A.M. Evaluating Peat Substrates Amended with Pine Wood Fiber for Nitrogen Immobilization and Effects on Plant Performance with Container-grown Petunia. HortTechnology 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Mu, B.; Wang, F.; Feng, N.; Zheng, D. Nitrogen limitation affects carbon and nitrogen metabolism in mung bean (Vigna radiata L.). J. Plant Physiol. 2023, 290, 154105. [Google Scholar] [CrossRef]
- Mulo, P. Chloroplast-targeted ferredoxin-NADP+ oxidoreductase (FNR): Structure, function and location. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 927–934. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. The energy flux theory 35 years later: Formulations and applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef]
- Takahashi, S.; Milward, S.E.; Fan, D.Y.; Chow, W.S.; Badger, M.R. How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol. 2009, 149, 1560–1567. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Qiang, B.; Zhou, W.; Zhong, X.; Fu, C.; Cao, L.; Zhang, Y.; Jin, X. Effect of nitrogen application levels on photosynthetic nitrogen distribution and use efficiency in soybean seedling leaves. J. Plant Physiol. 2023, 287, 154051. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Ologundudu, A.F.; Adelusi, A.A. Effect of nitrogen nutritional stress on some mineral nutrients and photosynthetic apparatus of Zea mays L. and Vigna unguiculata L. Not. Sci. Biol. 2013, 5, 376–382. [Google Scholar] [CrossRef]
- Liang, X.; Wang, H.; Zhang, Y.; Yang, R.; Zhang, D.; Zhou, W.; Qi, Z.; Lin, W. The Substitution of Sphagnum for Peat as a Culture Substrate Reduces N2O Emissions from Vegetable Production Systems. Agronomy 2024, 14, 369. [Google Scholar] [CrossRef]
- Li, P.; Zhu, Y.; Song, X.; Song, F. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiol. Biochem. 2020, 155, 93–104. [Google Scholar] [CrossRef]
- Berry, J.A.; Downton, W.J.S. Environmental Regulation of Photosynthesis. In Photosynthesis; Academic Press: New York, NY, USA, 1982. [Google Scholar]



| Form | HN | MN | LN |
|---|---|---|---|
| Nutrient solution nitrogen concentration (mg N·L−1) | 210.30 | 127.60 | 62.50 |
| Nutrient solution input per pot (L) | 0.32 | 0.32 | 0.32 |
| Total nitrogen input to single pot (mg) | 67.30 | 40.32 | 20.00 |
| Wood fiber nitrogen immobilization capacity (mg N·L−1) | 115.00 | ||
| Volume of wood fiber in single pot substrate (L) | 0.16 | ||
| 4-day nitrogen immobilization potential of single-pot substrates (mg) | 18.40 | ||
| Capacity (g·cm−3) | Porosity (%) | pH | EC (ms·cm−1) | Nutrients (g·kg−1 Dry Weight) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Porosity | Water-Holding Porosity | Air Porosity | Quick-Acting Nitrogen | Total Nitrogen | Total Phosphorus | Total Potassium | ||||
| Control substrate | 0.21 | 0.72 | 0.68 | 0.04 | 6.26 | 0.46 | 0.30 | 0.82 | 0.01 | 2.2 |
| Wood fiber | 0.18 | 0.8 | 0.72 | 0.07 | 6.09 | 0.42 | 0.24 | 0.65 | 0 | 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Li, R.; Liu, J.; Cui, W.; Qi, Z.; Zhou, W. Nitrogen Immobilization by Wood Fiber Substrates Strongly Affects the Photosynthetic Performance of Lettuce. Plants 2025, 14, 1518. https://doi.org/10.3390/plants14101518
Wu L, Li R, Liu J, Cui W, Qi Z, Zhou W. Nitrogen Immobilization by Wood Fiber Substrates Strongly Affects the Photosynthetic Performance of Lettuce. Plants. 2025; 14(10):1518. https://doi.org/10.3390/plants14101518
Chicago/Turabian StyleWu, Lingyi, Ruohan Li, Juncheng Liu, Wenzhong Cui, Zhiyong Qi, and Wanlai Zhou. 2025. "Nitrogen Immobilization by Wood Fiber Substrates Strongly Affects the Photosynthetic Performance of Lettuce" Plants 14, no. 10: 1518. https://doi.org/10.3390/plants14101518
APA StyleWu, L., Li, R., Liu, J., Cui, W., Qi, Z., & Zhou, W. (2025). Nitrogen Immobilization by Wood Fiber Substrates Strongly Affects the Photosynthetic Performance of Lettuce. Plants, 14(10), 1518. https://doi.org/10.3390/plants14101518








