Response of Photosynthesis and Chlorophyll Fluorescence to Nitrogen Changes in Rice with Different Nitrogen Use Efficiencies
Abstract
1. Introduction
2. Results
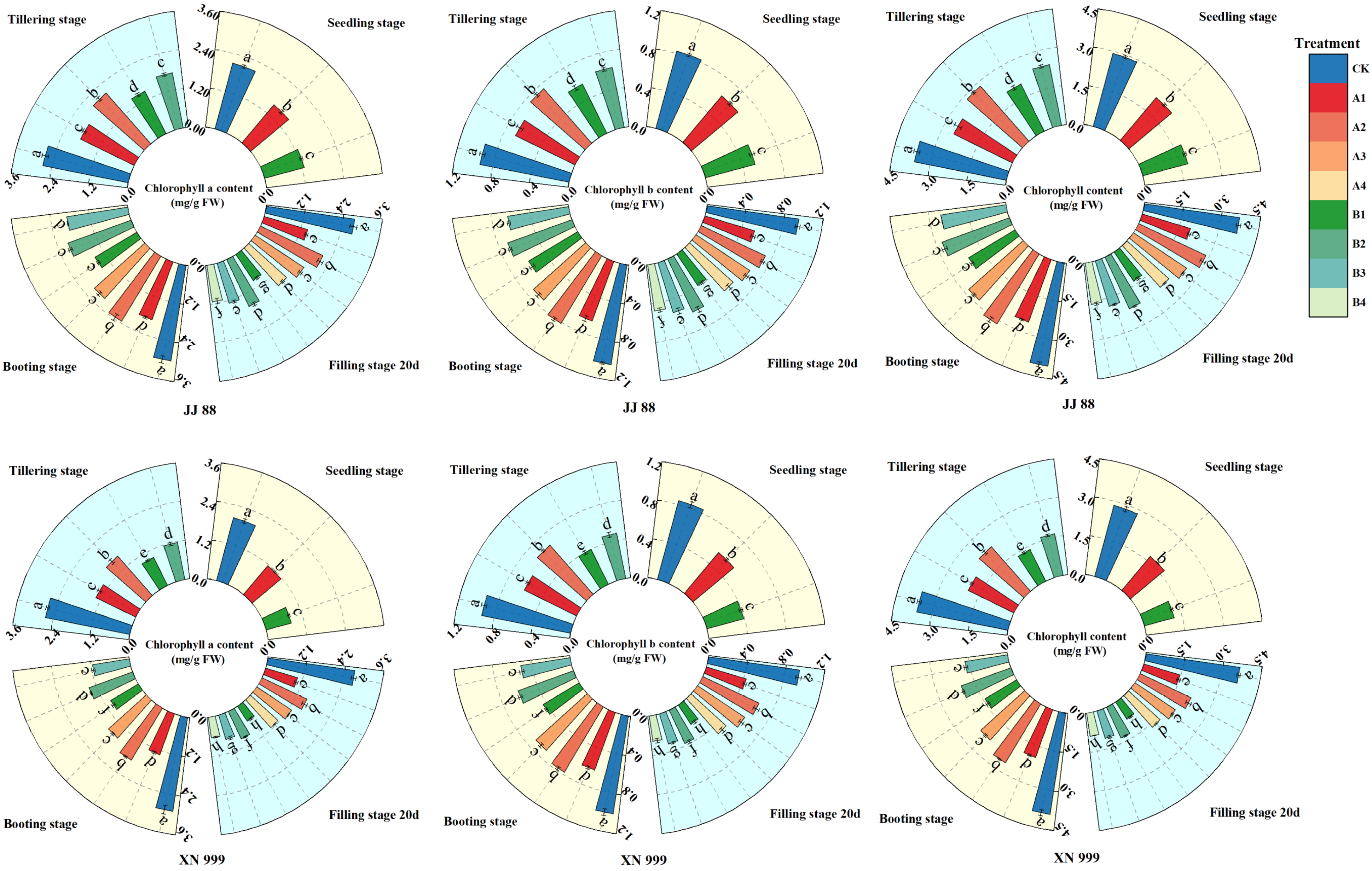
2.1. Chlorophyll Content
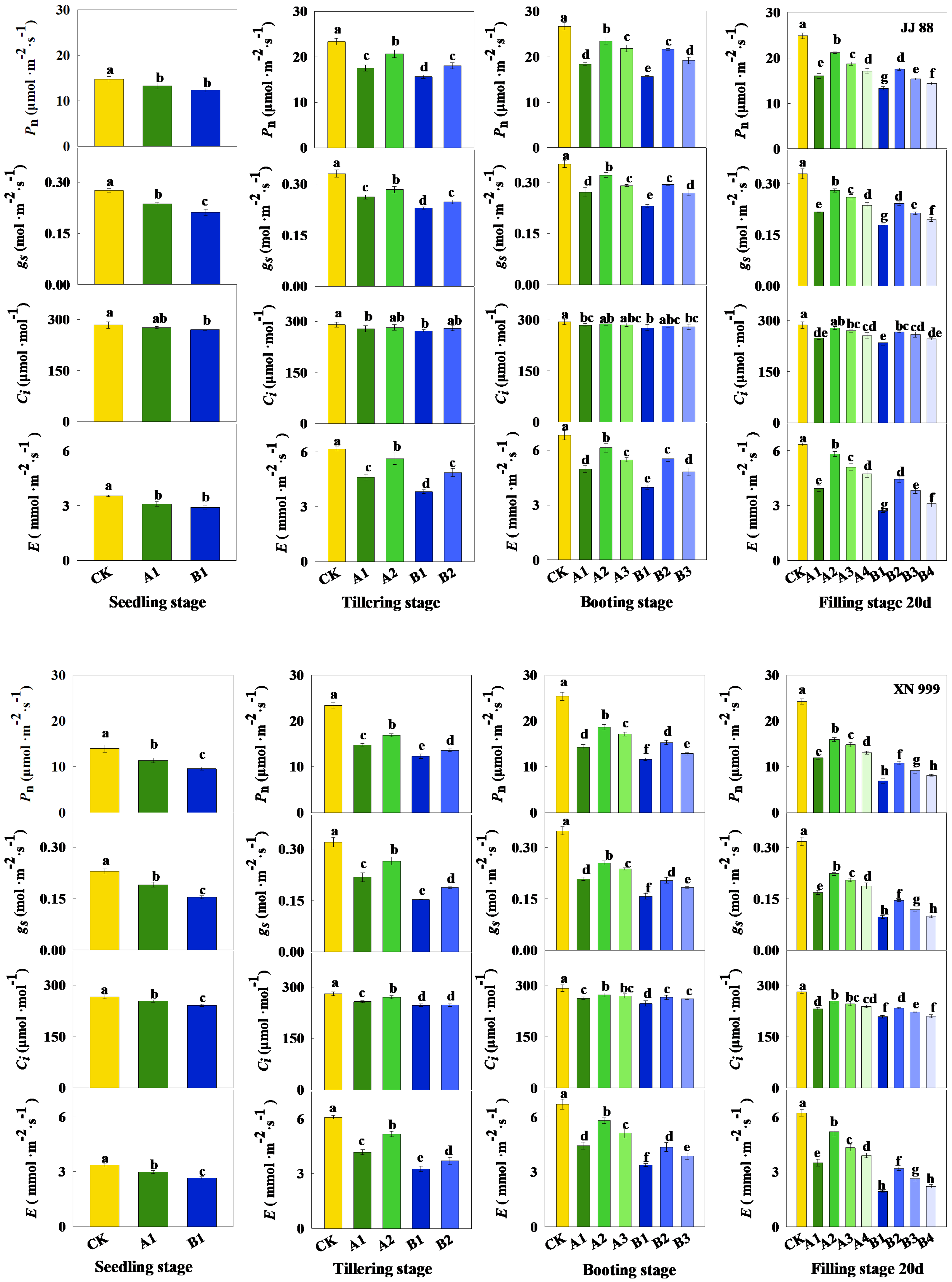
2.2. Parameters Related to Photosynthesis
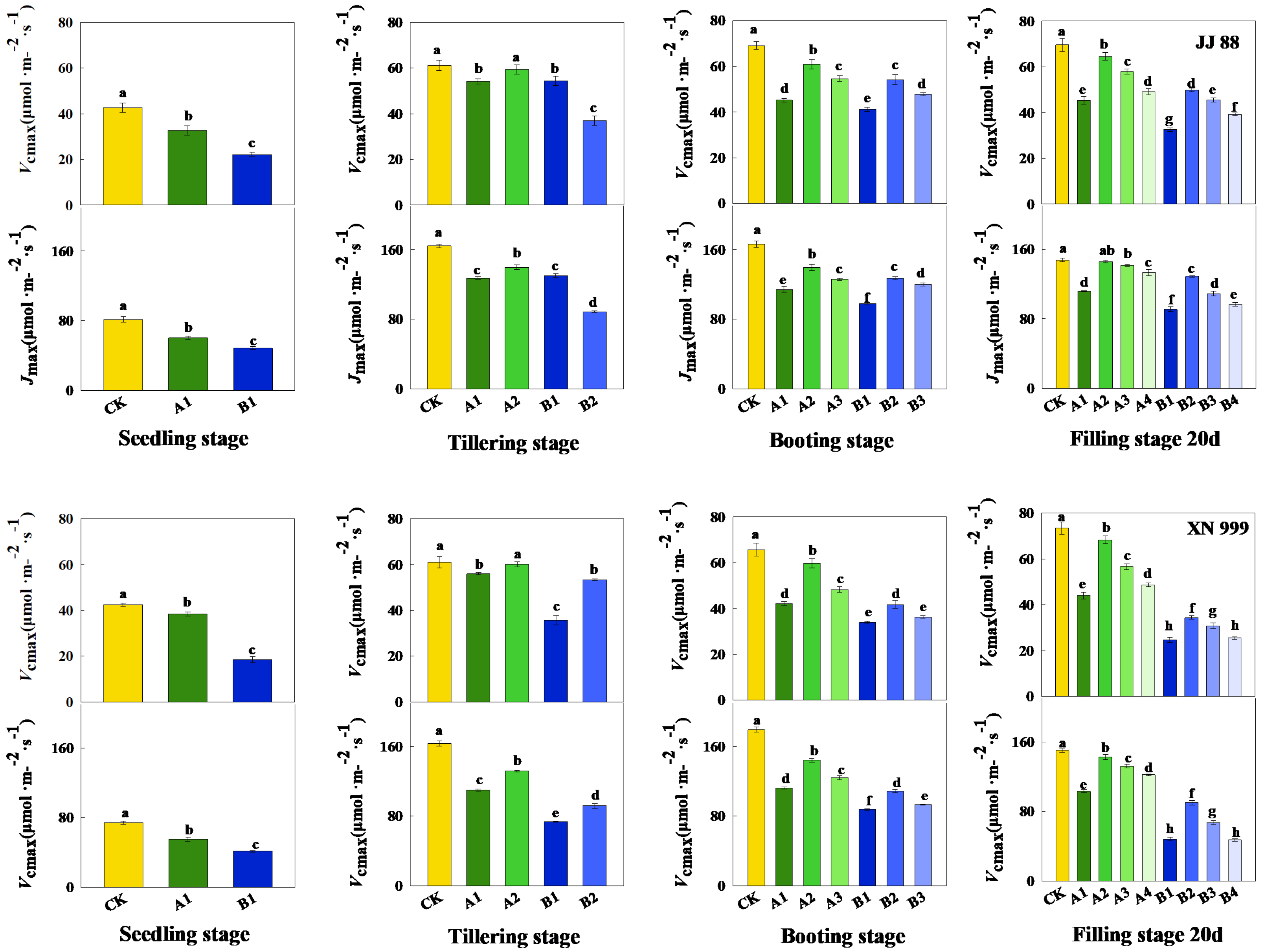
2.3. Carbon Dioxide Response Parameters
2.4. Chlorophyll Fluorescence Parameters
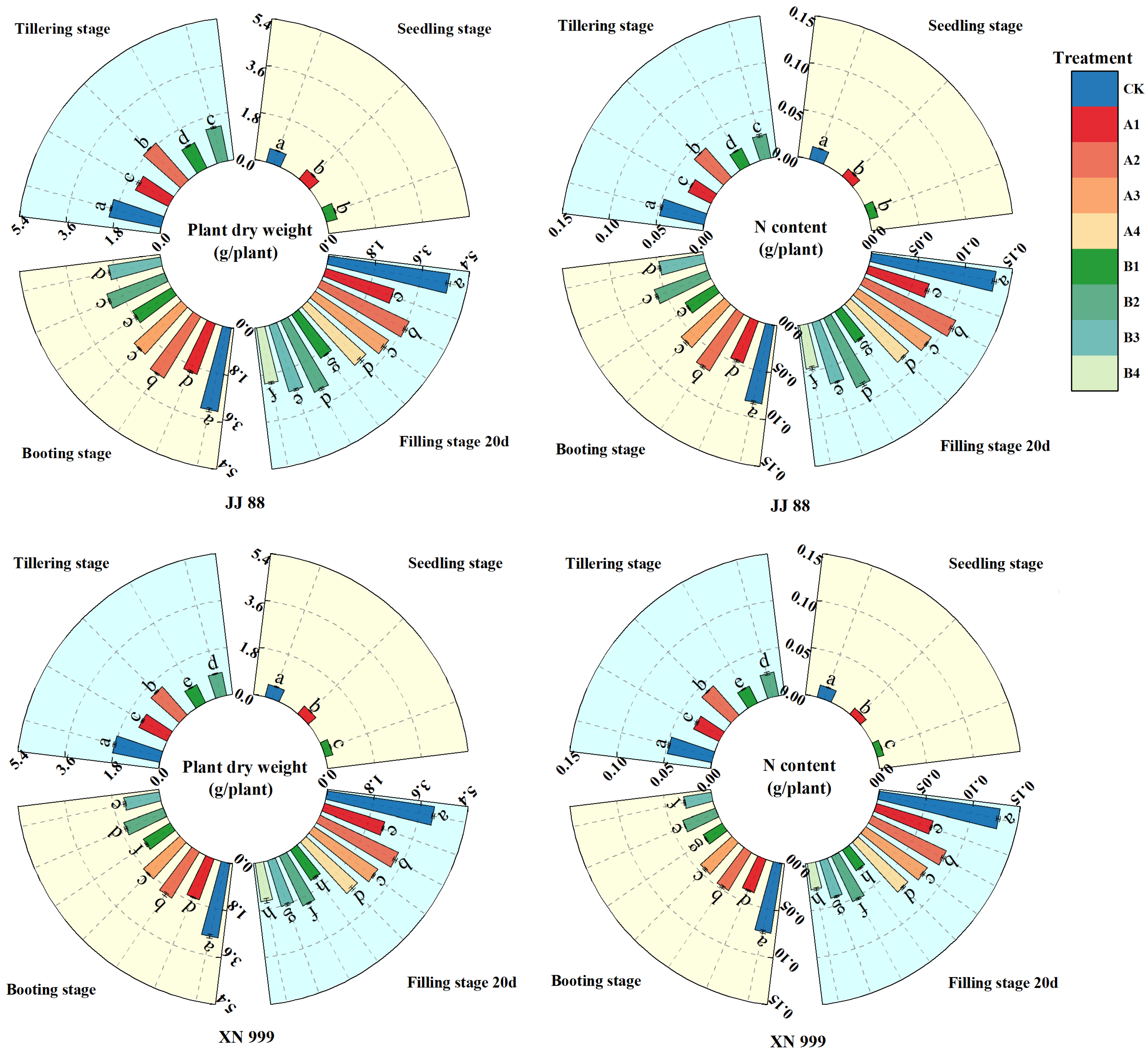
2.5. Dry Matter and Nitrogen Accumulation
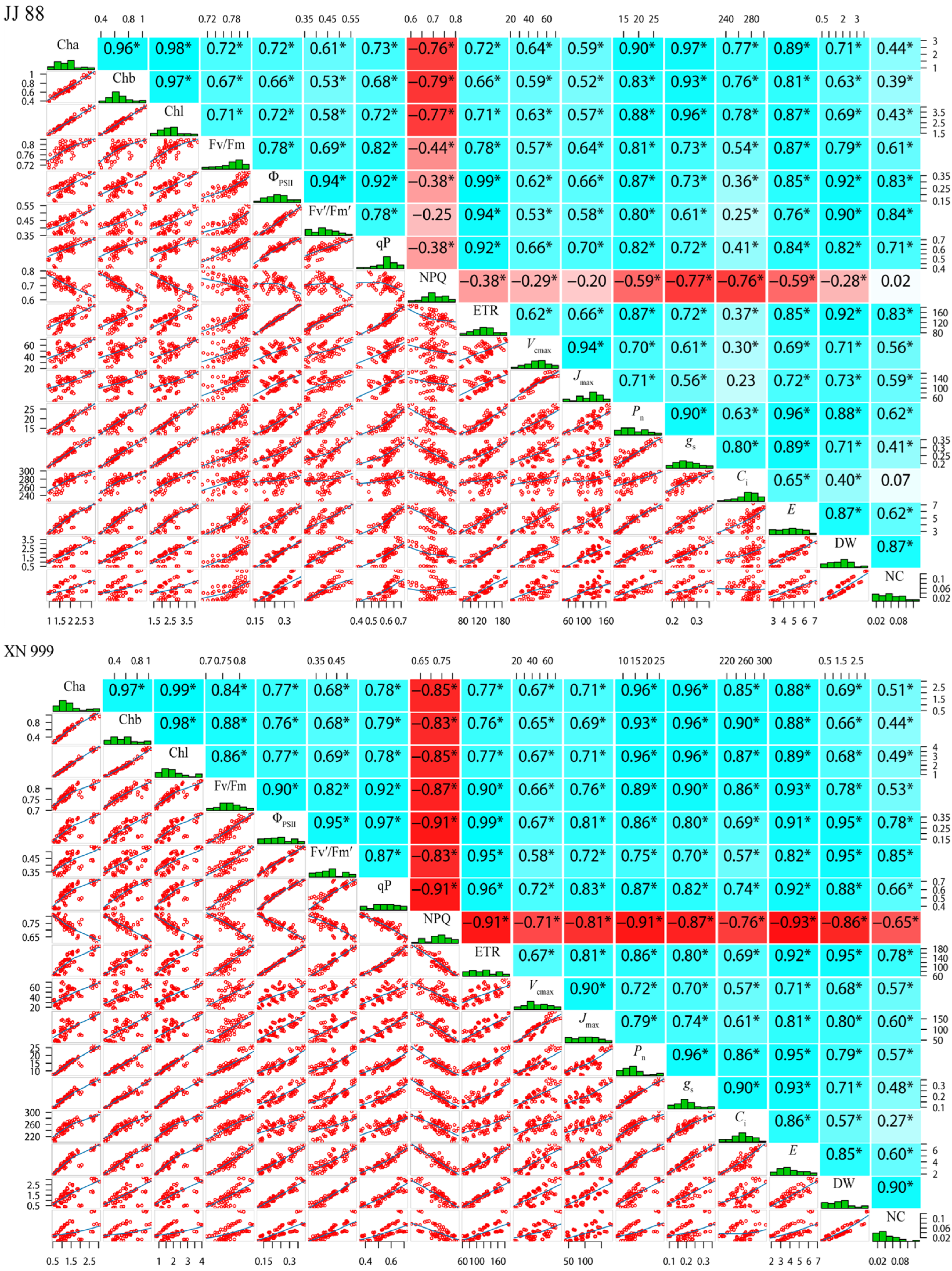
2.6. Correlation Analysis
2.7. Redundancy Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Measurement Indicators and Methods
4.3.1. Dry Matter Determination
4.3.2. Determination of Photosynthetic Parameters
4.3.3. Carbon Dioxide Response Parameter Measurement
4.3.4. Chlorophyll Content Determination
4.3.5. Nitrogen Content Determination
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S. Recent advances on nitrogen use efficiency in rice. Agronomy 2021, 11, 753. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Xu, X.; Shen, Q.; Guo, S. Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J. Exp. Bot. 2009, 60, 2351–2360. [Google Scholar] [CrossRef]
- Yamori, W.; Nagai, T.; Makino, A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 2011, 34, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Nakae, T.; Uchida, M.; Soda, K.; Takai, T.; Oi, T.; Yamamoto, T.; Ookawa, T.; Miyake, H.; Yano, M.; et al. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. J. Exp. Bot. 2013, 64, 1061–1072. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Yin, X.; Harbinson, J.; Struik, P.C. Mathematical review of literature to assess alternative electron transports and interphotosystem excitation partitioning of steady-state C3 photosynthesis under limiting light. Plant Cell Environ. 2006, 29, 1771–1782. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Von Caemmerer; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis: Mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef]
- Grassi, G.; Colom, M.R.; Minotta, G. Effects of nutrient supply on photosynthetic acclimation and photoinhibition of one-year-old foliage of Picea abies. Physiol. Plant. 2001, 111, 245–254. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits—Vcmax and Jmax—To leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.G. Increased needle nitrogen contents did not improve shoot photosynthetic performance of mature nitrogen-poor scots pine trees. Front. Plant Sci. 2016, 7, 1051. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ. 2001, 24, 597–609. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of nitrogen application rate on the photosynthetic pigment, leaf fluorescence characteristics, and yield of indica hybrid rice and their interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Domingues, T.; Ishida, F.Y.; Feldpausch, T.R.; Grace, J.; Meir, P.; Saiz, G.; Sene, O.; Schrodt, F.; Sonké, B.; Taedoumg, H.; et al. Biome-specific effects of nitrogen and phosphorus on the photosynthetic characteristics of trees at a forest-savanna boundary in Cameroon. Oecologia 2015, 178, 659–672. [Google Scholar] [CrossRef]
- Mujawamariya, M.; Wittemann, M.; Manishimwe, A.; Ntirugulirwa, B.; Zibera, E.; Nsabimana, D.; Wallin, G.; Uddling, J.; Dusenge, M.E. Complete or overcompensatory thermal acclimation of leaf dark respiration in African tropical trees. New Phytol. 2021, 229, 2548–2561. [Google Scholar] [CrossRef]
- Martindale, W.; Leegood, R.C. Acclimation of photosynthesis to low temperature in Spinacia oleracea L. II. Effects of nitrogen supply. J. Exp. Bot. 1997, 48, 1873–1880. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Crous, K.Y.; De Kauwe, M.G.; Verryckt, L.T.; Goll, D.; Zaehle, S.; Bloomfield, K.J.; Ciais, P.; Cernusak, L.A.; Domingues, T.F.; et al. Convergence in phosphorus constraints to photosynthesis in forests around the world. Nat. Commun. 2022, 13, 5005. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Nakano, H.; Mae, T. Responses of ribulose-1,5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes to leaf nitrogen in rice, and their relationships to photosynthesis. New Phytol. 1994, 105, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H. The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. New Phytol. 2002, 115, 191–198. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Chen, G.Y.; Gong, Z.Y.; Chen, J.; Yong, Z.H.; Zhu, J.G.; Xu, D.Q. Ribulose-1,5-bisphosphate regeneration limitation in rice leaf photosynthetic acclimation to elevated CO2. Plant Sci. 2008, 175, 348–355. [Google Scholar] [CrossRef]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, Regulatory interactions, and Possibilities for a Better Enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–475. [Google Scholar] [CrossRef]
- Tantray, A.Y.; Bashir, S.S.; Ahmad, A. Low nitrogen stress regulates chlorophyll fluorescence in coordination with photosynthesis and Rubisco efficiency of rice. Physiol. Mol. Biol. Plants 2020, 26, 83–94. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Naumann, J.C. Linking leaf chlorophyll fluorescence properties to physiological responses for detection of salt and drought stress in coastal plant species. Physiol. Plant. 2007, 131, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Horiguchi, S.; Makino, A.; Shinzaki, Y.; Yamamoto, H.; Tomizawa, K.I. Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of Chl fluorescence in tobacco leaves. Plant Cell Physiol. 2005, 46, 1819–1830. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hu, Y.G.; Ren, C.Z.; Guo, L.C.; Wang, C.L.; Jiang, Y.; Wang, X.J.; Phendukani, H.; Zeng, Z.H. Effects of nitrogen application on chlorophyll fluorescence parameters and leaf gas exchange in naked oat. J. Integr. Agric. 2013, 12, 2164–2171. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Loos, R.A.; Silva, E.A.; Loureiro, M.E. Limitations to photosynthesis in Coffea canephoraas a result of nitrogen and water availability. J. Plant Physiol. 2002, 159, 975–981. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 2000, 151, 135–143. [Google Scholar] [CrossRef]
- Kumagai, E.; Araki, T.; Kubota, F. Effects of nitrogen supply restriction on gas exchange and photosystem 2 function in flag leaves of a traditional low-yield cultivar and a recently improved high-yield cultivar of rice (Oryza sativa L.). Photosynthetica 2007, 45, 489–495. [Google Scholar] [CrossRef]
- Kumagai, E.; Araki, T.; Ueno, O. Effect of nitrogen-deficiency on midday photoinhibition in flag leaves of different rice (Oryza sativa L.) cultivars. Photosynthetica 2009, 47, 241–246. [Google Scholar] [CrossRef]
- Kumagai, E.; Araki, T.; Ueno, O. Comparison of susceptibility to photoinhibition and energy partitioning of absorbed light in photosystem II in flag leaves of two rice (Oryza sativa L.) cultivars that differ in their responses to nitrogen-deficiency. Plant Prod. Sci. 2010, 13, 11–20. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Jiang, J.; Xu, J.; Wei, X.; Fu, X.; Liu, C.; He, Y.; Wang, G. Genetic architecture of natural variation in rice nonphotochemical quenching capacity revealed by genome-wide association study. Front. Plant Sci. 2017, 8, 1773. [Google Scholar] [CrossRef]
- Murchie, E.H.; Hubbart, S.; Peng, S.; Horton, P. Acclimation of photosynthesis to high irradiance in rice: Gene expression and interactions with leaf development. J. Exp. Bot. 2005, 56, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Osmond, B. Solubilization of ribulose-1,5-bisphosphate carboxylase from the membrane fraction of pea leaves. Photosynth. Res. 1991, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Osone, Y. A paradox of leaf-trait convergence: Why is leaf nitrogen concentration higher in species with higher photosynthetic capacity? J. Plant Res. 2009, 122, 245–251. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, S. The dynamic relation between fertilization, precipitation and the diffusion of agricultural non-point pollution in Hainan Island. Front. Environ. Sci. 2024, 12, 1419912. [Google Scholar] [CrossRef]
- Ethier, G.J.; Livingston, N.J.; Harrison, D.L.; Black, T.A.; Moran, J.A. Low stomatal and internal conductance to CO2 versus rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant Cell Environ. 2006, 29, 2168–2184. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef]
- Kumagai, E.; Araki, T.; Kubota, F. Characteristics of gas exchange and chlorophyll fluorescence during senescence of flag leaf in different rice (Oryza sativa L.) cultivars grown under nitrogen-deficient condition. Plant Prod. Sci. 2009, 12, 285–292. [Google Scholar] [CrossRef]
- Chen, L.S.; Cheng, L. Both xanthophyll cycle-dependent thermal dissipation and the antioxidant system are up-regulated in grape (Vitis labrusca L. cv. Concord) leaves in response to N limitation. J. Exp. Bot. 2003, 54, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Mori, S.; Degl’Innocenti, E.; Pecchia, S. Effects of ozone exposure or fungal pathogen on white lupin leaves as determined by imaging of chlorophyll a fluorescence. Plant Physiol. Bioch. 2007, 45, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Zheng, E.N.; Wang, C.M.; Fu, N.H. Effect of different water and nitrogen levels on chlorophyll fluorescence parameters and photosynthetic characteristics of rice. Trans. Chin. Soc Agric. Mach. 2017, 48, 176–183. [Google Scholar]
- Fu, H.; Cui, D.; Shen, H. Effects of nitrogen forms and application rates on nitrogen uptake, photosynthetic characteristics and yield of double-cropping rice in South China. Agronomy 2021, 10, 158. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Li, Y.; Gu, X.; Li, Y.; Fang, H.; Chen, P. Ridge-furrow mulching combined with appropriate nitrogen rate for enhancing photosynthetic efficiency, yield and water use efficiency of summer maize in a semi-arid region of China. Agric. Water Manag. 2023, 287, 108450. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, X.; Hou, X.; Wu, J.; Wang, T. Effect of different levels of nitrogen deficiency on switchgrass seedling growth. Crop J. 2014, 2, 223–234. [Google Scholar] [CrossRef]
- Lin, Z.H.; Zhong, Q.S.; Chen, C.S.; Ruan, Q.C.; Chen, Z.H.; You, X.M. Carbon dioxide assimilation and photosynthetic electron transport of tea leaves under nitrogen deficiency. Photosynth. Res. 2016, 57, 37. [Google Scholar] [CrossRef]
- Schmierer, M.; Knopf, O.; Asch, F. Growth and photosynthesis responses of a super dwarf rice genotype to shade and nitrogen supply. Rice Sci. 2021, 28, 178–190. [Google Scholar] [CrossRef]
- Cisse, A.; Zhao, X.; Fu, W.; Kim, R.E.R.; Chen, T.; Tao, L.; Feng, B. Non-photochemical quenching involved in the regulation of photosynthesis of rice leaves under high nitrogen conditions. Int. J. Mol. Sci. 2020, 21, 2115. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Gao, H.; Zhang, L.; Yang, C.; Liu, P.; Meng, Q. Characterization of photosynthetic performance during senescence in stay-green and quick-leaf-senescence Zea mays L. inbred lines. PLoS ONE 2012, 7, e42936. [Google Scholar] [CrossRef] [PubMed]
- Swarna, R.; Leela, R.; Sreenivas, G.; Raji, R.; Madhavi, A. Growth performance and radiation use efficiency of transplanted rice under varied plant densities and nitrogen levels. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1429–1437. [Google Scholar] [CrossRef]
- Bama, K.S.; Babu, K.R.; Sharmila, R.; Anuradha, A. Influence of nitrogen application on direct seeded rice in old and new Cauvery Delta zone of Tamil Nadu, India. J. Appl. Nat. Sci. 2021, 13, 1462–1469. [Google Scholar] [CrossRef]
- Qi, Z.; Ling, F.; Jia, D.; Cui, J.; Zhang, Z.; Xu, C.; Yu, L.; Guan, C.; Wang, Y.; Zhang, M.; et al. Effects of low nitrogen on seedling growth, photosynthetic characteristics and antioxidant system of rice varieties with different nitrogen efficiencies. Sci. Rep. 2023, 13, 19780. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z. Experimental Techniques in Plant Physiology; Jilin University Press: Changchun, China, 2008; pp. 50–66. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Xu, C.; Tang, R.; Zhang, Q.; Sun, W.; Guan, C.; Wang, Y.; Zhang, M.; Ding, J.; Zhang, Y.; et al. Response of Photosynthesis and Chlorophyll Fluorescence to Nitrogen Changes in Rice with Different Nitrogen Use Efficiencies. Plants 2025, 14, 1465. https://doi.org/10.3390/plants14101465
Qi Z, Xu C, Tang R, Zhang Q, Sun W, Guan C, Wang Y, Zhang M, Ding J, Zhang Y, et al. Response of Photosynthesis and Chlorophyll Fluorescence to Nitrogen Changes in Rice with Different Nitrogen Use Efficiencies. Plants. 2025; 14(10):1465. https://doi.org/10.3390/plants14101465
Chicago/Turabian StyleQi, Zexin, Chen Xu, Rui Tang, Qiang Zhang, Wenzheng Sun, Chenglong Guan, Ye Wang, Mengru Zhang, Jiale Ding, Yuankai Zhang, and et al. 2025. "Response of Photosynthesis and Chlorophyll Fluorescence to Nitrogen Changes in Rice with Different Nitrogen Use Efficiencies" Plants 14, no. 10: 1465. https://doi.org/10.3390/plants14101465
APA StyleQi, Z., Xu, C., Tang, R., Zhang, Q., Sun, W., Guan, C., Wang, Y., Zhang, M., Ding, J., Zhang, Y., Yang, H., Yang, Y., Liu, X., Zhang, Z., & Ling, F. (2025). Response of Photosynthesis and Chlorophyll Fluorescence to Nitrogen Changes in Rice with Different Nitrogen Use Efficiencies. Plants, 14(10), 1465. https://doi.org/10.3390/plants14101465





