Effects of Seedling Substrate and Hydroponic Versus Aquaponic Nutrient Solution on Growth, Nutrient Uptake, and Eco-Physiological Response of Lemon Basil (Ocimum × citriodorum)
Abstract
1. Introduction
2. Results
2.1. Experiment 1
2.1.1. Seed Germination and Seedling Growth
2.1.2. Cluster Heatmap Analysis
2.2. Experiment 2
2.2.1. Plant Growth
2.2.2. Gas Exchanges and Chl a Fluorescence Emission
2.2.3. Leaf Photosynthetic Pigments
2.2.4. Mineral Content (Leaf, Stem, and Root)
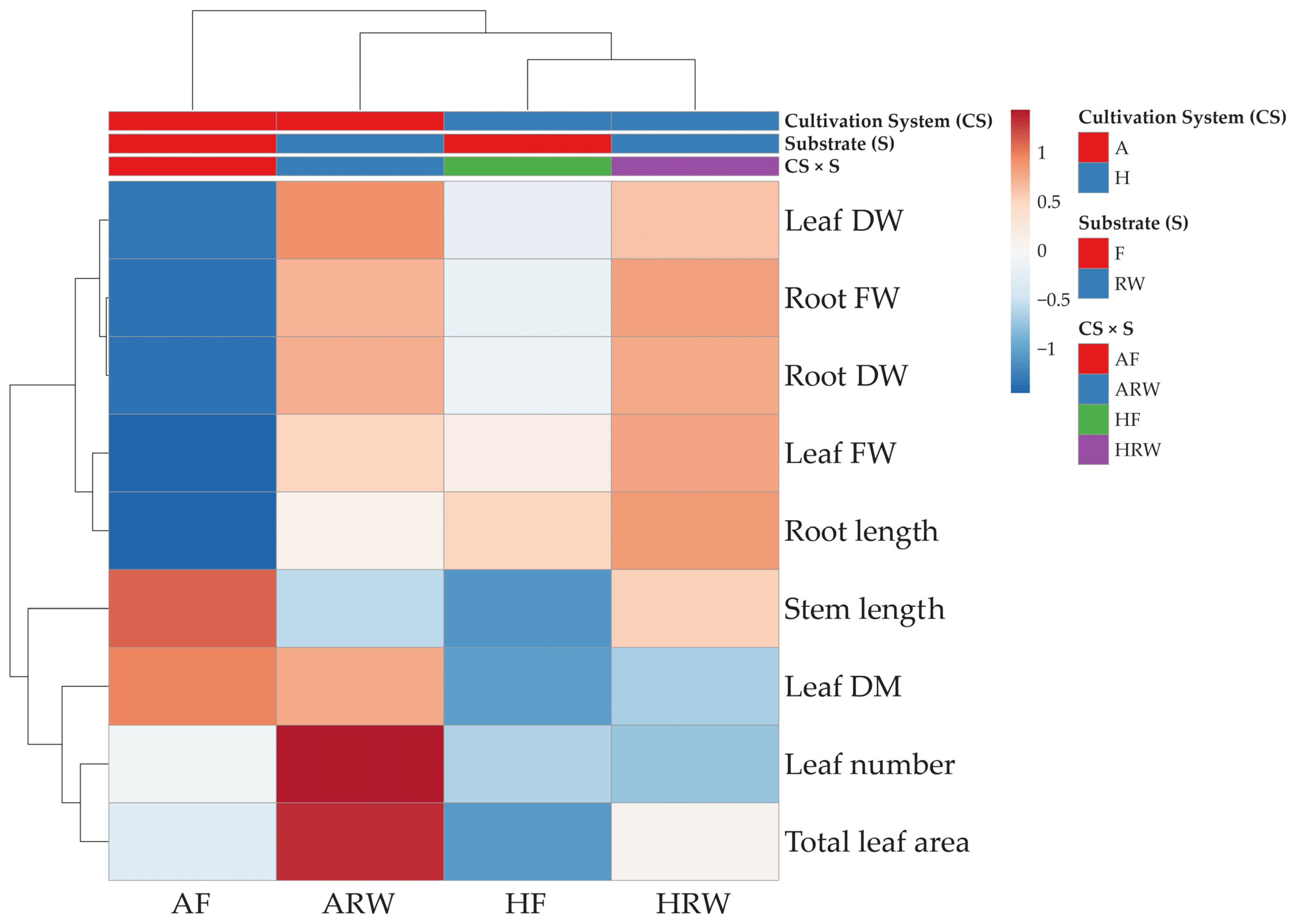
2.2.5. Cluster Heatmap Analysis
3. Discussion
4. Materials and Methods
4.1. Experiment 1: Seed Germination and Seedling Growth
4.1.1. Experimental Site and Duration
4.1.2. Plant Material and Experimental Conditions
4.1.3. Substrates and Sowing Procedure
4.1.4. Germination and Seedling Management
4.1.5. Seedling Growth Measurements
4.2. Experiment 2: Comparison of Aquaponic and Hydroponic Systems
4.2.1. Experimental Site, Duration, and Setup
4.2.2. Greenhouse and Cultivation Systems Description
4.2.3. Decoupled Aquaponic and Hydroponic Nutrient Solution Management
4.2.4. Plant Growth Measurements
4.2.5. Leaf Vegetation Indices
4.2.6. Gas Exchanges and Chlorophyll Fluorescence Measurements
4.2.7. Leaf, Stem, and Root Mineral Content Analyses
4.2.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spence, C. Sweet Basil: An Increasingly Popular Culinary Herb. Int. J. Gastron. Food Sci. 2024, 36, 100927. [Google Scholar] [CrossRef]
- Boronat, Ò.; Mora, M.; Romeo-Arroyo, E.; Vázquez-Araújo, L. Unraveling the Mediterranean Cuisine. What Ingredients Drive the Flavor of the Gastronomies Included in the Mediterranean Diet? Int. J. Gastron. Food Sci. 2023, 34, 100802. [Google Scholar] [CrossRef]
- Matarazzo, N.; Imbrenda, V.; Coluzzi, R.; Pace, L.; Samela, C.; Lanfredi, M. ‘Industrializzazione Dell’ Agricoltura Nella Piana Del Sele: Una Prospettiva Geografica Basata Sull’ Urban Atlas Copernicus. Bull. Dell’assoc. Ital. Cartogr. 2022, 175, 101–113. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil Degradation in the European Mediterranean Region: Processes, Status and Consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef] [PubMed]
- Stolte, J.; Tesfai, M.; Øygarden, L.; Hessel, R.; Panagos, P.; Kværnø, S.; Verheijen, F.; Ballabio, C.; Stolte, J.; Keizer, J. Soil Threats in Europe; Publications Office: Luxemburg, 2015. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morra, L.; Zaccardelli, M.; Alfani, A. Cadmium Accumulation in Leaves of Leafy Vegetables. Ecotoxicol. Environ. Saf. 2016, 123, 89–94. [Google Scholar] [CrossRef]
- Junge, R.; Schmautz, Z.; Milliken, S. Toward Nutrient Cycling from Organic Waste Streams for Soilless Cultivation. Curr. Opin. Food Sci. 2025, 61, 101257. [Google Scholar] [CrossRef]
- Manimozhi, R.; Krishnamoorthy, G. Innovative Techniques in Agriculture: Transitioning From Traditional Farming to Precision and Hydroponic Agriculture. Environ. Qual. Manag. 2025, 34, e70047. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Baganz, D.; Schmidt, U.; Kloas, W. An Innovative Suction Filter Device Reduces Nitrogen Loss in Double Recirculating Aquaponic Systems. Aquac. Eng. 2018, 82, 63–72. [Google Scholar] [CrossRef]
- Yen, H.Y.; Chou, J.H. Water Purification by Oyster Shell Bio-Medium in a Recirculating Aquaponic System. Ecol. Eng. 2016, 95, 229–236. [Google Scholar] [CrossRef]
- Mansour, E.; Loxton, C.; Elias, R.M.; Ormondroyd, G.A. Assessment of Health Implications Related to Processing and Use of Natural Wool Insulation Products. Environ. Int. 2014, 73, 402–412. [Google Scholar] [CrossRef]
- Modarelli, G.C.; Vanacore, L.; Rouphael, Y.; Langellotti, A.L.; Masi, P.; De Pascale, S.; Cirillo, C. Hydroponic and Aquaponic Floating Raft Systems Elicit Differential Growth and Quality Responses to Consecutive Cuts of Basil Crop. Plants 2023, 12, 1355. [Google Scholar] [CrossRef]
- Körner, O.; Bisbis, M.B.; Baganz, G.F.; Baganz, D.; Staaks, G.B.; Monsees, H.; Goddek, S.; Keesman, K.J. Environmental impact assessment of local decoupled multi-loop aquaponics in an urban context. J. Clean. Prod. 2021, 313, 127735. [Google Scholar] [CrossRef]
- Fussy, A.; Papenbrock, J. Techniques—Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Vanacore, L.; El-nakhel, C.; Modarelli, G.C.; Rouphael, Y. Growth, Ecophysiological Responses, and Leaf Mineral Composition of Lettuce and Curly Endive in Hydroponic and Aquaponic Systems. Plants 2024, 13, 2852. [Google Scholar] [CrossRef] [PubMed]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- He, L.; Ding, X.; Jin, H.; Zhang, H.; Cui, J.; Chu, J.; Li, R.; Zhou, Q.; Yu, J. Comparison of Rockwool and Coir for Greenhouse Cucumber Production: Chemical Element, Plant Growth, and Fruit Quality. Heliyon 2022, 8, e10930. [Google Scholar] [CrossRef]
- Inden, H.; Torres, A. Comparison of Four Substrates on the Growth and Quality of Tomatoes. Acta Hortic. 2004, 644, 205–210. [Google Scholar] [CrossRef]
- Kloas, W.; Groß, R.; Baganz, D.; Graupner, J.; Monsees, H.; Schmidt, U.; Staaks, G.; Suhl, J.; Tschirner, M.; Wittstock, B.; et al. A New Concept for Aquaponic Systems to Improve Sustainability, Increase Productivity, and Reduce Environmental Impacts. Aquac. Environ. Interact. 2015, 7, 179–192. [Google Scholar] [CrossRef]
- Francisco, E.C.; Freato, T.A.; Piolli, A.L.; Poz, M.E.S.D. Analysis of the Aquaponic System Sustainability via System Dynamics Modelling—FEW Nexus Approach. Circ. Econ. Sustain. 2024. [Google Scholar] [CrossRef]
- Chiquito-Contreras, R.G.; Hernandez-Adame, L.; Alvarado-Castillo, G.; Martínez-Hernández, M.d.J.; Sánchez-Viveros, G.; Chiquito-Contreras, C.J.; Hernandez-Montiel, L.G. Aquaculture—Production System and Waste Management for Agriculture Fertilization—A Review. Sustainability 2022, 14, 7257. [Google Scholar] [CrossRef]
- Lobanov, V.; Keesman, K.J.; Joyce, A. Plants Dictate Root Microbial Composition in Hydroponics and Aquaponics. Front. Microbiol. 2022, 13, 848057. [Google Scholar] [CrossRef]
- Braglia, R.; Costa, P.; Di Marco, G.; D’Agostino, A.; Redi, E.L.; Scuderi, F.; Gismondi, A.; Canini, A. Phytochemicals and Quality Level of Food Plants Grown in an Aquaponics System. J. Sci. Food Agric. 2022, 102, 844–850. [Google Scholar] [CrossRef]
- Flores-Aguilar, P.S.; Rico-Chávez, A.K.; Rodriguez-deLeón, E.; Aguirre-Becerra, H.; Zamora-Castro, S.A.; Soto-Zarazúa, G.M. Bioactive Compounds of Endemic Medicinal Plants (Cuphea spp.) Cultured in Aquaponic Systems: A Short Study. Agriculture 2023, 13, 2018. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Ouerghi, Z.; Navari-Izzo, F. Antioxidative Responses of Ocimum basilicum to Sodium Chloride or Sodium Sulphate Salinization. Plant Physiol. Biochem. 2010, 48, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: New York, NY, USA, 2019; pp. 1–620. [Google Scholar] [CrossRef]
- Cramer, G.R.; Läuchli, A.; Epstein, E. Effects of NaCl and CaCl2 on Ion Activities in Complex Nutrient Solutions and Root Growth of Cotton. Plant Physiol. 1986, 81, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, M.; Formisano, L.; Kyriacou, M.C.; Carillo, P.; Scognamiglio, L.; De Pascale, S.; Rouphael, Y. Morpho-Physiological and Biochemical Responses of Hydroponically Grown Basil Cultivars to Salt Stress. Antioxidants 2022, 11, 2207. [Google Scholar] [CrossRef] [PubMed]
- Mourantian, A.; Aslanidou, M.; Mente, E.; Katsoulas, N.; Levizou, E. Basil Functional and Growth Responses When Cultivated via Different Aquaponic and Hydroponics Systems. PeerJ 2023, 11, e15664. [Google Scholar] [CrossRef]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae 2023, 9, 1077. [Google Scholar] [CrossRef]
- Eck, M.; Szekely, I.; Massart, S.; Jijakli, M.H. Microorganisms in Aquaponics: Insights on the Composition of the Root Microbiome of Lettuces of Varying Age. Acta Hortic. 2021, 1321, 213–219. [Google Scholar] [CrossRef]
- Kasozi, N.; Abraham, B.; Kaiser, H.; Wilhelmi, B. The Complex Microbiome in Aquaponics: Significance of the Bacterial Ecosystem. Ann. Microbiol. 2021, 71, 1. [Google Scholar] [CrossRef]
- Tuckeldoe, R.B.; Maluleke, M.K.; Adriaanse, P. The Effect of Coconut Coir Substrate on the Yield and Nutritional Quality of Sweet Peppers (Capsicum annuum) Varieties. Sci. Rep. 2023, 13, 2742. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta—Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality Through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Germination | Shoot DM | Root DM | Plant Height | Root Length |
|---|---|---|---|---|---|
| Substrate | % | cm Plant−1 | |||
| F50:P50 | 63.89 ± 3.67 b | 8.41 ± 0.32 b | 4.43 ± 0.08 b | 7.12 ± 0.28 a | 13.67 ± 0.91 |
| F70:P30 | 80.56 ± 5.55 a | 8.86 ± 0.23 b | 4.30 ± 0.1 b | 7.47 ± 0.31 a | 14.71 ± 1.51 |
| F90:P10 | 66.67 ± 2.41 b | 9.79 ± 0.15 ab | 4.75 ± 0.05 b | 7.13 ± 0.35 a | 13.08 ± 0.9 |
| RW 100% | 81.94 ± 5.55 a | 11.09 ± 0.46 a | 6.57 ± 0.73 a | 5.83 ± 0.06 b | 16.17 ± 1.18 |
| Significance | 0.04 * | 0.00 ** | 0.01 ** | 0.01 * | 0.32 ns |
| Treatment | Shoot FW | Shoot DW | Leaf Number | Total Leaf Area |
|---|---|---|---|---|
| Substrate | g Plant−1 | n Plant−1 | cm2 Plant−1 | |
| F50:P50 | 1.09 ± 0.08 a | 0.10 ± 0.01 | 7.58 ± 0.22 a | 24.78 ± 2.10 a |
| F70:P30 | 1.13 ± 0.07 a | 0.10 ± 0.01 | 7.83 ± 0.2 a | 24.55 ± 0.81 a |
| F90:P10 | 0.97 ± 0.05 ab | 0.10 ± 0.01 | 7.58 ± 0.17 a | 20.89 ± 0.66 ab |
| RW 100% | 0.78 ± 0.02 b | 0.09 ± 0.00 | 6.17 ± 0.17 b | 16.98 ± 0.39 b |
| Significance | 0.01 * | 0.50 ns | 0.00 ** | 0.01 * |
| Treatments | Leaf Number | Leaf FW | Leaf DW | Root FW | Total Leaf Area | |
|---|---|---|---|---|---|---|
| Cultivation System (CS) | Substrate (S) | (n Plant−1) | (g Plant−1) | (cm2 Plant−1) | ||
| A | F | 127.55 ± 7.29 | 40.56 ± 4.36 | 3.81 ± 0.38 | 26.33 ± 2.79 | 1098.02 ± 78.09 |
| RW | 143.77 ± 13.66 | 53.22 ± 4.86 | 5.02 ± 0.49 | 33.56 ± 2.94 | 1307.38 ± 103.30 | |
| Mean | 135.67 | 46.89 | 4.42 | 29.94 | 1202.70 | |
| H | F | 125.44 ± 12.10 | 53.67 ± 8.06 | 4.64 ± 0.76 | 31.11 ± 3.66 | 1060.47 ± 135.46 |
| RW | 121.77 ± 6.59 | 55.78 ± 5.21 | 4.85 ± 0.48 | 34.67 ± 3.57 | 1147.14 ± 92.41 | |
| Mean | 123.61 | 54.72 | 4.75 | 32.89 | 1103.81 | |
| Significance | ||||||
| Cultivation system (CS) | 0.25 ns | 0.18 ns | 0.56 ns | 0.37 ns | 0.35 ns | |
| Substrates (S) | 0.55 ns | 0.21 ns | 0.20 ns | 0.11 ns | 0.17 ns | |
| CS × S | 0.46 ns | 0.25 ns | 0.42 ns | 0.29 ns | 0.37 ns | |
| Treatments | Root DW | Leaf DM | Stem Length | Root Length | ||
|---|---|---|---|---|---|---|
| Cultivation System (CS) | Substrate (S) | (g Plant−1) | (%) | (cm Plant−1) | ||
| A | F | 1.71 ± 0.14 | 9.49 ± 0.22 a | 34.36 ± 2.57 | 13.704 ± 2.16 | |
| RW | 2.31 ± 0.18 | 9.40 ± 0.28 a | 31.73 ± 2.45 | 15.46 ± 0.88 | ||
| Mean | 2.01 | 9.45 | 33.05 | 14.58 | ||
| H | F | 2.06 ± 0.18 | 8.51 ± 0.31 b | 30.80 ± 2.08 | 17.42 ± 1.68 | |
| RW | 2.32 ± 0.19 | 8.68 ± 0.25 b | 32.93 ± 1.62 | 18.57 ± 1.33 | ||
| Mean | 2.19 | 8.59 | 31.87 | 18.00 | ||
| Significance | ||||||
| Cultivation system (CS) | 0.31 ns | 0.03 * | 0.59 ns | 0.04 * | ||
| Substrates (S) | 0.02 * | 0.88 ns | 0.91 ns | 0.37 ns | ||
| CS × S | 0.06 ns | 0.03 * | 0.69 ns | 0.16 ns | ||
| Treatments | A | WUEi | ΦPSII | Fv/Fm | NPQ | |
|---|---|---|---|---|---|---|
| Cultivation System (CS) | Substrate (S) | (µmol CO2 m−2 s−1) | (µmol CO2 m−2 s−1/mol H2O m−2 s−1) | |||
| A | F | 9.27 ± 0.89 | 107.67 ± 10.97 | 0.62 ± 0.04 | 0.84 ± 0.00 | 1.07 ± 0.09 |
| RW | 8.67 ± 0.86 | 127.53 ± 14.44 | 0.49 ± 0.07 | 0.83 ± 0.01 | 1.48 ± 0.21 | |
| Mean | 8.97 | 117.6 | 0.55 | 0.84 | 1.28 | |
| H | F | 8.24 ± 0.48 | 112.56 ± 8.55 | 0.49 ± 0.06 | 0.83 ± 0.01 | 1.31 ± 0.14 |
| RW | 8.78 ± 1.02 | 97.56 ± 12.05 | 0.57 ± 0.05 | 0.83 ± 0.01 | 1.36 ± 0.12 | |
| Mean | 8.51 | 105.06 | 0.53 | 0.83 | 1.34 | |
| Significance | ||||||
| Cultivation system (CS) | 0.58 ns | 0.30 ns | 0.72 ns | 0.82 ns | 0.68 ns | |
| Substrates (S) | 0.97 ns | 0.84 ns | 0.67 ns | 0.87 ns | 0.14 ns | |
| CS × S | 0.50 ns | 0.15 ns | 0.07 ns | 0.96 ns | 0.24 ns | |
| Treatments | FlvM | CCla | |
|---|---|---|---|
| Cultivation System (CS) | Substrate (S) | Index | |
| A | F | 0.92 ± 0.08 | 21.54 ± 1.33 |
| RW | 0.93 ± 0.03 | 21.64 ± 2.23 | |
| Mean | 0.93 | 21.60 | |
| H | F | 0.66 ± 0.09 | 22.89 ± 2.00 |
| RW | 0.56 ± 0.03 | 24.6 ± 200 | |
| Mean | 0.61 | 23.75 | |
| Significance | |||
| Cultivation system (CS) | 0.00 *** | 0.27 ns | |
| Substrates (S) | 0.51 ns | 0.64 ns | |
| CS × S | 0.4 ns | 0.68 ns | |
| Treatments | NO3 | P | K | Ca | Mg | S | |
|---|---|---|---|---|---|---|---|
| Cultivation System (CS) | Substrate (S) | mg kg−1 f.w. | g Plant−1 d.w. | ||||
| A | F | 2451.67 ± 631.40 | 17.67 ± 1.67 | 153.67 ± 13.22 | 63.67 ± 3.84 | 18.67 ± 1.45 | 7.33 ± 1.20 |
| RW | 5457.67 ± 208.73 | 23.00 ± 2.00 | 181.67 ± 24.18 | 82.00 ± 7.00 | 25.00 ± 1.53 | 18.67 ± 1.76 | |
| Mean | 3954.67 | 20.34 | 167.67 | 72.83 | 21.83 | 13.00 | |
| H | F | 3402.00 ± 1480.88 | 18.33 ± 9.24 | 211.00 ± 48.40 | 59.00 ± 12.74 | 18.00 ± 4.04 | 17.33 ± 5.04 |
| RW | 1643.33 ± 815.76 | 16.67 ± 6.98 | 224.67 ± 10.81 | 66.67 ± 2.85 | 19.33 ± 0.67 | 20.33 ± 14.44 | |
| Mean | 2522.67 | 17.50 | 217.84 | 62.83 | 18.67 | 18.83 | |
| Significance | |||||||
| Cultivation system (CS) | 0.154 ns | 0.646 ns | 0.115 ns | 0.228 ns | 0.206 ns | 0.472 ns | |
| Substrates (S) | 0.512 ns | 0.765 ns | 0.484 ns | 0.128 ns | 0.135 ns | 0.380 ns | |
| CS × S | 0.08 ns | 0.877 ns | 0.356 ns | 0.245 ns | 0.201 ns | 0.648 ns | |
| Treatments | NO3 | P | K | Ca | Mg | |
|---|---|---|---|---|---|---|
| Cultivation System (CS) | Substrate(S) | mg kg−1 f.w. | g plant−1 d.w. | |||
| A | F | 2485.00 ± 694.26 | 2.00 ± 0.00 | 94.00 ± 3.06 | 7.00 ± 0.00 | 4.00 ± 0.00 a |
| RW | 5783.00 ± 3230.14 | 1.67 ± 0.33 | 102.00 ± 8.72 | 6.00 ± 0.58 | 3.33 ± 0.33 a | |
| Mean | 4134.00 | 1.83 | 98.00 | 6.50 | 3.67 | |
| H | F | 3712.00 ± 452.27 | 1.67 ± 0.33 | 91.00 ± 12.12 | 5.33 ± 0.67 | 2.67 ± 0.33 b |
| RW | 2020.67 ± 397.76 | 1.67 ± 0.33 | 87.33 ± 3.18 | 5.33 ± 0.33 | 2.67 ± 0.33 b | |
| Mean | 2866.33 | 1.67 | 89.17 | 5.33 | 2.67 | |
| Significance | ||||||
| Cultivation system (CS) | 0.472 ns | 0.580 ns | 0.289 ns | 0.038 * | 0.009 ** | |
| Substrates (S) | 0.645 ns | 0.580 ns | 0.788 ns | 0.320 ns | 0.282 ns | |
| CS × S | 0.44 ns | 0.802 ns | 0.609 ns | 0.109 ns | 0.032 * | |
| Treatments | NO3 | P | K | Ca | Mg | |
|---|---|---|---|---|---|---|
| Cultivation System (CS) | Substrate (S) | mg kg−1 f.w. | g Plant−1 d.w. | |||
| A | F | 2998.70 ± 63.19 | 0.58 ± 0.12 | 75.94 ± 6.77 | 4.28 ± 0.22 | 10.25 ± 0.63 |
| RW | 2476.03 ± 158.60 | 0.68 ± 0.25 | 81.95 ± 10.90 | 3.17 ± 0.24 | 11.43 ± 1.69 | |
| Mean | 2737.36 | 0.63 | 78.95 | 3.73 | 10.84 | |
| H | F | 2356.53 ± 125.05 | 0.68 ± 0.10 | 77.77 ± 13.13 | 3.73 ± 0.07 | 7.70 ± 1.33 |
| RW | 2491.60 ± 178.48 | 0.54 ± 0.18 | 85.25 ± 4.71 | 3.46 ± 0.37 | 8.04 ± 0.49 | |
| Mean | 2424.07 | 0.61 | 81.51 | 3.60 | 7.86 | |
| Significance | ||||||
| Cultivation system (CS) | 0.053 ns | 0.915 ns | 0.793 ns | 0.620 ns | 0.032 * | |
| Substrates (S) | 0.199 ns | 0.920 ns | 0.497 ns | 0.020 * | 0.527 ns | |
| CS × S | 0.050 ns | 0.920 ns | 0.897 ns | 0.066 ns | 0.141 ns | |
| System | NO3 | PO4 | SO4 | K | Ca | Mg | Na | Cl |
|---|---|---|---|---|---|---|---|---|
| H | 390.28 | 45.70 | 80.43 | 95.77 | 113.64 | 35.98 | 10.35 | 7.63 |
| A | 469.93 | 50.04 | 60.04 | 40.65 | 107.46 | 31.77 | 41.23 | 59.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorini, L.; Modarelli, G.C.; Di Pierro, P.; Langellotti, A.L.; Cirillo, C.; De Pascale, S.; Masi, P. Effects of Seedling Substrate and Hydroponic Versus Aquaponic Nutrient Solution on Growth, Nutrient Uptake, and Eco-Physiological Response of Lemon Basil (Ocimum × citriodorum). Plants 2025, 14, 1929. https://doi.org/10.3390/plants14131929
Signorini L, Modarelli GC, Di Pierro P, Langellotti AL, Cirillo C, De Pascale S, Masi P. Effects of Seedling Substrate and Hydroponic Versus Aquaponic Nutrient Solution on Growth, Nutrient Uptake, and Eco-Physiological Response of Lemon Basil (Ocimum × citriodorum). Plants. 2025; 14(13):1929. https://doi.org/10.3390/plants14131929
Chicago/Turabian StyleSignorini, Linda, Giuseppe Carlo Modarelli, Prospero Di Pierro, Antonio Luca Langellotti, Chiara Cirillo, Stefania De Pascale, and Paolo Masi. 2025. "Effects of Seedling Substrate and Hydroponic Versus Aquaponic Nutrient Solution on Growth, Nutrient Uptake, and Eco-Physiological Response of Lemon Basil (Ocimum × citriodorum)" Plants 14, no. 13: 1929. https://doi.org/10.3390/plants14131929
APA StyleSignorini, L., Modarelli, G. C., Di Pierro, P., Langellotti, A. L., Cirillo, C., De Pascale, S., & Masi, P. (2025). Effects of Seedling Substrate and Hydroponic Versus Aquaponic Nutrient Solution on Growth, Nutrient Uptake, and Eco-Physiological Response of Lemon Basil (Ocimum × citriodorum). Plants, 14(13), 1929. https://doi.org/10.3390/plants14131929













