Microstructure and Microorganisms Alternation of Paddy Soil: Interplay of Biochar and Water-Saving Irrigation
Abstract
1. Introduction
2. Results
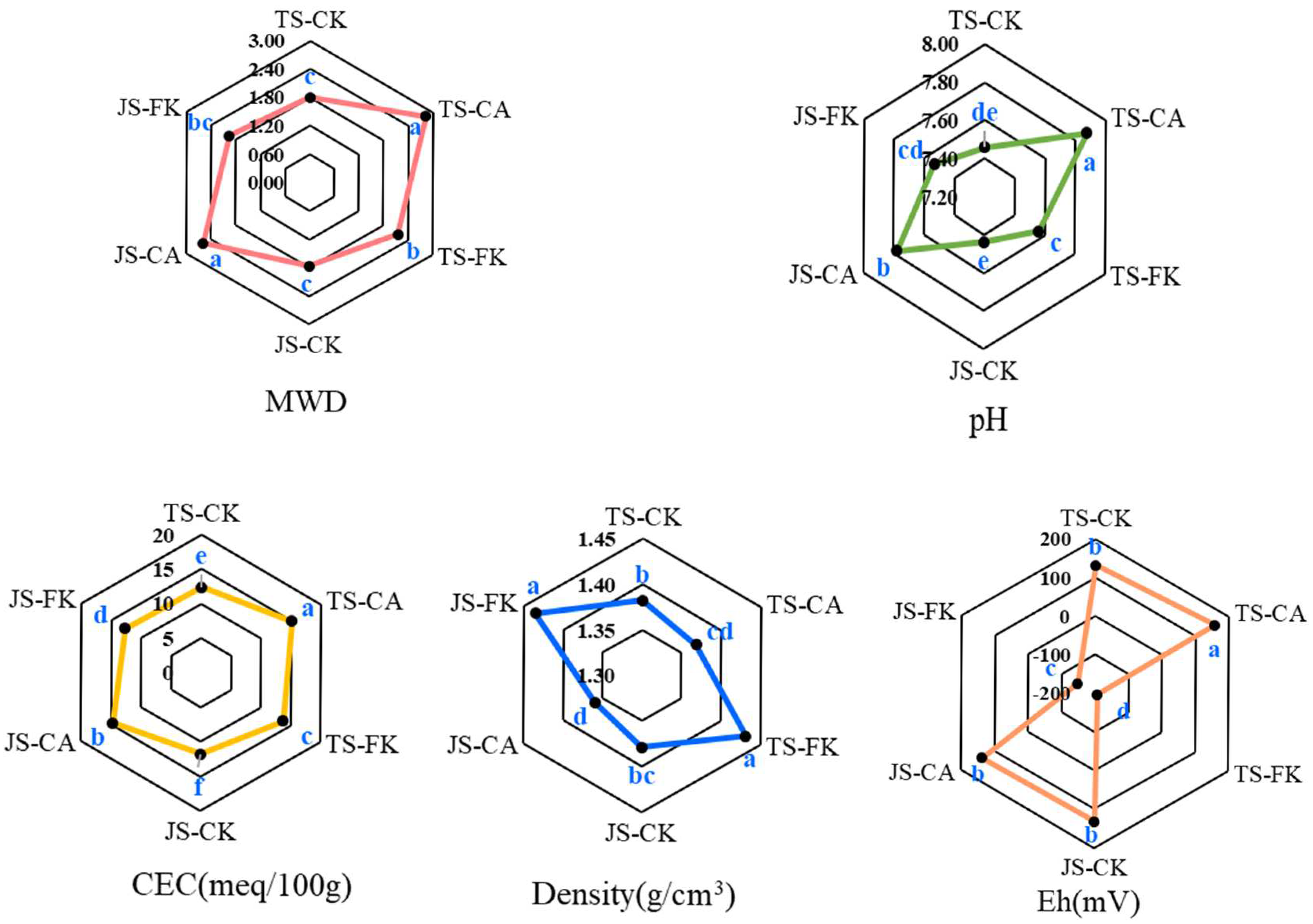
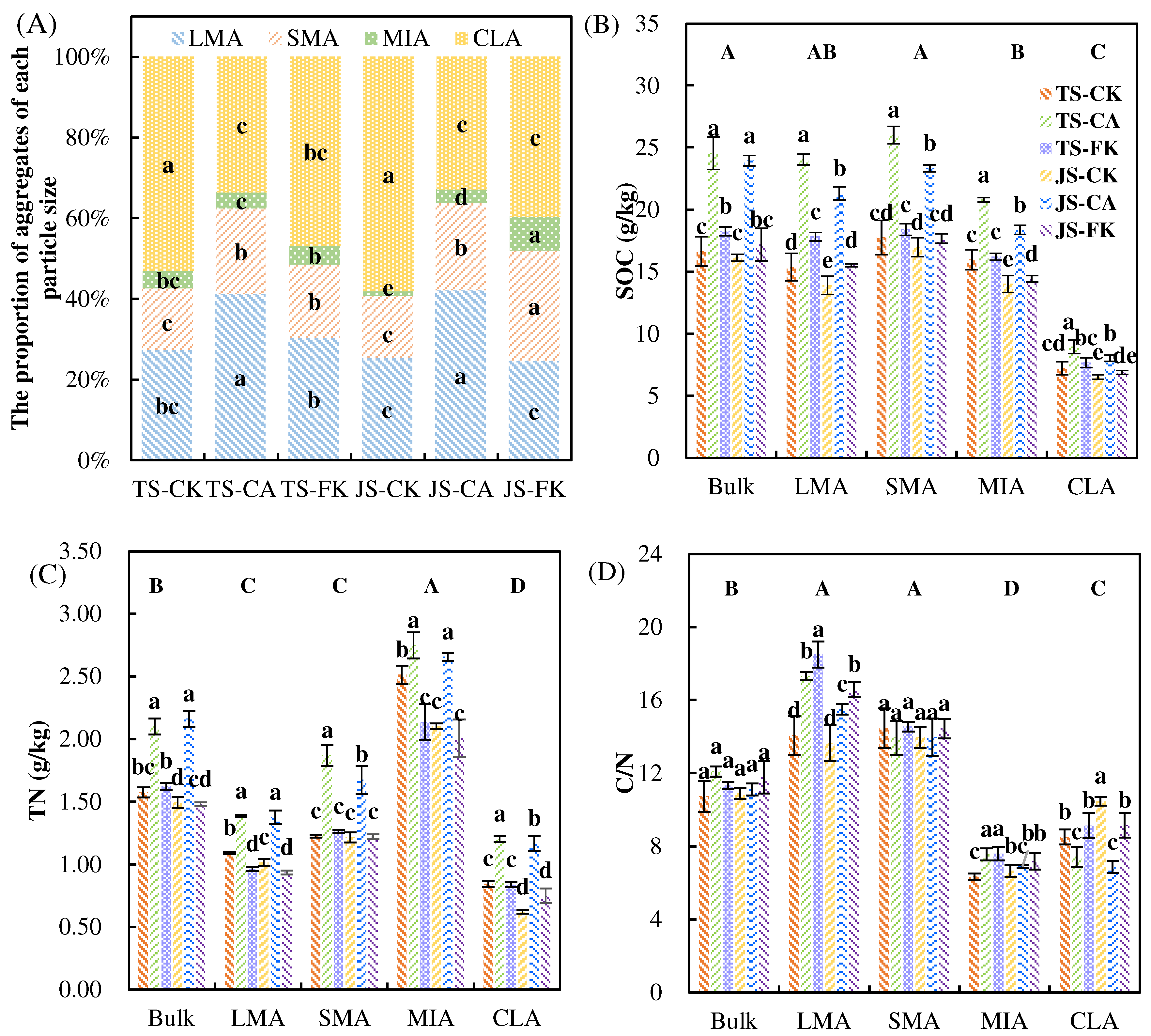
2.1. Soil Physicochemical Characteristics
2.1.1. Soil Basic Properties
2.1.2. SOC, and TN in Bulk Soil and Aggregate Classes
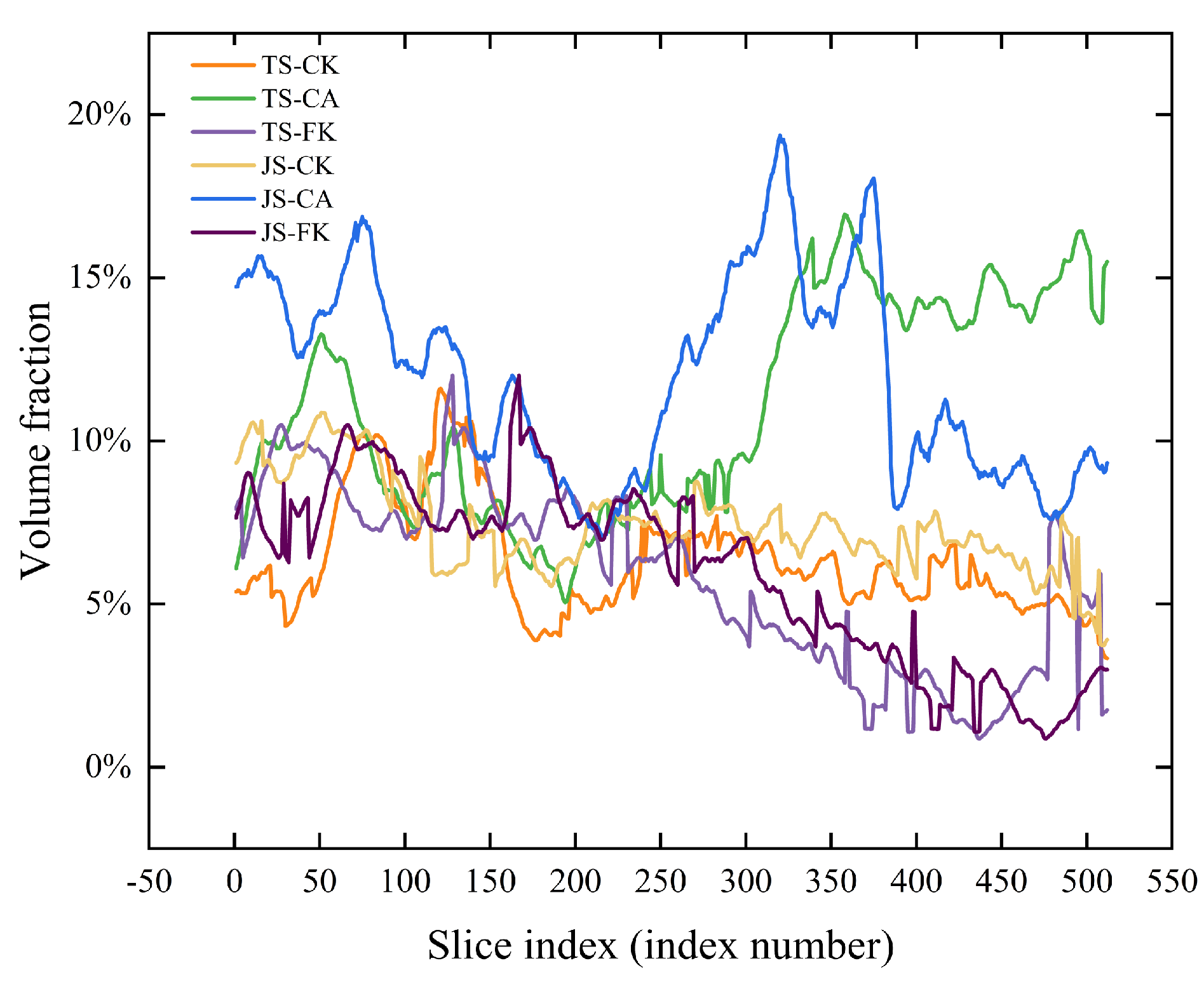
2.2. CT-Measured Microstructure Characteristics
2.2.1. Pore Structure
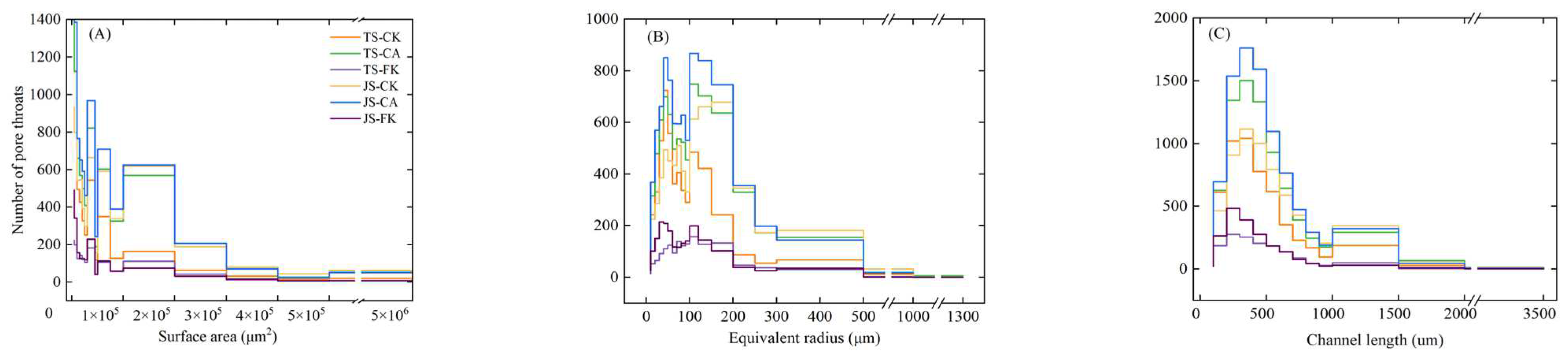
2.2.2. Pore Throat Structure
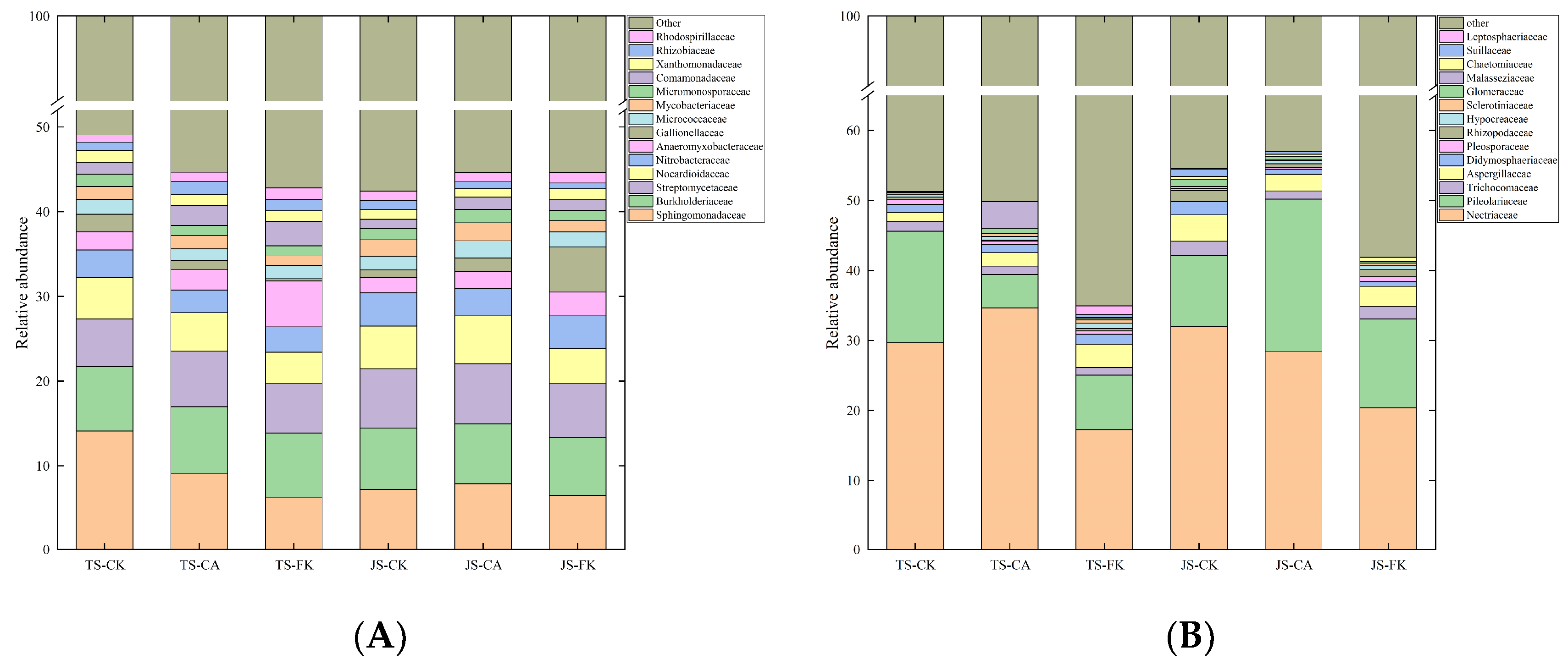
2.3. Composition and Functional Properties of Soil Bacterial and Fungi Communities
2.3.1. Community Structure of Bacterial and Fungi Genes
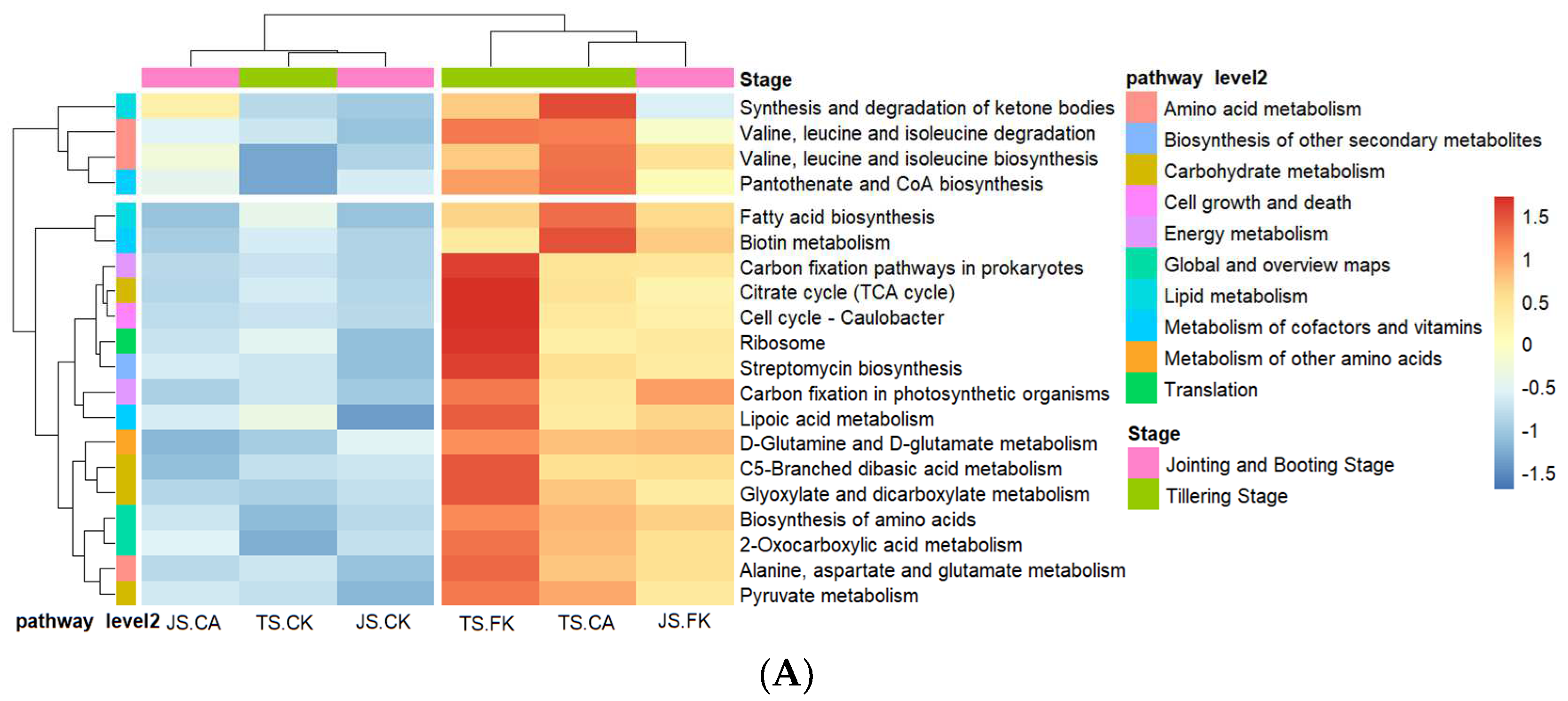
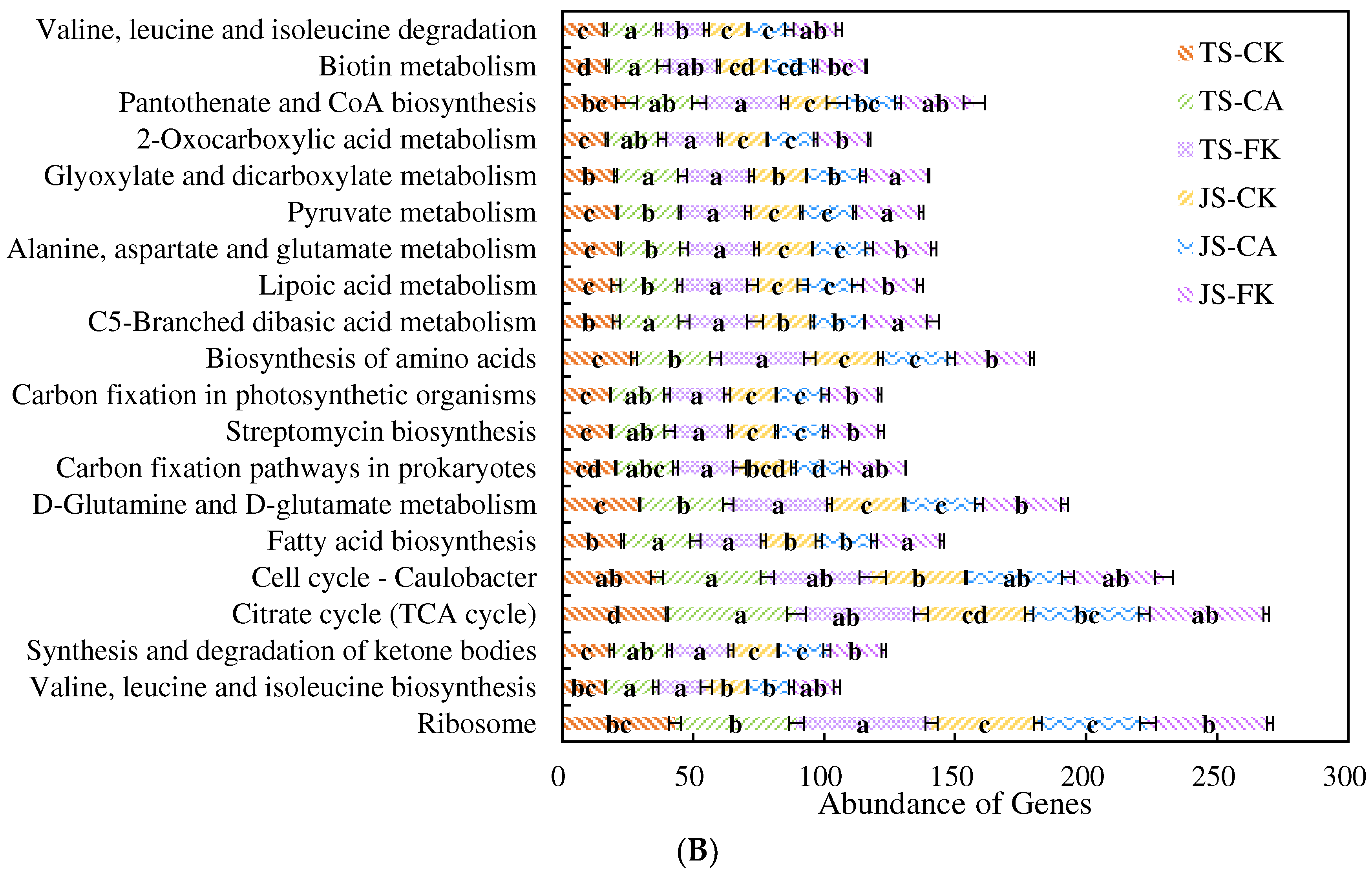
2.3.2. Functional Categories of Microorganisms
3. Discussion
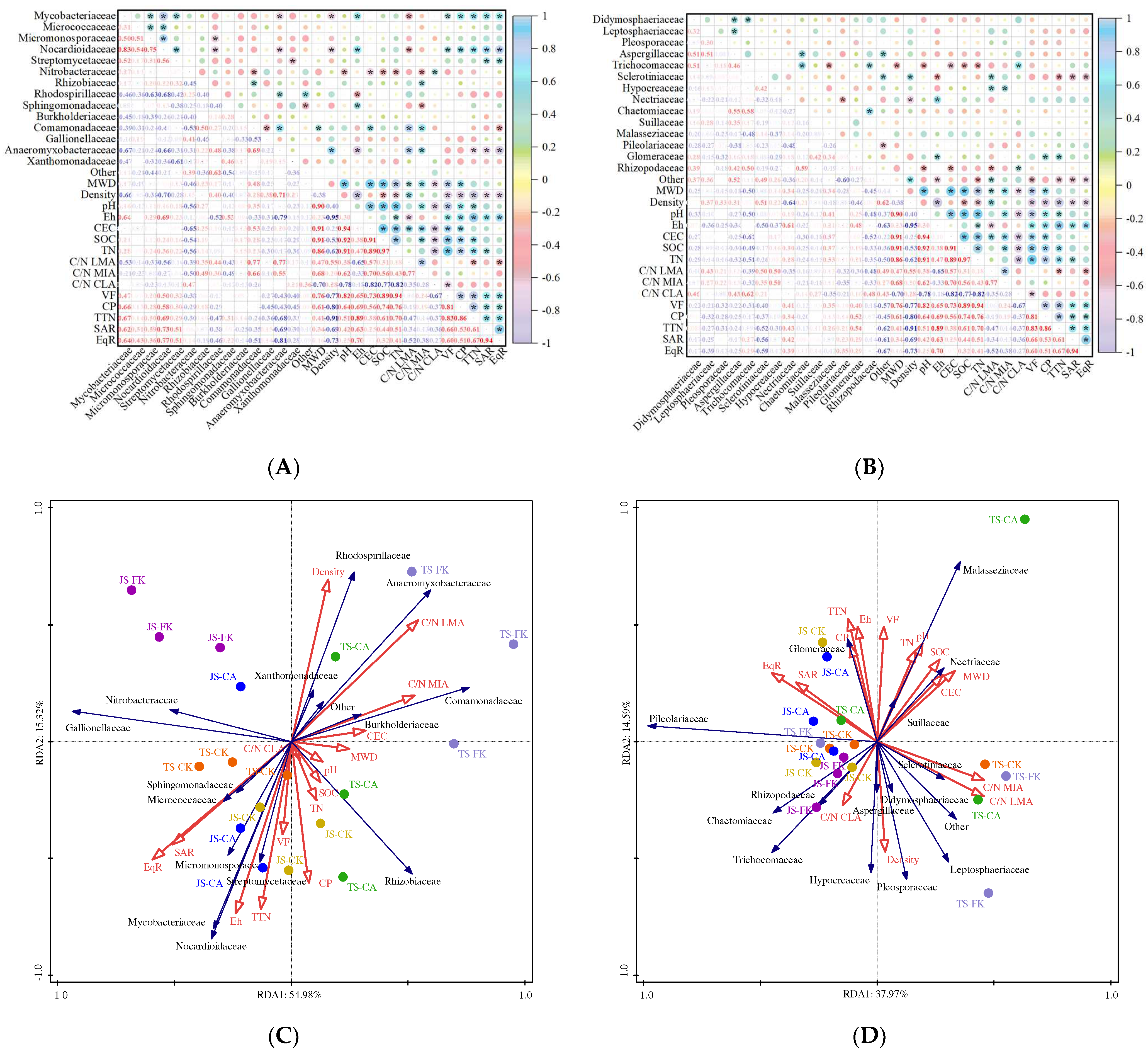
3.1. Influence of Soil Physicochemical Properties on Soil Microstructure Under Biochar and Water-Saving Irrigation
3.2. Impact of Soil Microstructure on Soil Microbial Composition and Function with Biochar and Water-Saving Irrigation
4. Materials and Methods
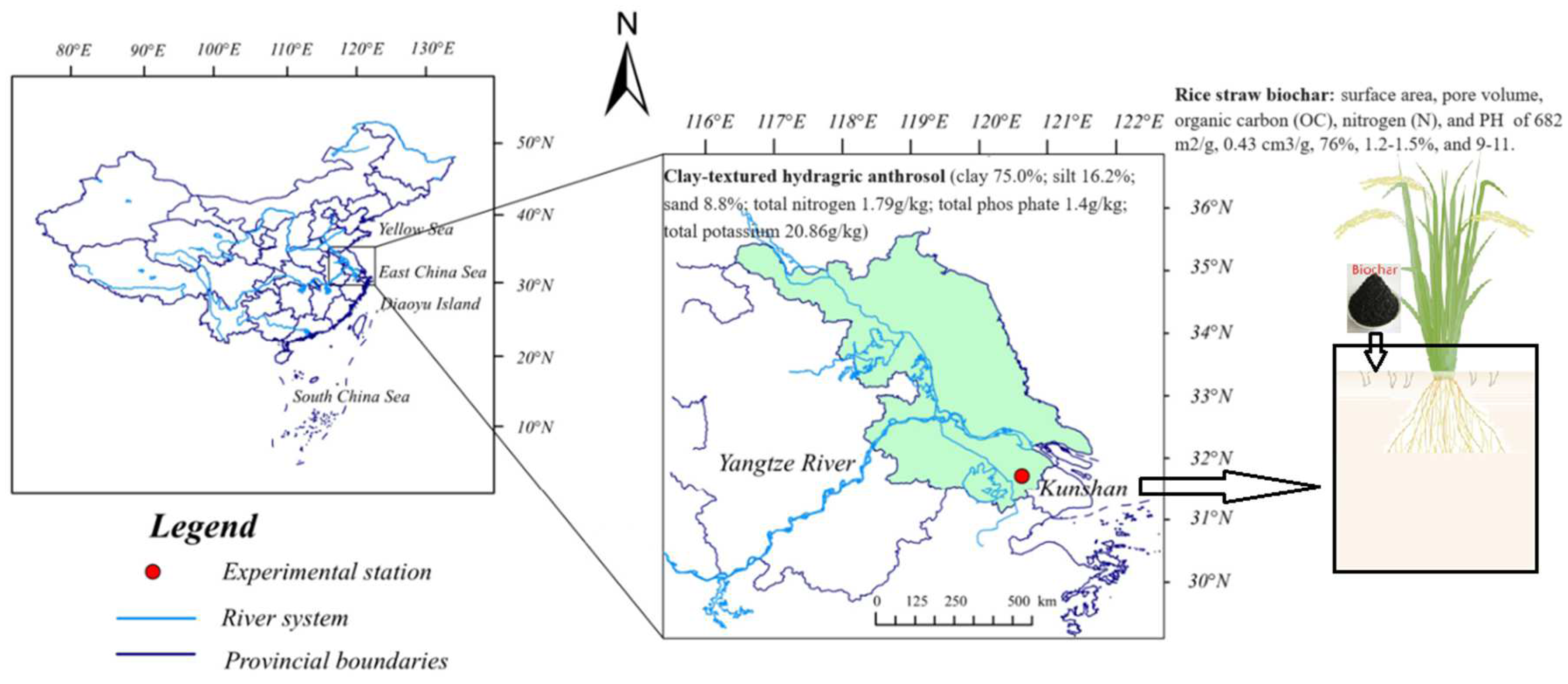
4.1. Experimental Site and Design
4.2. Soil Sampling and Properties
4.3. Determination of Physical and Chemical Properties of Soil
4.4. CT Scanning and Image Processing
4.5. Determination of Soil Microbial Community Diversity, Composition, and Metabolite Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Tao, W.; Li, J.; Li, W.; Wen, C.; Gao, S.; Wang, Y.; Liu, D.; Xu, L.; Jiang, Y.; Liu, Z.; et al. Higher rice productivity and lower paddy nitrogen loss with optimized irrigation and fertilization practices in a rice-upland system. Agric. Ecosyst. Environ. 2024, 374, 109176. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Mooney, S.J.; Peng, X.H. Effects of long-term inorganic and organic fertilizations on the soil micro and macro structures of rice paddies. Geoderma 2016, 266, 66–74. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Yang, S.H.; Pang, Q.Q.; Xu, Y.; Chen, X.; Sun, X.; Qi, S.T.; Yu, W.Q. Biochar improved soil health and mitigated greenhouse gas emission from controlled irrigation paddy field: Insights into microbial diversity. J. Clean. Prod. 2021, 318, 128595. [Google Scholar] [CrossRef]
- Xiang, S.; Doyle, A.; Holden, P.A.; Schimel, J.P. Drying and rewetting effects on C and N mineralization and microbial activity in surface and subsurface California grassland soils. Soil Biol. Biochem. 2008, 40, 2281–2289. [Google Scholar] [CrossRef]
- Zhang, W.J.; Munkholm, L.J.; Liu, X.; An, T.T.; Xu, Y.D.; Ge, Z.; Xie, N.H.; Li, A.M.; Dong, Y.Q.; Peng, C.; et al. Soil aggregate microstructure and microbial community structure mediate soil organic carbon accumulation: Evidence from one-year field experiment. Geoderma 2023, 430, 116324. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Fernández-Ugalde, O.; Barré, P.; Hubert, F.; Virto, I.; Girardin, C.; Ferrage, E.; Caner, L.; Chenu, C. Clay mineralogy differs qualitatively in aggregate-size classes: Clay-mineral-based evidence for aggregate hierarchy in temperate soils. Eur. J. Soil Sci. 2013, 64, 406–422. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Zhang, Q.; Zou, M.; Yin, Q.; Qiu, Y.; Qin, W. Effect of organic material addition on active soil organic carbon and microbial diversity: A meta-analysis. Soil Tillage Res. 2024, 241, 106128. [Google Scholar] [CrossRef]
- Ortas, I.; Akpinar, C.; Lal, R. Long-term impacts of organic and inorganic fertilizers on carbon sequestration in aggregates of an Entisol in Mediterranean Turkey. Soil Sci. 2013, 178, 12–23. [Google Scholar] [CrossRef]
- Zhao, R.; Kuzyakov, Y.; Zhang, H.; Wang, Z.; Li, T.; Shao, L.; Jiang, L.; Wang, R.; Li, M.; Sun, O.J.; et al. Labile carbon inputs offset nitrogen-induced soil aggregate destabilization via enhanced growth of saprophytic fungi in a meadow steppe. Geoderma 2024, 443, 116841. [Google Scholar] [CrossRef]
- Du, M.; Minasny, B.; Rabbi, S.M.F. Carbon to nitrogen stoichiometry of organic amendments influences the improvement of aggregate stability of a cropping vertisol. Soil Use Manag. 2024, 40, e13087. [Google Scholar] [CrossRef]
- Cao, W.; Zou, Y.; Li, G.; Shan, Y.; Wang, J.; Singh, B.K. The impact of agricultural land use on the linkages between soil microbial communities and agroecosystem functioning is depth-dependent. Agric. Ecosyst. Environ. 2025, 379, 109330. [Google Scholar] [CrossRef]
- Ananyeva, K.; Wang, W.; Smucker, A.J.M.; Rivers, M.L.; Kravchenko, A.N. Can intra-aggregate pore structures affect the aggregate’s effectiveness in protecting carbon? Soil Biol. Biochem. 2013, 57, 868–875. [Google Scholar] [CrossRef]
- Trost, B.; Ellmer, F.; Baumecker, M.; Meyer-Aurich, A.; Prochnow, A.; Drastig, K. Effects of irrigation and nitrogen fertilizer on yield, carbon inputs from above-ground harvest residues and soil organic carbon contents of a sandy soil in Germany. Soil Use Manag. 2014, 30, 538–546. [Google Scholar] [CrossRef]
- Juarez, S.; Nunan, N.; Duday, A.; Pouteau, V.; Schmidt, S.; Hapca, S.; Falconer, R.; Otten, W.; Chenu, C. Effects of different soil structures on the decomposition of native and added organic carbon. Eur. J. Soil Biol. 2013, 58, 81–90. [Google Scholar] [CrossRef]
- Lugato, E.; Morari, F.; Nardi, S.; Berti, A.; Giardini, L. Relationship between aggregate pore size distribution and organic-humic carbon in contrasting soils. Soil Tillage Res. 2009, 103, 153–157. [Google Scholar] [CrossRef]
- Livsey, J.; Katterer, T.; Vico, G.; Lyon, S.W.; Lindborg, R.; Scaini, A.; Da, C.T.; Manzoni, S. Do alternative irrigation strategies for rice cultivation decrease water footprints at the cost of long-term soil health? Environ. Res. Lett. 2019, 14, 074011. [Google Scholar] [CrossRef]
- Bouckaret, L.; Sleutel, S.; Van Loo, D.; Brabant, L.; Cnudde, V.; Van Hoorebeke, L.; De Neve, S. Carbon mineralization and pore size classes in undisturbed soil cores. Soil Res. 2013, 51, 14–22. [Google Scholar] [CrossRef]
- Wang, R.Z.; Hu, X. Pore structure characteristics and organic carbon distribution of soil aggregates in alpine ecosystems in the Qinghai Lake basin on the Qinghai-Tibet Plateau. Catena 2023, 231, 107359. [Google Scholar] [CrossRef]
- Hartman, K.; Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar]
- Mummey, D.; Holben, W.; Six, J.; Stahl, P. Spatial stratification of soil bacterial populations in aggregates of diverse soils. Microb. Ecol. 2006, 51, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ranjard, L.; Poly, F.; Combrisson, J.; Richaume, A.; Gourbière, F.; Thioulouse, J.; Nazaret, S. Heterogeneous cell density and genetic structure of bacterial pools associated with various soil microenvironments as determined by enumeration and DNA fingerprinting approach (RISA). Microb. Ecol. 2000, 39, 263–272. [Google Scholar] [PubMed]
- Daynes, C.N.; Field, D.J.; Saleeba, J.A.; Cole, M.A.; McGee, P.A. Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol. Biochem. 2013, 57, 683–694. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil aggregate microbial communities: Towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Sessitsch, A.; Weilharter, A.; Gerzabek, M.H.; Kirchmann, H.; Kandeler, E. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl. Environ. Microbiol. 2001, 67, 4215–4224. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Zeeshan, M.; Farooq, S.; Ali, I.; Zhou, X.B. Irrigation and nitrogen fertilization alter soil bacterial communities, soil enzyme activities, and nutrient availability in maize crop. Front Microbiol. 2022, 13, 105. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Wang, J.; Zhang, J.; Wang, Z. Drip irrigation shapes the soil bacterial communities and enhances jujube yield by regulating the soil moisture content and nutrient levels. Agric. Water Manag. 2023, 289, 108563. [Google Scholar] [CrossRef]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.M.; de Moraes, J.C.; Albrecht, A. Soil organic matter, biota and aggregation in temperate and tropical soils-effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- Namatsheve, T.; Martinsen, V.; Obia, A.; Mulder, J. Grain yield and nitrogen cycling under conservation agriculture and biochar amendment in agroecosystems of sub-Saharan Africa: A meta-analysis. Agric. Ecosyst. Environ. 2024, 376, 109243. [Google Scholar] [CrossRef]
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent developments in understanding biochar’s physical–chemistry. Agronomy 2021, 11, 615. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Situ, G.M.; Zhao, Y.L.; Zhang, L.; Yang, X.Q.; Chen, D.; Li, S.H.; Wu, Q.F.; Xu, Q.F.; Chen, J.H.; Qin, H. Linking the chemical nature of soil organic carbon and biological binding agent in aggregates to soil aggregate stability following biochar amendment in a rice paddy. Sci. Total Environ. 2022, 847, 157460. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, S. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Khodadad, C.L.M.; Zimmerman, A.R.; Green, S.J.; Uthandi, S.; Foster, J.S. Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol. Biochem. 2011, 43, 385–392. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef]
- Dhaliwal, J.K.; Kumar, S. 3D-visualization and quantification of soil porous structure using X-ray micro-tomography scanning under native pasture and crop-livestock systems. Soil Tillage Res. 2022, 218, 105305. [Google Scholar] [CrossRef]
- Flannery, B.P.; Devasher, J.; Roberge, W.G.; D’Amico, K.L. Three-dimensional microtomography. Science 1987, 237, 1439–1444. [Google Scholar] [CrossRef]
- Ma, R.; Jiang, Y.; Liu, B.; Fan, H. Effects of pore structure characterized by synchrotron-based micro-computed tomography on aggregate stability of black soil under freeze-thaw cycles. Soil Tillage Res. 2021, 207, 104855. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Guber, A.K.; Razavi, B.S.; Koestel, J.; Quigley, M.Y.; Robertson, G.P.; Kuzyakov, Y. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 2019, 10, 3121. [Google Scholar] [CrossRef] [PubMed]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Byun, Y.; Seo, C.; Yun, T.; Joo, Y.; Young Jo, H. Prediction of Na- and Ca-montmorillonite contents and swelling properties of clay mixtures using Vis-NIR spectroscopy. Geoderma 2023, 430, 116294. [Google Scholar] [CrossRef]
- Lan, X.J.; Shan, J.; Huang, Y.; Liu, X.M.; Lv, Z.Z.; Ji, J.H.; Hou, H.Q.; Xia, W.J.; Liu, Y.R. Effects of long-term manure substitution regimes on soil organic carbon composition in a red paddy soil of southern China. Soil Tillage Res. 2022, 221, 105395. [Google Scholar] [CrossRef]
- Peng, L.; Deng, S.; Wu, Y.; Yi, W.; Zhang, Y.; Yao, X.; Xing, P.; Gu, Q.; Qi, J.; Tang, X. A rapid increase of soil organic carbon in paddy fields after applying organic fertilizer with reduced inorganic fertilizer and water-saving irrigation is linked with alterations in the structure and function of soil bacteria. Agric. Ecosyst. Environ. 2025, 379, 109353. [Google Scholar] [CrossRef]
- Grunwald, D.; Kaiser, M.; Ludwig, B. Effect of biochar and organic fertilizers on C mineralization and macro-aggregate dynamics under different incubation temperatures. Soil Tillage Res. 2016, 164, 11–17. [Google Scholar] [CrossRef]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Bossuyt, H.; Frey, S.D.; Elliott, E.T.; Merckx, R.; Paustian, K. Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol. Biochem. 2001, 33, 1599–1611. [Google Scholar] [CrossRef]
- Feng, X.; Xia, X.; Chen, S.; Lin, Q.; Zhang, X.; Cheng, K.; Liu, X.; Bian, R.; Zheng, J.; Li, L.; et al. Amendment of crop residue in different forms shifted micro-pore system structure and potential functionality of macroaggregates while changed their mass proportion and carbon storage of paddy topsoil. Geoderma 2022, 409, 115643. [Google Scholar] [CrossRef]
- Quigley, M.Y.; Kravchenko, A.N. Inputs of root-derived carbon into soil and its losses are associated with pore-size distributions. Geoderma 2022, 410, 115667. [Google Scholar] [CrossRef]
- Ruamps, L.S.; Nunan, N.; Chenu, C. Microbial biogeography at the soil pore scale. Soil Biol. Biochem. 2011, 43, 280–286. [Google Scholar] [CrossRef]
- Pires, L.F.; Ghanbarian, B.; Lin, Q. Physical, topological and hydraulic properties of an Oxisol under conservation practices: X-ray tomography imaging and pore-network simulation. Soil Tillage Res. 2024, 239, 106055. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Sarauer, J.L.; Page-Dumroese, D.S.; Coleman, M.D. Soil greenhouse gas, carbon content, and tree growth response to biochar amendment in western United States forests. GCB Bioenergy 2019, 11, 660–671. [Google Scholar] [CrossRef]
- Islam, M.U.; Jiang, F.; Guo, Z.; Peng, X. Does biochar application improve soil aggregation? A meta-analysis. Soil Tillage Res. 2021, 209, 104926. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, W.Q.; Peñuelas, J.; Sardans, J.; Chen, X.X.; Fang, Y.Y.; Alrefaei, A.F.; Zeng, F.J.; Tariq, A. Effects of nitrogen-enriched biochar on subtropical paddy soil organic carbon pool dynamics. Sci. Total Environ. 2022, 851, 158322. [Google Scholar] [CrossRef]
- Fomin, D.S.; Yudina, A.V.; Romanenko, K.A.; Abrosimov, K.N.; Karsanina, M.V.; Gerke, K.M. Soil pore structure dynamics under steady-state wetting-drying cycle. Geoderma 2023, 432, 116401. [Google Scholar] [CrossRef]
- Liu, J.J.; Lu, S.G. Amendment of different biochars changed pore characteristics and permeability of Ultisol macroaggregates identified by X-ray computed tomography (CT). Geoderma 2023, 434, 116470. [Google Scholar] [CrossRef]
- Rooney, E.C.; Bailey, V.L.; Patel, K.F.; Dragila, M.; Battu, A.K.; Buchko, A.C.; Gallo, A.C.; Hatten, J.; Possinger, A.R.; Qafoku, O.; et al. Soil pore network response to freeze-thaw cycles in permafrost aggregates. Geoderma 2022, 411, 115674. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Letuma, P.; Zhao, H.; Zhang, Z.X.; Lin, W.W.; Chen, H.F.; Lin, W.X. A positive response of rice rhizosphere to alternate moderate wetting and drying irrigation at grain filling stage. Agric. Water Manag. 2018, 207, 26–36. [Google Scholar] [CrossRef]
- Daynes, C.N.; Zhang, N.; Saleeba, J.A.; McGee, P.A. Soil aggregates formed in vitro by saprotrophic Trichocomaceae have transient water-stability. Soil Biol. Biochem. 2012, 48, 151–161. [Google Scholar] [CrossRef]
- Haque, A.N.A.; Uddin, M.K.; Sulaiman, M.F.; Amin, A.M.; Hossain, M.; Solaiman, Z.M.; Mosharrof, M. Biochar with Alternate Wetting and Drying Irrigation: A Potential Technique for Paddy Soil Management. Agriculture 2021, 11, 367. [Google Scholar] [CrossRef]
- Wang, R.; Wei, S.; Jia, P.; Liu, T.; Hou, D.; Xie, R.; Lin, Z.; Ge, J.; Qiao, Y.; Chang, X.; et al. Biochar significantly alters rhizobacterial communities and reduces Cd concentration in rice grains grown on Cd-contaminated soils. Sci. Total Environ. 2019, 676, 627–638. [Google Scholar] [CrossRef] [PubMed]
- De Tender, C.A.; Debode, J.; Vandecasteele, B.; D’Hose, T.; Cremelie, P.; Haegeman, A.; Ruttink, T.; Dawyndt, P.; Maes, M. Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl. Soil Ecol. 2016, 107, 1–121. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Lew, B.; Cohen, E. Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe. Eur. J. Soil Sci. 2013, 65, 162–172. [Google Scholar] [CrossRef]
- Song, Y.; Yao, S.; Li, X.; Wang, T.; Jiang, X.; Bolan, N.; Warren, C.R.; Northen, T.R.; Chang, S.X. Deciphering underground metabolic webs in terrestrial ecosystems. Eco-Environ. Health 2024, 3, 227–237. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2017, 180, 11. [Google Scholar] [CrossRef]
- Krause, L.; Rodionov, A.; Schweizer, S.A.; Siebers, N.; Lehndorff, E.; Klumpp, E.; Amelung, W. Microaggregate stability and storage of organic carbon is affected by clay content in arable Luvisols. Soil Tillage Res. 2018, 182, 123–129. [Google Scholar] [CrossRef]
- Zhang, M.R.; Xu, Y.; Wang, J.; Hu, J.Z.; Qi, S.T.; Jiang, Z.W.; Yang, S.H. Impact of biochar on the antibiotic resistome and associated microbial functions in rhizosphere and bulk soil in water-saving and flooding irrigated paddy fields. Environ. Pollut. 2024, 342, 123026. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Riaz, M.; Zhang, M.Y.; Liu, B.; Li, Y.; El-Desouki, Z. Biochar-N fertilizer interaction increases N utilization efficiency by modifying soil C/N component under N fertilizer deep placement modes. Chemosphere 2022, 286, 131594. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Z.; Zhang, S.N.; Yang, S.H.; Wim, M.C.; Qi, S.T.; Jiang, Z.W.; Qiu, H.N.; Xu, Y. Balance rice high-yielding, high-quality and high-economic by changing the irrigation and fertilization management for sustainable production in China. Environ. Exp. Bot. 2024, 220, 105675. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zhang, J.; Liang, X.; Wang, H.; Yang, Z.; Tang, R.; Yu, Q.; Zhang, Y. Micro-nano aerated subsurface drip irrigation and biochar promote photosynthesis, dry matter accumulation and yield of cucumbers in greenhouse. Agric. Water Manag. 2025, 308, 109295. [Google Scholar] [CrossRef]
- Rong, F.L.; Cai, Z.W.; Qin, S.S.; Zhang, K.; Wu, L.Q.; Yang, S.Y.; Xiao, Z.H.; Ren, B.; Lin, Y.S.; Chen, F.L. Effects of biochar on growth and yield of rice in an acidic paddy field: Findings from a five-year field trial. Acta Ecol. Sin. 2020, 40, 4413–4424. [Google Scholar]
- Malobane, M.E.; Nciizah, A.D.; Bam, L.C.; Mudau, F.N.; Wakindiki, I.I.C. Soil microstructure as affected by tillage, rotation and residue management in a sweet sorghum-based cropping system in soils with low organic carbon content in South Africa. Soil Tillage Res. 2021, 209, 104972. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Cui, X.; Yuan, J.; Yang, X.; Wei, C.; Bi, Y.; Sun, Q.; Meng, J.; Han, X. Biochar application alters soil metabolites and nitrogen cycle-related microorganisms in a soybean continuous cropping system. Sci. Total Environ. 2024, 917, 170522. [Google Scholar] [CrossRef]
- Chao, A. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
| Volume Fraction | Equivalent Diameter (μm) | Area (µm2) | Connected Porosity | Fractal Dimension | |
|---|---|---|---|---|---|
| TS-CK | 6.37% ± 0.12% d | 103.79 ± 3.42 a | 137,114.59 ± 16,469.29 a | 4.17% ± 0.92% cd | 2.47 ± 0.01 a |
| TS-CA | 11.02% ± 1.03% b | 114.67 ± 18.4 a | 326,084.22 ± 227,949.58 a | 8.08% ± 1.33% ab | 2.5 ± 0.02 a |
| TS-FK | 5.83% ± 0.16% d | 84.74 ± 17.28 a | 98,976.97 ± 25,775.96 a | 3.7% ± 1.73% d | 2.5 ± 0.09 a |
| JS-CK | 7.43% ± 0.31% c | 83.61 ± 26.95 a | 161,231.53 ± 113,774.71 a | 6.04% ± 0.19% bc | 2.49 ± 0.06 a |
| JS-CA | 11.97% ± 0.58% a | 125.17 ± 70.94 a | 401,916.56 ± 304,676.99 a | 8.84% ± 1.6% a | 2.5 ± 0.1 a |
| JS-FK | 6.02% ± 0.22% d | 99.82 ± 13.53 a | 148,756.48 ± 8843.36 a | 2.15% ± 1.06% d | 2.42 ± 0.04 a |
| Total Throat Number | Surface Area (µm2) × 100 | Equivalent Radius (µm) | Channel Length (µm) | |
|---|---|---|---|---|
| TS-CK | 5219 ± 766 c | 46,824.49 ± 5888.94 b | 97.68 ± 4.87 b | 450.68 ± 80.72 a |
| TS-CA | 7619 ± 781 ab | 50,335.27 ± 6330.48 ab | 99.13 ± 4.94 b | 478.92 ± 85.78 a |
| TS-FK | 1476 ± 257 d | 32,764.93 ± 4120.72 c | 80.39 ± 4.01 c | 445.98 ± 79.88 a |
| JS-CK | 6260 ± 1452 bc | 47,890 ± 6022.95 b | 100.15 ± 4.99 b | 447.26 ± 80.11 a |
| JS-CA | 8836 ± 1123 a | 59,962.5 ± 7541.26 a | 109.78 ± 5.47 a | 516.79 ± 92.56 a |
| JS-FK | 1937 ± 517 d | 35,344.49 ± 4809.52 c | 82.87 ± 4.76 c | 404.33 ± 74.72 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Yang, S.; Cornelis, W.M.; Zhang, M.; Huang, Q.; Qiu, H.; Qi, S.; Jiang, Z.; Xu, Y.; Zhu, L. Microstructure and Microorganisms Alternation of Paddy Soil: Interplay of Biochar and Water-Saving Irrigation. Plants 2025, 14, 1498. https://doi.org/10.3390/plants14101498
Hu J, Yang S, Cornelis WM, Zhang M, Huang Q, Qiu H, Qi S, Jiang Z, Xu Y, Zhu L. Microstructure and Microorganisms Alternation of Paddy Soil: Interplay of Biochar and Water-Saving Irrigation. Plants. 2025; 14(10):1498. https://doi.org/10.3390/plants14101498
Chicago/Turabian StyleHu, Jiazhen, Shihong Yang, Wim M. Cornelis, Mairan Zhang, Qian Huang, Haonan Qiu, Suting Qi, Zewei Jiang, Yi Xu, and Lili Zhu. 2025. "Microstructure and Microorganisms Alternation of Paddy Soil: Interplay of Biochar and Water-Saving Irrigation" Plants 14, no. 10: 1498. https://doi.org/10.3390/plants14101498
APA StyleHu, J., Yang, S., Cornelis, W. M., Zhang, M., Huang, Q., Qiu, H., Qi, S., Jiang, Z., Xu, Y., & Zhu, L. (2025). Microstructure and Microorganisms Alternation of Paddy Soil: Interplay of Biochar and Water-Saving Irrigation. Plants, 14(10), 1498. https://doi.org/10.3390/plants14101498









