Digitally Quantifying Growth and Verdancy of Lolium Plants In Vitro
Abstract
1. Introduction
2. Results
2.1. Greenness Index
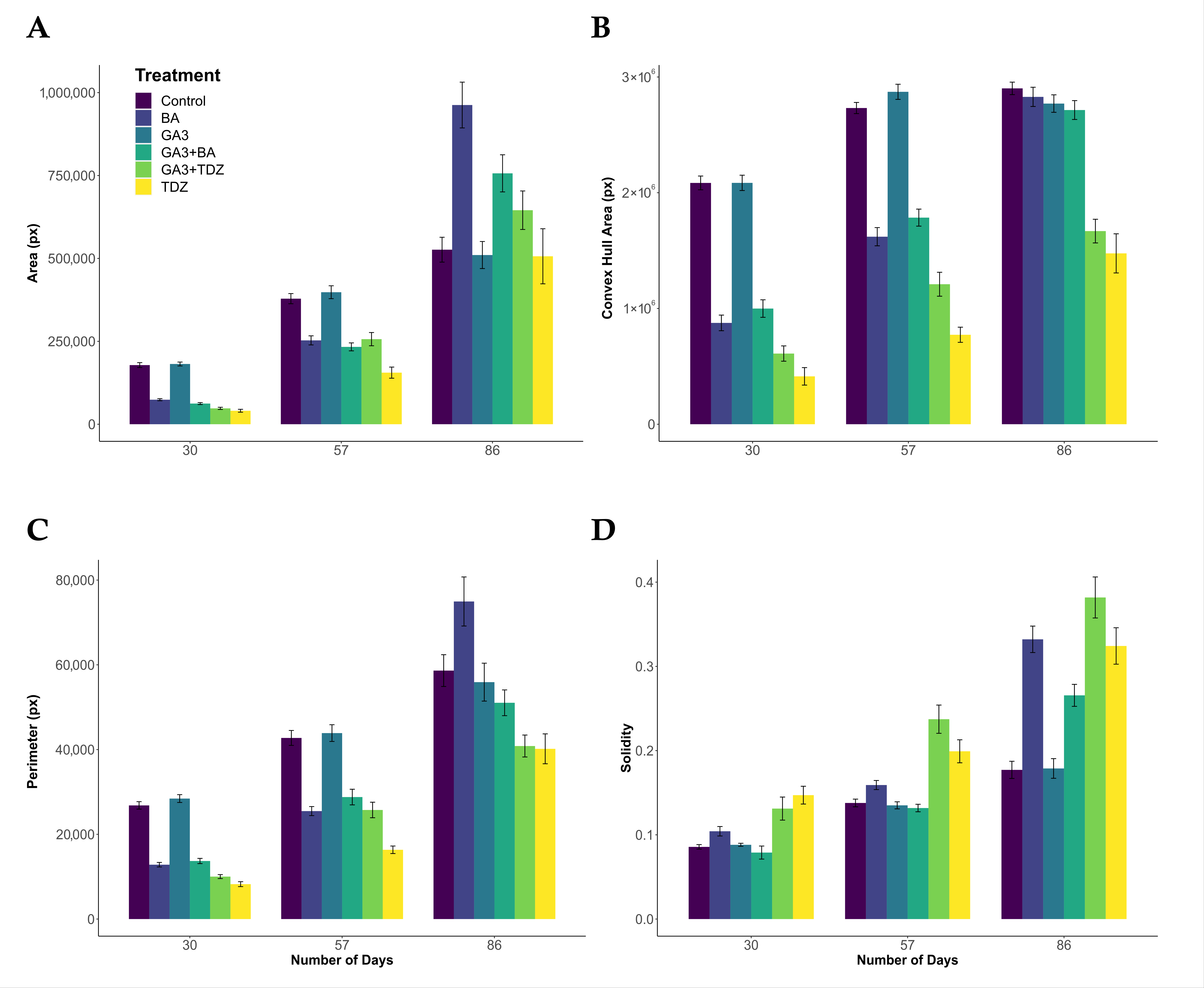
2.2. Area
2.3. Convex Hull Area
2.4. Perimeter
2.5. Solidity
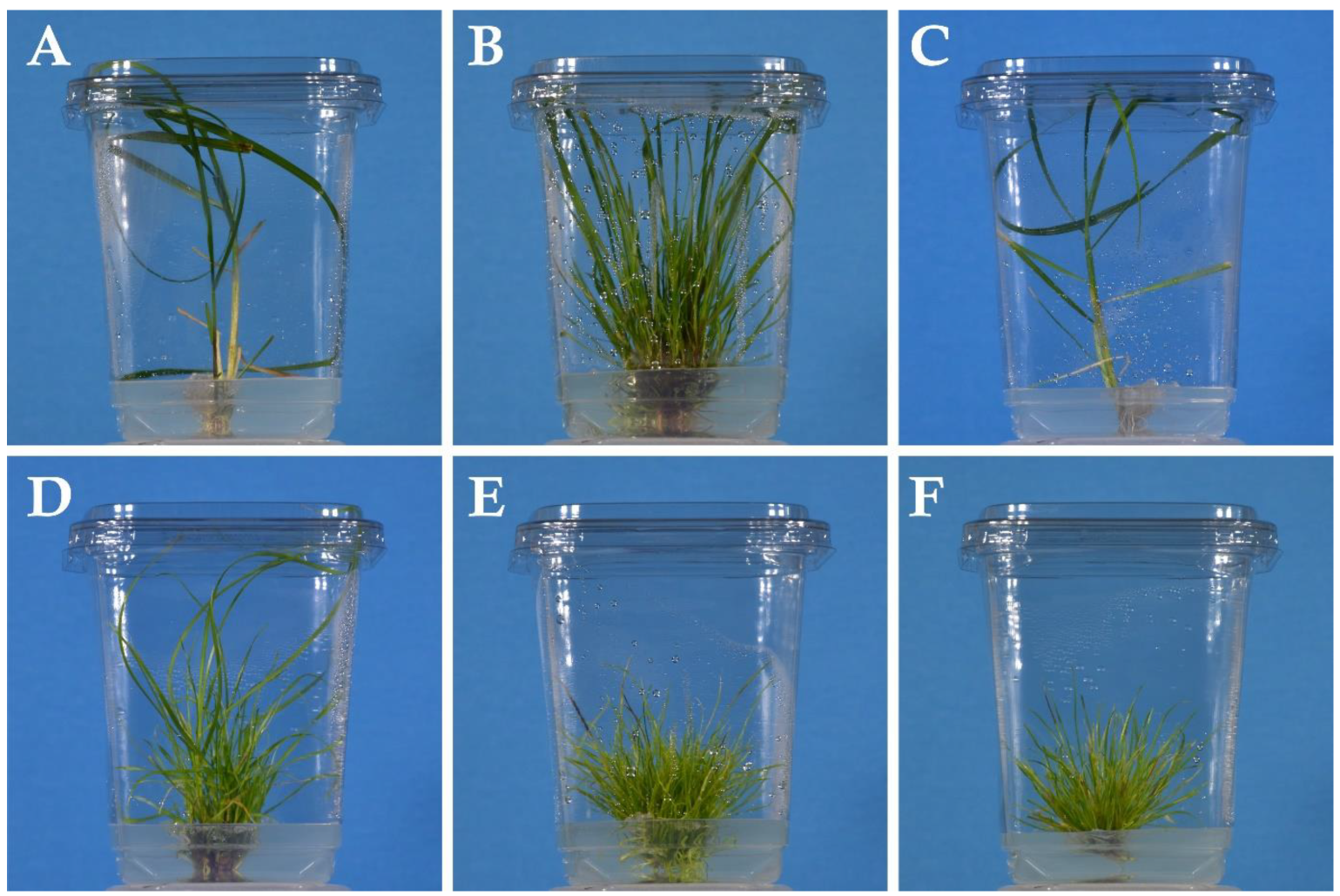
2.6. General Survival
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Seed Sterilisation
4.3. Seed Germination
4.4. Hormone Treatments
4.5. Collection of Images
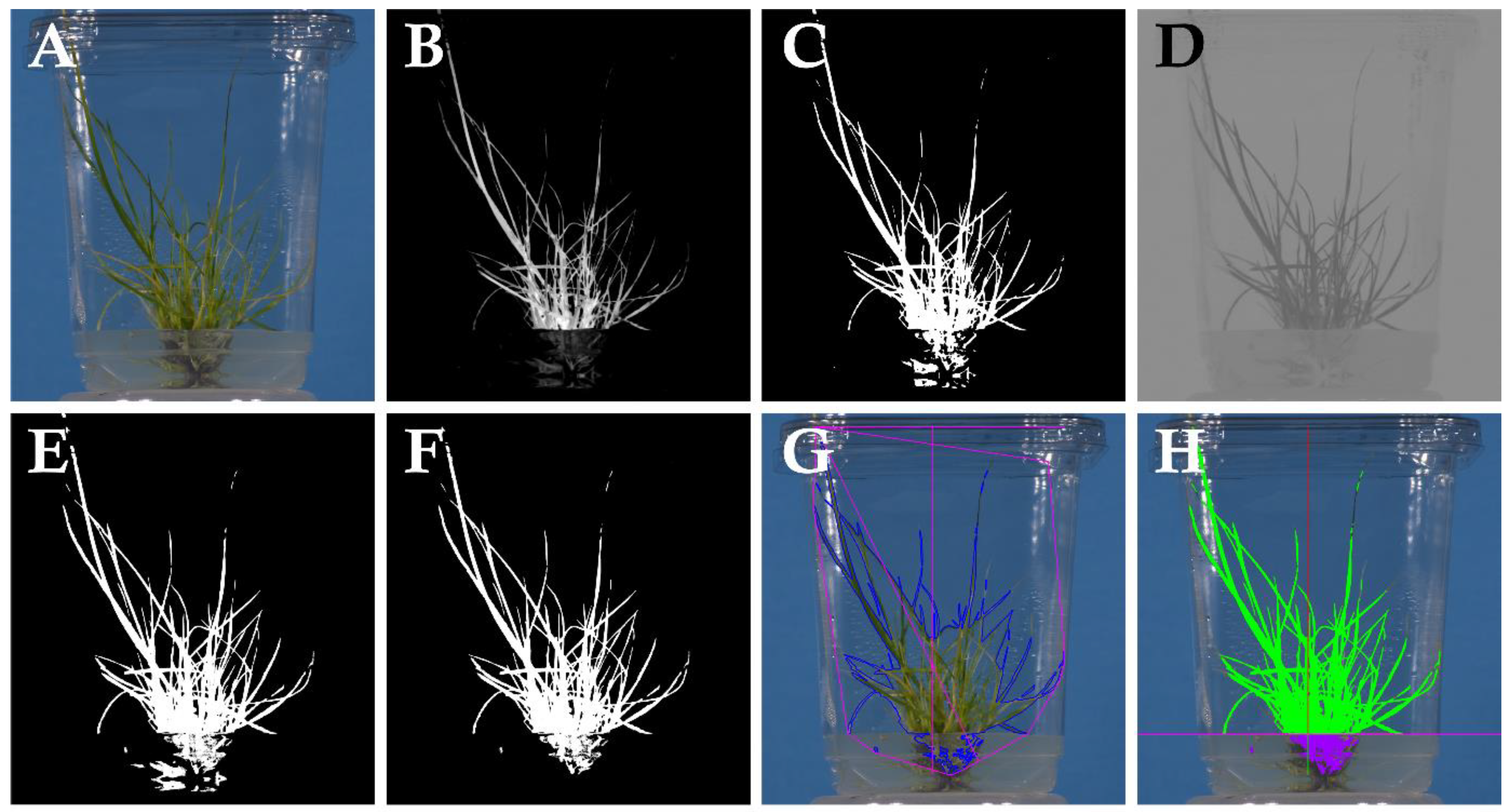
4.6. Image Analysis
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.A.L.; Spimer, L.A.; Meyer, M.J.; McClelland, M.T. Non-Invasive Image Analysis Evaluation of Growth during Plant Micropropagation. Plant Cell Tiss. Org. 1989, 19, 91–102. [Google Scholar] [CrossRef]
- Fahlgren, N.; Feldman, M.; Gehan, M.A.; Wilson, M.S.; Shyu, C.; Bryant, D.W.; Hill, S.T.; McEntee, C.J.; Warnasooriya, S.N.; Kumar, I.; et al. A Versatile Phenotyping System and Analytics Platform Reveals Diverse Temporal Responses to Water Availability in Setaria. Mol. Plant 2015, 8, 1520–1535. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Suen, C.Y. A Fast Parallel Algorithm for Thinning Digital Patterns. Commun. ACM 1984, 27, 239. [Google Scholar] [CrossRef]
- Sakeef, N.; Scandola, S.; Kennedy, C.; Lummer, C.; Chang, J.; Uhrig, R.G.; Lin, G. Machine Learning Classification of Plant Genotypes Grown under Different Light Conditions through the Integration of Multi-Scale Time-Series Data. Comput. Struct. Biotechnol. J. 2023, 21, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Al-Lami, M.K.; Nguyen, D.; Oustriere, N.; Burken, J.G. High Throughput Screening of Native Species for Tailings Eco-Restoration Using Novel Computer Visualization for Plant Phenotyping. Sci. Total Environ. 2021, 780, 146490. [Google Scholar] [CrossRef]
- Dhondt, S.; Gonzalez, N.; Blomme, J.; De Milde, L.; Van Daele, T.; Van Akoleyen, D.; Storme, V.; Coppens, F.; TS Beemster, G.; Inze, D. High-Resolution Time-Resolved Imaging of in Vitro Arabidopsis Rosette Growth. Plant J. 2014, 80, 172–184. [Google Scholar] [CrossRef]
- Faragó, D.; Sass, L.; Valkai, I.; Andrasi, N.; Szabados, L. PlantSize Offers an Affordable, Non-Destructive Method to Measure Plant Size and Color in Vitro. Front. Plant Sci. 2018, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Mestre, D.; Fonseca, J.M.; Mora, A. Monitoring of in Vitro Plant Cultures Using Digital Image Processing and Random Forests. In Proceedings of the 8th International Conference of Pattern Recognition Systems (ICPRS 2017), Madrid, Spain, 11–13 July 2017; pp. 1–6. [Google Scholar]
- Young, C.; Hume, D.; McCulley, R. Forages and Pastures Symposium: Fungal Endophytes of Tall Fescue and Perennial Ryegrass: Pasture Friend or Foe? J. Anim. Sci. 2013, 91, 2379–2394. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Badenhorst, P.; Wang, J.; Jacobs, J.; Spangenberg, G.; Smith, K. An Object-Based Image Analysis Approach to Assess Persistence of Perennial Ryegrass (Lolium perenne L.) in Pasture Breeding. Agronomy 2019, 9, 501. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.X.; Yuan, J.G.; Xi, J.B.; Du, X.L.; Yang, Z.Y. Callus Induction and Plant Regeneration in Eleven Perennial Ryegrass Cultivars. Biotechnol. Biotechnol. Equip. 2014, 20, 30–37. [Google Scholar] [CrossRef]
- Cao, M.X.; Huang, J.Q.; He, Y.L.; Liu, S.J.; Wang, C.L.; Jiang, W.Z.; Wei, Z.M. Transformation of Recalcitrant Turfgrass Cultivars through Improvement of Tissue Culture and Selection Regime. Plant Cell Tissue Organ Cult. 2006, 85, 307–316. [Google Scholar] [CrossRef]
- Josekutty, P.; Potlakayala, S.D.; Templin, R.; Rudrabhatla, S. In Vitro Flowering Studies with Nine Cultivars of Perennial Ryegrass (Lolium perenne L.). Floric. Ornam. Biotechnol. 2011, 5, 45–49. [Google Scholar]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant Hormones and Plant Growth Regulators in Plant Tissue Culture. Vitr. Cell. Dev. Biol.-Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Noodén, L.D.; Leopold, A.C. Senescence and Aging in Plants; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Graebe, J.E. Gibberellin Biosynthesis and Control. Annu. Rev. Plant Physiol. 1987, 38, 419–465. [Google Scholar] [CrossRef]
- Joshi, R.; Shukla, A.; Kumar, P. In Vitro Flowering in Hill Maize: A Novel Technique for Future. Indian J. Plant Physiol. 2009, 16, 299–302. [Google Scholar]
- Devi, P.; Zhong, H.; Sticklen, M.B. In Vitro Morphogenesis of Pearl Millet [Pennisetum glaucum (L.) R. Br.]: Efficient Production of Multiple Shoots and Inflorescences from Shoot Apices. Plant Cell Rep. 2000, 19, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.M.; Elahinia, A. Optimization of Medium Composition for Apple Rootstocks. Afr. J. Biotechnol. 2011, 10, 3594–3601. [Google Scholar]
- King, R.W.; Blundell, C.; Evans, L.T. The Behaviour of Shoot Apices of Lolium temulentum in Vitro as the Basis of an Assay System for Florigenic Extracts. Funct. Plant Biol. 1993, 3, 337–348. [Google Scholar] [CrossRef]
- McDaniel, C.N.; Hartnett, L.K. Flowering as Metamorphosis: Two Sequential Signals Regulate Floral Initiation in Lolium temulentum. Development 1996, 122, 3661–3668. [Google Scholar] [CrossRef]
- McDaniel, C.N.; King, R.W.; Evans, L.T. Floral Determination and in Vitro Floral Differentiation in Isolated Shoot Apices of Lolium temulentum L. Planta 1991, 185, 9–16. [Google Scholar] [CrossRef]
- Rao, I.V.R.; Rao, I.U.; Narang, V.; Jerath, R.; Pillai, K.G. Mass Propagation of Bamboos from Somatic Embryos and Their Successful Transfer to the Forest. In Bamboos Current Research, Proceedings of the International Bamboo Workshop; Kerala Forest Research Institute, India and International Development Research Institute, Canada: Cochin, India, 1990; pp. 167–172. ISBN 981-00-2084-8. [Google Scholar]
- Rout, G.R.; Das, P. Somatic Embryogenesis and in Vitro Flowering of 3 Species of Bamboo. Plant Cell Rep. 1994, 13, 683–686. [Google Scholar] [CrossRef]
- Britto, S.J.; Natarajan, E.; Arockiasamy, D.I. In Vitro Flowering and Shoot Multiplication from Nodal Explants of Ceropegia bulbosa Roxb. var. bulbosa. Taiwania 2003, 48, 106–111. [Google Scholar]
- Isogai, S.; Touno, K.; Shimomura, K. Gibberellic Acid Improved Shoot Multiplication in Cephaelis Ipecacuanha. Vitr. Cell. Dev. Biol. Plant 2008, 44, 216–220. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–Cytokinin Interaction Regulates Meristem Development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Agarwal, A.; Dutta Gupta, S. Assessment of Spinach Seedling Health Status and Chlorophyll Content by Multivariate Data Analysis and Multiple Linear Regression of Leaf Image Features. Comput. Electron. Agric. 2018, 152, 281–289. [Google Scholar] [CrossRef]
- Gaba, V.P. Plant Growth Regulators in Plant Tissue Culture and Development; Trigiano, R.N., Dennis, J.G., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Capelle, S.C.; Mok, D.W.; Kirchner, S.C.; Mok, M.C. Effects of Thidiazuron on Cytokinin Autonomy and the Metabolism of N 6-(Δ2-Isopentenyl)[8-14C] Adenosine in Callus Tissues of Phaseolus lunatus L. Plant Physiol. 1983, 73, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Bethge, H.; Winkelmann, T.; Ludeke, P.; Rath, T. Low-Cost and Automated Phenotyping System “Phenomenon” for Multi-Sensor in Situ Monitoring in Plant in Vitro Culture. Plant Methods 2023, 19, 42. [Google Scholar] [CrossRef]
- Hamuda, E.; Glavin, M.; Jones, E. A Survey of Image Processing Techniques for Plant Extraction and Segmentation in the Field. Comput. Electron. Agric. 2016, 125, 184–199. [Google Scholar] [CrossRef]
- Gehan, M.A.; Fahlgren, N.; Abbasi, A.; Berry, J.C.; Callen, S.T.; Chavez, L.; Doust, A.N.; Feldman, M.J.; Gilbert, K.B.; Hodge, J.G.; et al. PlantCV v2: Image Analysis Software for High-Throughput Plant Phenotyping. PeerJ 2017, 5, e4088. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Zhong, X.; Preng, S.; Sheehy, J.E.; Visperas, R.M.; Liu, H. Relationship between Tillering and Leaf Area Index: Quantifying Critical Leaf Area Index for Tillering in Rice. J. Agric. Sci. 2002, 138, 269–279. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Gaion, L.A.; Galati, V.C.; Cardoso, A.C.R.; Rossatto, D.R.; Carvalho, R.F. Shifting the Anthocyanins from Light to Shade: What Roles Do These Pigments Play? Theor. Exp. Plant Physiol. 2025, 37, 16. [Google Scholar] [CrossRef]
- Agarwal, A.; Colwell, F.D.J.; Galvis, V.A.C.; Hill, T.R.; Boonham, N.; Prashar, A. Two-Fold Red Excess (TREx): A Simple and Novel Digital Color Index That Enables Non-Invasive Real-Time Monitoring of Green-Leaved as Well as Anthocyanin-Rich Crops. Plant Methods 2025, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Dongre, P.K.; Dutta Gupta, S. Smartphone-Assisted Real-Time Estimation of Chlorophyll and Carotenoid Concentrations and Ratio Using the Inverse of Red and Green Digital Color Features. Theor. Exp. Plant Physiol. 2021, 33, 293–302. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.; Miller, R.; Ojimi, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Amiri, M.E.; Elahinia, A. Influence of Medium Compositions on Growth of Apple Rootstocks (“M9”, “M27”, ’MM106’) in in Vitro Condition. Acta Hortic. 2011, 923, 139–146. [Google Scholar] [CrossRef]
- Ansari, S.A.; Kumar, S.; Palanisamy, K. Peroxidase Activity in Relation to in Vitro Rhizogenesis and Precocious Flowering in Bambusa Arundinacea. Curr. Sci. 1996, 71, 358–359. [Google Scholar]
- International Commission on Illumination. Colorimetry, 4th ed.; International Commission on Illumination: Vienna, Austria, 2018. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 7 April 2025).
- Lenth, R. V Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://rvlenth.github.io/emmeans/ (accessed on 7 April 2025).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
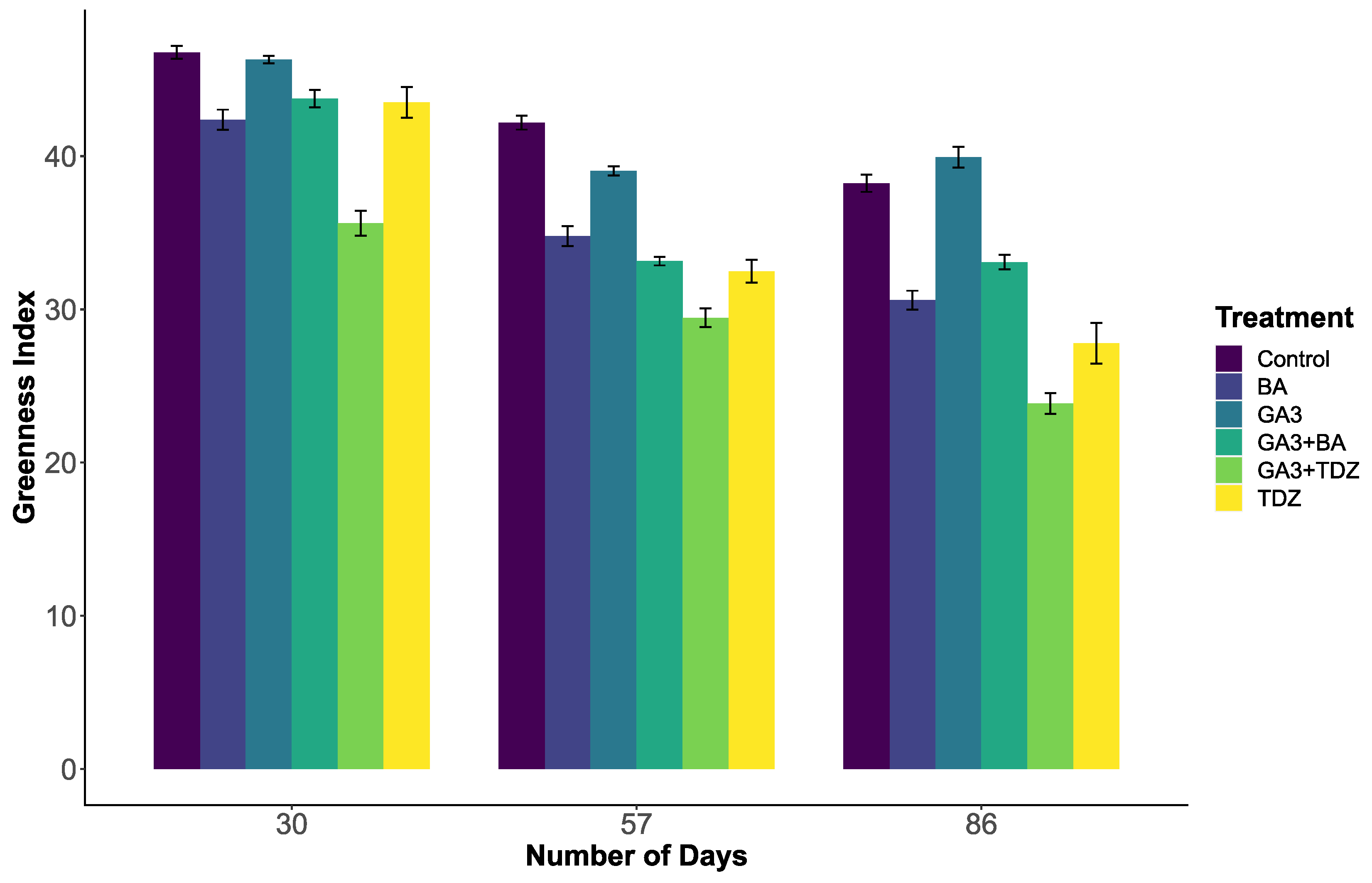


| Response Variable | R2 | Factor | χ2 | DF | Pr (>χ2) | q-Value |
|---|---|---|---|---|---|---|
| Area | 0.62 | day | 310.412 | 2 | <0.0001 | <0.0001 |
| treatment | 15.873 | 5 | 0.007 | 0.007 | ||
| day:treatment | 55.043 | 10 | <0.0001 | <0.0001 | ||
| Convex Hull Area | 0.73 | day | 233.200 | 2 | <0.0001 | <0.0001 |
| treatment | 363.638 | 5 | <0.0001 | <0.0001 | ||
| day:treatment | 46.099 | 10 | <0.0001 | <0.0001 | ||
| Greenness | 0.69 | day | 289.825 | 2 | <0.0001 | <0.0001 |
| treatment | 255.439 | 5 | <0.0001 | <0.0001 | ||
| day:treatment | 30.877 | 10 | 0.0006 | 0.0019 | ||
| Perimeter | 0.56 | day | 217.197 | 2 | <0.0001 | <0.0001 |
| treatment | 58.236 | 5 | <0.0001 | <0.0001 | ||
| day:treatment | 30.118 | 10 | 0.0008 | 0.0018 | ||
| Solidity | 0.59 | day | 235.852 | 2 | <0.0001 | <0.0001 |
| treatment | 87.632 | 5 | <0.0001 | <0.0001 | ||
| day:treatment | 37.406 | 10 | <0.0001 | 0.0002 |
| Designation | Plant Growth Regulator(s) | Concentration (µM) |
|---|---|---|
| Control | NA | 0 |
| BA | N6-benzylaminopurine | 8.8 |
| GA3 | Gibberellic acid | 2.8 |
| TDZ | Thidiazuron | 9 |
| GA3 + TDZ | Gibberellic acid | 2.8 |
| Thidiazuron | 9 | |
| GA3 + BA | Gibberellic acid | 2.8 |
| N6-benzylaminopurine | 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depetris, M.B.; Dimech, A.M.; Guthridge, K.M. Digitally Quantifying Growth and Verdancy of Lolium Plants In Vitro. Plants 2025, 14, 1499. https://doi.org/10.3390/plants14101499
Depetris MB, Dimech AM, Guthridge KM. Digitally Quantifying Growth and Verdancy of Lolium Plants In Vitro. Plants. 2025; 14(10):1499. https://doi.org/10.3390/plants14101499
Chicago/Turabian StyleDepetris, Mara B., Adam M. Dimech, and Kathryn M. Guthridge. 2025. "Digitally Quantifying Growth and Verdancy of Lolium Plants In Vitro" Plants 14, no. 10: 1499. https://doi.org/10.3390/plants14101499
APA StyleDepetris, M. B., Dimech, A. M., & Guthridge, K. M. (2025). Digitally Quantifying Growth and Verdancy of Lolium Plants In Vitro. Plants, 14(10), 1499. https://doi.org/10.3390/plants14101499








