Toxicity of Essential Oils of Origanum vulgare, Salvia rosmarinus, and Salvia officinalis Against Aculops lycopersici
Abstract
1. Introduction
2. Results
2.1. Precision Aromatic Crop (PAC) Techniques
2.2. Analyses of Essential Oils
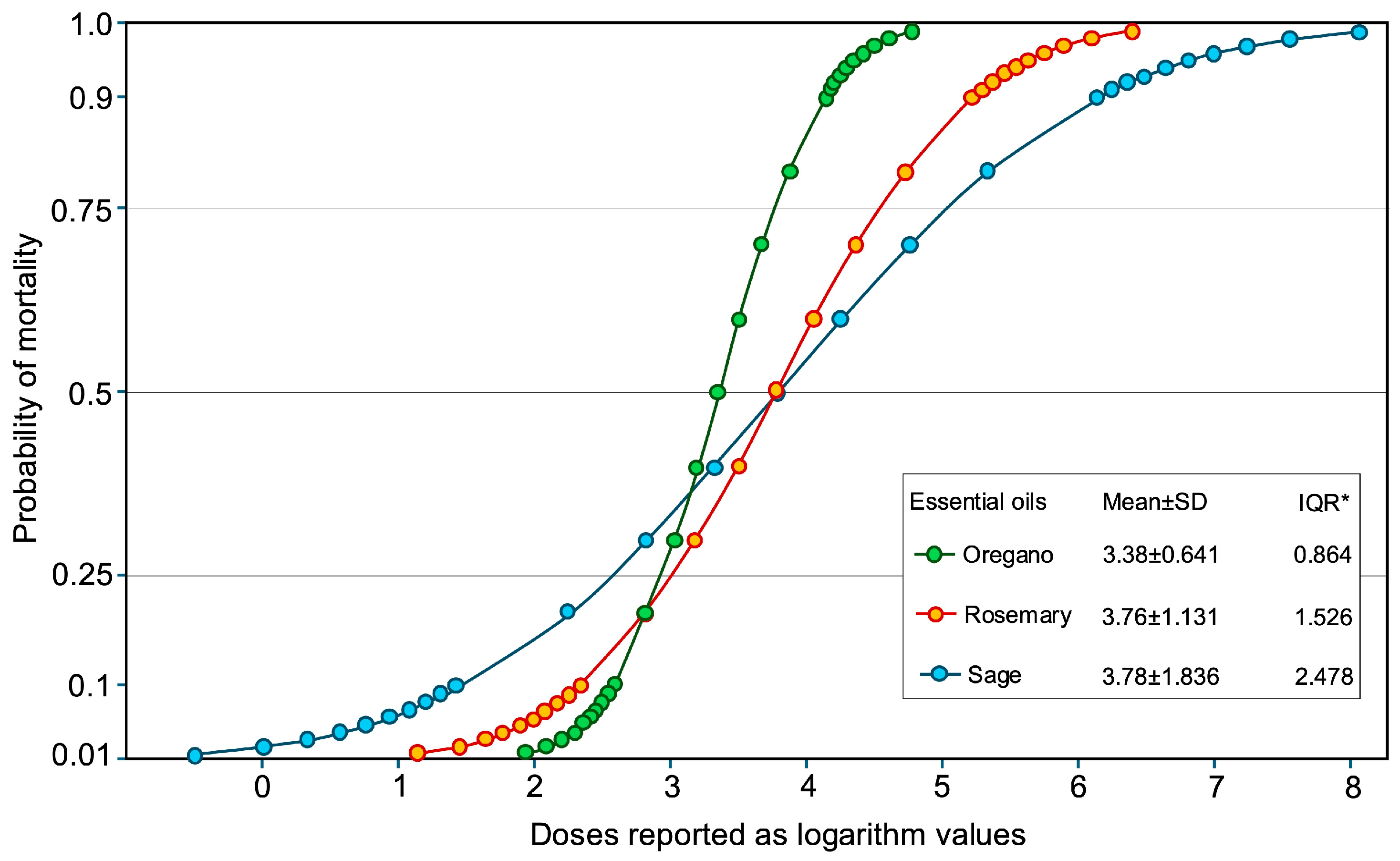
2.3. Toxicity of O. vulgare, S. rosmarinus, and S. officinalis EOs Against A. lycopersici
3. Discussion
Challenges in Field Application
4. Materials and Methods
4.1. Cultivation of Officinal Plants with Precision Aromatic Crops (PAC) Techniques, and Essential Oil Extraction Methods
4.1.1. Plants Cultivation Method
4.1.2. Precision Aromatic Crop (PAC) Techniques
4.1.3. Extraction and Analyses of Essential Oils
4.2. Aculops lycopersici Experimental Set-Up
4.2.1. Solanum Nigrum Seedlings for A. lycopersici Breeding
4.2.2. Adult Cohort for the Experiments
4.2.3. Experimental Units
4.2.4. Effects of Essential Oils on A. lycopersici
4.2.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lindquist, E.E.; Sabelis, M.W.; Bruin, J. Eriophyoid Mites. Their Biology, Natural Enemies and Control; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Pfaff, A.; Gabriel, D.; Böckmann, E. Observation and Restriction of Aculops lycopersici Dispersal in Tomato Layer Cultivation. J. Plant Dis. Prot. 2024, 131, 155–166. [Google Scholar] [CrossRef]
- Royalty, R.N.; Per Ring, T.M. Morphological Analysis of Damage to Tomato Leaflets by Tomato Russet Mite (Acari: Eriophyidae). J. Econ. Entomol. 1988, 81, 816–820. [Google Scholar] [CrossRef]
- Haque, M.M. Population Growth of Tomato Russet Mite, Aculops lycopersici (Acari: Eriophyidae) and Its Injury Effect on the Growth of Tomato Plants. J. Acarol. Soc. Jpn. 2002, 11, 1–10. [Google Scholar] [CrossRef]
- Luigi, M.; Tiberini, A.; Taglienti, A.; Bertin, S.; Dragone, I.; Sybilska, A.; Tarchi, F.; Goggioli, D.; Lewandowski, M.; Simoni, S. Molecular Methods for the Simultaneous Detection of Tomato Fruit Blotch Virus and Identification of Tomato Russet Mite, a New Potential Virus–Vector System Threatening Solanaceous Crops Worldwide. Viruses 2024, 16, 806. [Google Scholar] [CrossRef]
- Pfaff, A.L. Aculops lycopersici Tryon (Acari: Eriophyoidea) Monitoring, Control Options and Economic Relevance in German Tomato Cultivation. Ph.D. Thesis, Georg-August Universität Göttingen, Bad Hersfeld, Germany, April 2023. [Google Scholar]
- Ximénez-Embún, M. Drought-Stressed Tomato Plants Trigger Bottom-Up Effects on Key Mite Pests. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2017. [Google Scholar]
- Duso, C.; Castagnoli, M.; Simoni, S.; Angeli, G. The Impact of Eriophyoids on Crops: Recent Issues on Aculus schlechtendali, Calepitrimerus vitis and Aculops lycopersici. Exp. Appl. Acarol. 2010, 51, 151–168. [Google Scholar] [CrossRef]
- Marcic, D. Acaricides in Modern Management of Plant-Feeding Mites. J. Pest Sci. 2012, 85, 395–408. [Google Scholar] [CrossRef]
- Marčić, D.; Döker, I.; Tsolakis, H. Bioacaricides in Crop Protection—What Is the State of Play? Insects 2025, 16, 95. [Google Scholar] [CrossRef]
- Hessein, N.A.; Perring, T.M. Feeding Habits of the Tydeidae with Evidence of Homeopronematus Anconai (Acari: Tydeidae) Predation on Aculops lycopersici (Acari: Eriophyidae). Int. J. Acarol. 1986, 12, 215–221. [Google Scholar] [CrossRef]
- Park, H.-H.; Shipp, L.; Buitenhuis, R. Predation, Development, and Oviposition by the Predatory Mite Amblyseius swirkii (Acari: Phytoseiidae) on Tomato Russet Mite (Acari: Eriophyidae). J. Econ. Entomol. 2010, 103, 563–569. [Google Scholar] [CrossRef]
- van Houten, Y.; Glas, J.; Hoogerbrugge, H.; Rothe, J.; Bolckmans, K.; Simoni, S.; Van Arkel, J.; Alba, J.; Kant, M.; Sabelis, M. Herbivory-Associated Degradation of Tomato Trichomes and Its Impact on Biological Control of Aculops lycopersici. Exp. Appl. Acarol. 2013, 60, 127–138. [Google Scholar] [CrossRef]
- Pijnakker, J.; Hürriyet, A.; Petit, C.; Vangansbeke, D.; Duarte, M.V.; Arijs, Y.; Moerkens, R.; Sutter, L.; Maret, D.; Wäckers, F. Evaluation of Phytoseiid and Iolinid Mites for Biological Control of the Tomato Russet Mite Aculops lycopersici (Acari: Eriophyidae). Insects 2022, 13, 1146. [Google Scholar] [CrossRef]
- Gard, B.; Bardel, A.; Douin, M.; Perrin, B.; Tixier, M.-S. Laboratory and Field Studies to Assess the Efficacy of the Predatory Mite Typhlodromus (Anthoseius) recki (Acari: Phytoseiidae) Introduced via Banker Plants to Control the Mite Pest Aculops lycopersici (Acari: Eriophyidae) on Tomato. BioControl 2025, 70, 179–191. [Google Scholar] [CrossRef]
- Al-Azzazy, M.M.; Al-Rehiayani, S.M.; Abdel-Baky, N.F. Life Tables of the Predatory Mite Neoseiulus cucumeris (Acari: Phytoseiidae) on Two Pest Mites as Prey, Aculops lycopersici and Tetranychus urticae. Arch. Phytopathol. Plant Prot. 2018, 51, 637–648. [Google Scholar] [CrossRef]
- Amaral, F.S.; Ferreira, M.M.; Lofego, A.C. Neoseiulus tunus (De Leon, 1967)(Acari: Phytoseiidae): Is This a Potential Natural Enemy of Aculops lycopersici (Massee, 1937) (Acari: Eriophyidae)? Entomol. Commun. 2021, 3, ec03033. [Google Scholar] [CrossRef]
- Castagnoli, M.; Simoni, S.; Liguori, M. Evaluation of Neoseiulus californicus (McGregor) (Acari: Phytoseiidae) as a candidate for the control of Aculops lycopersici (Tryon) (Acari: Eriophyoidea): A preliminary study. Redia 2003, 86, 97–100. [Google Scholar]
- Trottin-Caudal, Y.; Fournier, C.; Leyre, J.M. Biological control of Aculops lycopersici (Massee) using the predatory mites Neoseiulus californicus McGregor and Neoseiulus cucumeris (Oudemans) on tomato greenhouse crops. In Proceedings of the Colloque International Tomate Sous Abri, Protection Intégrée—Agriculture Biologique, Avignon, France, 17–19 September 2003; Roche, L., Edin, M., Mathieu, V., Laurens, F., Eds.; Centre De Balandran: Bellegarde, France, 2003; pp. 153–157. [Google Scholar]
- Fischer, S.; Klötzli, F.; Falquet, L.; Celle, O. An Investigation on Biological Control of the Tomato Russet Mite Aculops lycopersici (Massee) with Amblyseius andersoni (Chant). In Proceedings of the IOBC/WPRS Working Group “Integrated Control in Protected Crops, Turku, Finland, 10–14 April 2005; Enkegaard, A., Ed.; IOBC/WPRS: Darmstadt, Germany, 2005. IOBC/WPRS Bull. 28. pp. 99–102. [Google Scholar]
- Aysan, E.; Nabi, A.K. Tritrophic Relationships among Tomato Cultivars, the Rust Mite, Aculops lycopersici (Massee) (Eriophyidae), and Its Predators. Acarologia 2018, 58, 5–17. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical Insecticide Research: Many Publications, Limited Useful Data. Trends Plant Sci. 2013, 19, 140–145. [Google Scholar] [CrossRef]
- Jacobson, M. Botanical pesticides. Past, present, and future. In Insecticides of Plant Origin; Arnason, J.T., Philogène, B.J.R., Morand, P., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 1–10. Available online: https://pubs.acs.org/doi/pdf/10.1021/bk-1989-0387.ch001 (accessed on 1 May 2025).
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Rizzo, R.; Ragusa, E.; Benelli, G.; Lo Verde, G.; Zeni, V.; Maggi, F.; Petrelli, R.; Spinozzi, E.; Ferrati, M.; Sinacori, M.; et al. Lethal and Sublethal Effects of Carlina Oxide on Tetranychus urticae (Acari: Tetranychidae) and Neoseiulus californicus (Acari: Phytoseiidae). Pest Manag. Sci. 2023, 80, 967–977. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Francardi, V.; Simoni, S.; Roversi, P.F.; Ferrati, M.; Spinozzi, E.; Perinelli, D.R.; Bonacucina, G.; Maggi, F.; Tortorici, S.; et al. Role of Plant Essential Oil Nanoemulsions on Host Colonization by the Invasive Ambrosia Beetle Xylosandrus compactus. Ind. Crops Prod. 2023, 195, 116437. [Google Scholar] [CrossRef]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or Lures? Biological and Behavioral Effects of Plant Essential Oils on Tephritidae Fruit Flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef]
- Park, Y.-L.; Tak, J.-H. Chapter 6—Essential oils for arthropod pest management in agricultural production systems. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: San Diego, CA, USA, 2016; pp. 61–70. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G. Essential Oils as Green Pesticides: Potential and Constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Tsolakis, H.; Ragusa, S. Laboratory Evaluation of the Effect of Plant Extracts on Tetranychus urticae Koch (Acariformes, Tetranychidae). Phytophaga 2004, 14, 539–548. [Google Scholar]
- Laborda, R.; Manzano, I.; Gamon, M.; Gavidia, I.; Perez-Bermudez, P.; Boluda, R. Effects of Rosmarinus officinalis and Salvia officinalis Essential Oils on Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod. 2013, 48, 106–110. [Google Scholar] [CrossRef]
- Choi, W.-I.; Lee, S.-G.; Park, H.-M.; Ahn, Y.-J. Toxicity of Plant Essential Oils to Tetranychus Urticae (Acari: Tetranychidae) and Phytoseiulus Persimilis (Acari: Phytoseiidae). J. Econ. Entomol. 2004, 97, 553–558. [Google Scholar] [CrossRef]
- Mansour, F.; Ravid, U.; Putievsky, E. Studies of the Effects of Essential Oils Isolated from 14 Species of Labiatae on the Carmine Spider Mite, Tetranychus cinnabarinus. Phytoparasitica 1986, 14, 137–142. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential Oils Extracted from Different Species of the Lamiaceae Plant Family as Prospective Bioagents against Several Detrimental Pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of Vermicompost, Compost and Digestate as Commercial Alternative Peat-Based Substrates on Qualitative Parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Hardman, J.M.; Franklin, J.L.; Moreau, D.L.; Bostanian, N.J. An Index for Selective Toxicity of Miticides to Phytophagous Mites and Their Predators Based on Orchard Trials. Pest Manag. Sci. Former. Pestic. Sci. 2003, 59, 1321–1332. [Google Scholar] [CrossRef]
- Wang, Y.; Dunn, B.L.; Jiang, L.; Arnall, D.B. Characterization of Normalized Difference Vegetation Index of Eight Poinsettia (Euphorbia pulcherrima L.) Cultivars during Bract Color Development. J. Appl. Hortic. 2014, 16, 205–209. [Google Scholar] [CrossRef]
- Coelho, A.P.; de Faria, R.T.; Leal, F.T.; de Arruda Barbosa, J.; Dalri, A.B.; Rosalen, D.L. Estimation of Irrigated Oats Yield Using Spectral Indices. Agric. Water Manag. 2019, 223, 105700. [Google Scholar] [CrossRef]
- Coelho, A.P.; Rosalen, D.L.; de Faria, R.T. Índices de Vegetação Na Predição Da Produtividade de Biomassa e Grãos Da Aveia-Branca Sob Lâminas de Irrigação. Pesqui. Agropecuária Trop. 2018, 48, 109–117. [Google Scholar] [CrossRef]
- Chauhan, N.; Singh, S.; Haider, S.; Lohani, H. Influence of Phenological Stages on Yield and Quality of Oregano (Origanum vulgare L.) under the Agroclimatic Condition of Doon Valley (Uttarakhand). Indian J. Pharm. Sci. 2013, 75, 489. [Google Scholar] [CrossRef]
- Kizil, S.; Ipek, A.; ArslAN, N.; Khawar, K.M. Effect of Different Developing Stages on Some Agronomical Characteristics and Essential Oil Composition of Oregano (Origanum onites). N. Z. J. Crop Hortic. Sci. 2008, 36, 71–76. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zhang, Z.; Chen, J.; Shi, H.; Tian, M. Diagnosis of Nitrogen Nutrition and Recommended Model of Topdressing for Cotton. Nongye Jixie Xuebao=Trans. Chin. Soc. Agric. Mach. 2014, 45, 209–214. [Google Scholar]
- Costa, B.R.S.; Oldoni, H.; da Silva, T.M.M.; Farinassi, L.G.; Bognola, I.A.; Bassoi, L.H. How Similar Is the Zoning of Different Vegetation Indices: Defining the Optimal Framework for Monitoring Grapevines’ Growth within Vigorous Vineyards. Sci. Hortic. 2023, 322, 112404. [Google Scholar] [CrossRef]
- Kavats, O.; Khramov, D.; Sergieieva, K.; Vasyliev, V. Monitoring of Sugarcane Harvest in Brazil Based on Optical and SAR Data. Remote Sens. 2020, 12, 4080. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, F.; Jing, Y.; Jiang, X.; Yang, S.; Han, X. Multi-Temporal Detection of Rice Phenological Stages Using Canopy Spectrum. Rice Sci. 2014, 21, 108–115. [Google Scholar]
- Kazmierski, M.; Glémas, P.; Rousseau, J.; Tisseyre, B. Temporal Stability of Within-Field Patterns of NDVI in Non Irrigated Mediterranean Vineyards. Oeno One 2011, 45, 61–73. [Google Scholar] [CrossRef]
- Rodimtsev, S.; Pavlovskaya, N.; Vershinin, S.; Gorkova, I.; Gagarina, I. Assessment of the Vegetative Index NDVI as an Indicator of Crop Yield; Springer: Berlin/Heidelberg, Germany, 2022; pp. 637–645. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Zhu, W.; Atzberger, C.; Liu, Q. The Optimal Threshold and Vegetation Index Time Series for Retrieving Crop Phenology Based on a Modified Dynamic Threshold Method. Remote Sens. 2019, 11, 2725. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Luo, W.; Gao, W. Summer Maize Phenology Monitoring Based on Normalized Difference Vegetation Index Reconstructed with Improved Maximum Value Composite. Trans. Chin. Soc. Agric. Eng. 2019, 35, 159–165. [Google Scholar]
- Zutic, I.; Putievsky, E.; Dudai, N. Influence of Harvest Dynamics and Cut Height on Yield Components of Sage (Salvia officinalis L.). J. Herbs Spices Med. Plants 2004, 10, 49–61. [Google Scholar] [CrossRef]
- Frescura, V.D.-S.; Schmitt, O.J.; Trapp, K.C.; Andriolo, J.L.; Lopes, S.J.; Tedesco, S.B. Nitrogen Influence and Frequency of Collections in the Production of Leaves and Essential Oil of Rosemary (Rosmarinus officinalis). Rev. Bras. De Plantas Med. 2021, 23, 179–185. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Zheljazkov, V.D.; Schlegel, V.; Cantrell, C.L. Growing Spearmint, Thyme, Oregano, and Rosemary in Northern Wyoming Using Plastic Tunnels. Ind. Crops Prod. 2016, 94, 251–258. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M. Phytochemical Constituents, Biological Activities, and Health-promoting Effects of the Genus Origanum. Phytother. Res. 2020, 35, 95–121. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Cousyn, G.; Dalfrà, S.; Scarpa, B.; Geelen, J.; Anton, R.; Serafini, M.; Delmulle, L. Harmonizing the Use of Plants in Food Supplements in the European Union: Belgium, France and Italy—A First Step. Eur. Food Feed Law Rev. 2013, 3, 187–196. [Google Scholar]
- Orhan, F.; Ölmez, M. Effect of Herbal Mixture Supplementation to Fish Oiled Layer Diets on Lipid Oxidation of Egg Yolk, Hen Performance and Egg Quality. Ank. Üniversitesi Vet. Fakültesi Derg. 2011, 58, 33–39. [Google Scholar] [CrossRef]
- Liguori, G.; Greco, G.; Salsi, G.; Garofalo, G.; Gaglio, R.; Barbera, M.; Greco, C.; Orlando, S.; Fascella, G.; Mammano, M.M. Effect of the Gellan-Based Edible Coating Enriched with Oregano Essential Oil on the Preservation of the ‘Tardivo Di Ciaculli’Mandarin (Citrus reticulata Blanco Cv. Tardivo Di Ciaculli). Front. Sustain. Food Syst. 2024, 8, 1334030. [Google Scholar] [CrossRef]
- Cetin, H.; Cilek, J.E.; Aydin, L.; Yanikoglu, A. Acaricidal Effects of the Essential Oil of Origanum minutiflorum (Lamiaceae) against Rhipicephalus turanicus (Acari: Ixodidae). Vet. Parasitol. 2009, 160, 359–361. [Google Scholar] [CrossRef]
- Dolan, M.C.; Jordan, R.A.; Schulze, T.L.; Schulze, C.J.; Cornell Manning, M.; Ruffolo, D.; Schmidt, J.P.; Piesman, J.; Karchesy, J.J. Ability of Two Natural Products, Nootkatone and Carvacrol, to Suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in a Lyme Disease Endemic Area of New Jersey. J. Econ. Entomol. 2009, 102, 2316–2324. [Google Scholar] [CrossRef]
- Tong, F.; Gross, A.D.; Dolan, M.C.; Coats, J.R. The Phenolic Monoterpenoid Carvacrol Inhibits the Binding of Nicotine to the Housefly Nicotinic Acetylcholine Receptor. Pest Manag. Sci. 2012, 69, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant Essential Oils for Pest and Disease Management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Akhtar, W.; Shahzad, T.; Malik, A.; Shah, M.A.; Iqbal, S.; Rauf, A.; Zengin, G.; Bouyahya, A. Rosemary Species: A Review of Phytochemicals, Bioactivities and Industrial Applications. S. Afr. J. Bot. 2022, 151, 3–18. [Google Scholar] [CrossRef]
- Wanna, R.; Bozdoğan, H. Activity of Rosmarinus officinalis (Lamiales: Lamiaceae) Essential Oil and Its Main Constituent, 1, 8-Cineole, against Tribolium castaneum (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2025, 60, 86–106. [Google Scholar] [CrossRef]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening of the Essential Oil Composition of Wild Sicilian Rosemary. Biochem. Syst. Ecol. 2010, 38, 659–670. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Dunan, L.; Malanga, T.; Benhamou, S.; Papaiconomou, N.; Desneux, N.; Lavoir, A.-V.; Michel, T. Effects of Essential Oil-Based Formulation on Biopesticide Activity. Ind. Crops Prod. 2023, 202, 117006. [Google Scholar] [CrossRef]
- Pierozan, M.K.; Pauletti, G.F.; Rota, L.; Santos, A.C.; Lerin, L.A.; Di Luccio, M.; Mossi, A.J.; Atti-Serafini, L.; Cansian, R.L.; Oliveira, J.V. Chemical Characterization and Antimicrobial Activity of Essential Oils of Salvia L. Species. Food Sci. Technol. 2009, 29, 764–770. [Google Scholar] [CrossRef]
- Delamare, A.P.L.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial Activity of the Essential Oils of Salvia officinalis L. and Salvia triloba L. Cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Anderson, J.A.; Coats, J.R. Acetylcholinesterase Inhibition by Nootkatone and Carvacrol in Arthropods. Pestic. Biochem. Physiol. 2012, 102, 124–128. [Google Scholar] [CrossRef]
- Rguez, S.; Daami-Remadi, M.; Cheib, I.; Laarif, A.; Hamrouni, I. Composition Chimique, Activité Antifongique et Activité Insecticide de l’huile Essentielle de Salvia officinalis. Tunis. J. Med. Plants Nat. Prod. 2013, 9, 65–76. [Google Scholar]
- Aissaoui, A.B.; Zantar, S.; Elamrani, A. Chemical Composition and Potential Acaricide of Salvia officinalis and Eucalyptus globulus on Tetranychus urticae Koch (Acarina: Tetranychidae). J. Appl. Chem. Environ. Prot. 2019, 4, 1–15. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Chiasson, H.; Bélanger, A.; Bostanian, N.; Vincent, C.; Poliquin, A. Acaricidal Properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) Essential Oils Obtained by Three Methods of Extraction. J. Econ. Entomol. 2001, 94, 167–171. [Google Scholar] [CrossRef] [PubMed]
- da Camara, C.A.; Akhtar, Y.; Isman, M.B.; Seffrin, R.C.; Born, F.S. Repellent Activity of Essential Oils from Two Species of Citrus against Tetranychus urticae in the Laboratory and Greenhouse. Crop Prot. 2015, 74, 110–115. [Google Scholar] [CrossRef]
- de Carvalho Brito, R.; de Moura Pádua, L.E.; da Silva, L.R.; Briozo, M.E.; Silva, P.R.; de Carvalho, L.F.; de Andrade Dutra, K.; Navarro, D.M.; Barbosa, D.R.; Rojas, M.O.; et al. Use of Essential Oils and α-Pinene as Insecticides against Sitophilus zeamais and Their Effects on Maize Seed Germination. Agronomy 2024, 14, 2282. [Google Scholar] [CrossRef]
- Comparetti, A.; Greco, C.; Orlando, S.; Ciulla, S.; Mammano, M.M. Comparison of Mechanical, Assisted and Manual Harvest of Origanum vulgare L. Sustainability 2022, 14, 2562. [Google Scholar] [CrossRef]
- Greco, C.; Catania, P.; Orlando, S.; Vallone, M.; Mammano, M.M. Assessment of Vegetation Indices as Tool to Decision Support System for Aromatic Crops; Springer: Berlin/Heidelberg, Germany, 2024; pp. 322–331. [Google Scholar]
- Greco, C.; Gaglio, R.; Settanni, L.; Sciurba, L.; Ciulla, S.; Orlando, S.; Mammano, M.M. Smart Farming Technologies for Sustainable Agriculture: A Case Study of a Mediterranean Aromatic Farm. Agriculture 2025, 15, 810. [Google Scholar] [CrossRef]
- Greco, C.; Catania, P.; Orlando, S.; Calderone, G.; Mammano, M.M. Rosemary Biomass Estimation from UAV Multispectral Camera. In Biosystems Engineering Promoting Resilience to Climate Change—AIIA 2024—Mid Term; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2025; Volume 586. [Google Scholar]
- Mammano, M.M.; Comparetti, A.; Ciulla, S.; Greco, C.; Orlando, S. A Prototype of Photovoltaic Dryer for Nutraceutical and Aromatic Plants; Springer: Berlin/Heidelberg, Germany, 2022; pp. 677–685. [Google Scholar]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.-L.; Song, B.-W.; Zhu, P.-L.; Zhang, H.-W. Vegetation Pixels Extraction Based on Red-Band Enhanced Normalized Difference Vegetation Index; SPIE: Bellingham, WA, USA, 2016; Volume 10033, pp. 731–737. [Google Scholar]
- Yang, J.S.; Yang, G.J.; Xu, B.; Zhang, K.; Yang, X.; Li, Z.; Li, H.; Yang, H.; Liang, H. Reliability analysis and calibration environment of field crop NDVI measuring instruments. Trans. Chin. Soc. Agric. Eng. 2019, 35, 230–236. [Google Scholar] [CrossRef]
- Gozdowski, D.; Stępień, M.; Panek, E.; Varghese, J.; Bodecka, E.; Rozbicki, J.; Samborski, S. Comparison of Winter Wheat NDVI Data Derived from Landsat 8 and Active Optical Sensor at Field Scale. Remote Sens. Appl. Soc. Environ. 2020, 20, 100409. [Google Scholar] [CrossRef]
- Hangbin, Z.; Xiaoping, Y.; Jialin, L. MODIS Data Based NDVI Seasonal Dynamics in Agro-Ecosystems of South Bank Hangzhouwan Bay. Afr. J. Agric. Res. 2011, 6, 4025–4033. [Google Scholar]
- Li, M.; Wang, X.; Gao, Y.; Fu, Y.; Fan, W. Inter-Annual Variation in Vegetation Index and Analysis of Factors Affecting It in Daxing’an Mountains. J. Beijing For. Univ. 2015, 37, 1–10. [Google Scholar]
- Garofalo, G.; Ponte, M.; Greco, C.; Barbera, M.; Mammano, M.M.; Fascella, G.; Greco, G.; Salsi, G.; Orlando, S.; Alfonzo, A. Improvement of Fresh Ovine “Tuma” Cheese Quality Characteristics by Application of Oregano Essential Oils. Antioxidants 2023, 12, 1293. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, ed. 4.1. 2017. Available online: http://essentialoilcomponentsbygcms.com/ (accessed on 1 February 2025).
- Zito, P.; Sajeva, M.; Bruno, M.; Rosselli, S.; Maggio, A.; Senatore, F. Essential Oils Composition of Two Sicilian Cultivars of Opuntia ficus-indica (L.) Mill. (Cactaceae) Fruits (Prickly Pear). Nat. Prod. Res. 2013, 27, 1305–1314. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef]
- Johnson, N.; Kotz, S. Discrete Distributions. In Their Distributions in Statistics; Houghton Mifflin: Boston, MA, USA, 1969; Volume 16, 328p. [Google Scholar]
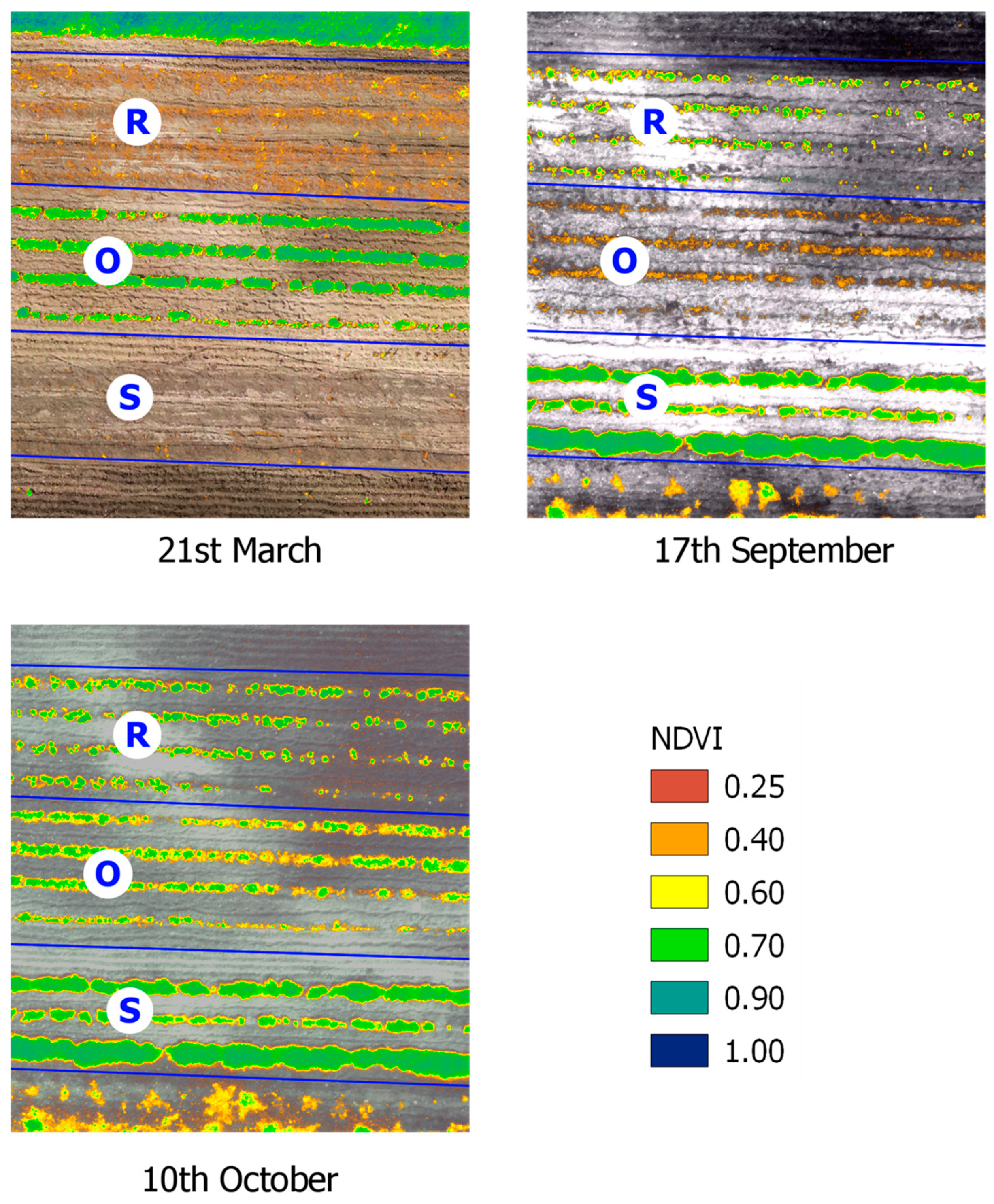
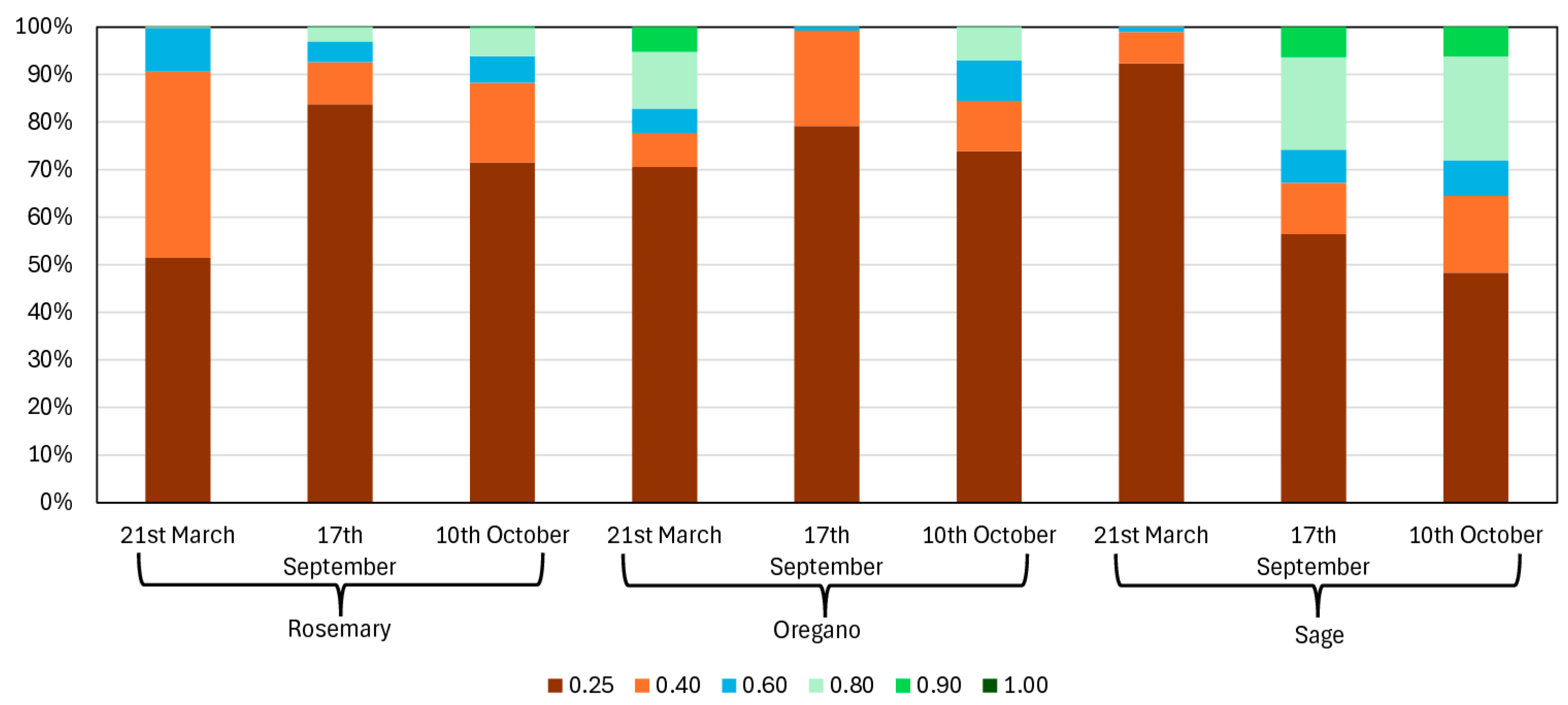

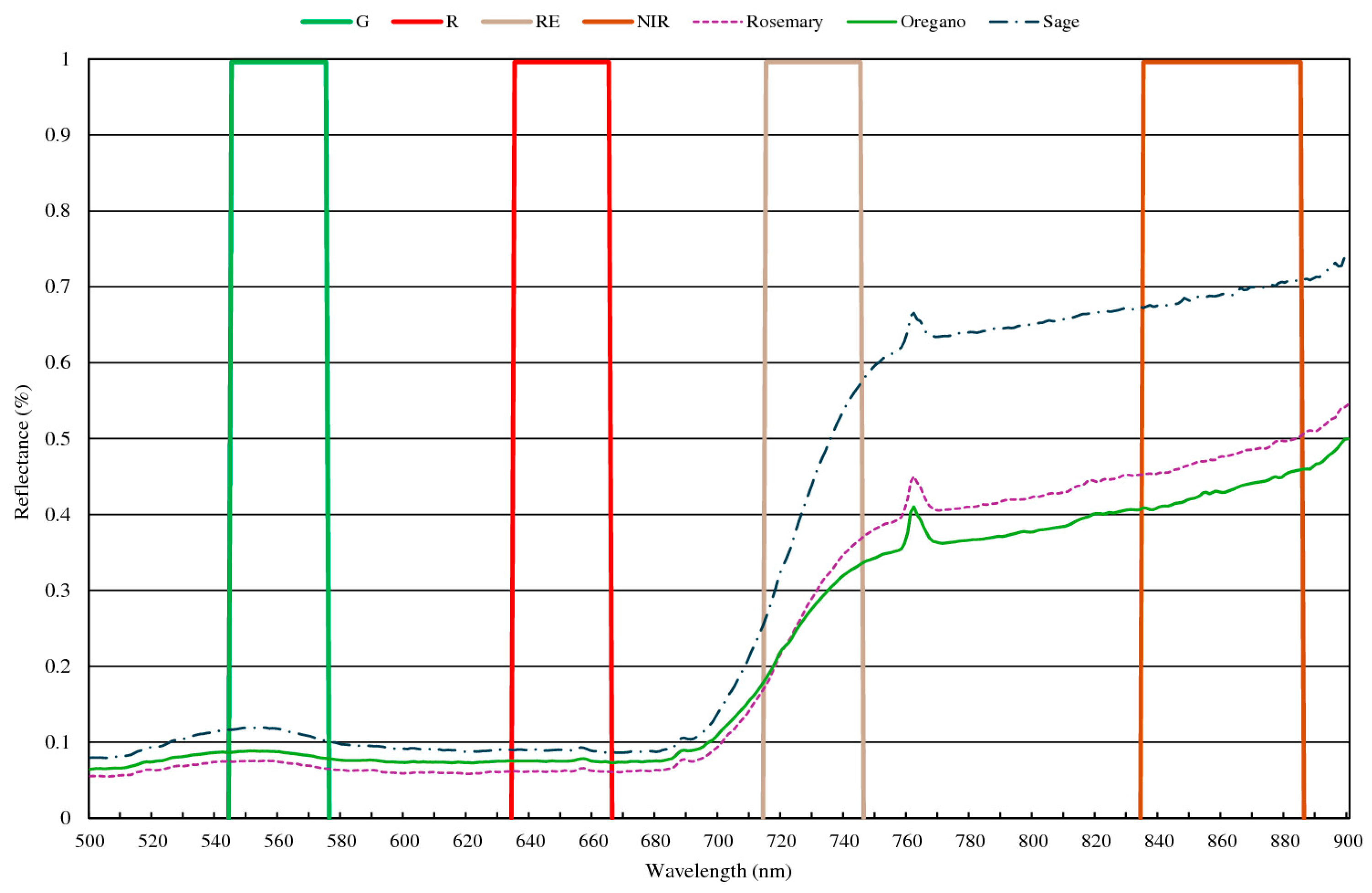
| Component/Chemical Class | Oregano EO (RW%) | Rosemary EO (RW%) | Sage EO (RW%) |
|---|---|---|---|
| Monoterpene phenol | |||
| Carvacrol | 83.42 | - | - |
| Thymol | 0.89 | - | - |
| Subtotal | 84.31 | - | - |
| Monoterpene hydrocarbons | |||
| ρ-Cymene | 3.06 | 0.80 | - |
| γ-Terpinene | 2.93 | 2.10 | - |
| β-Myrcene | 1.01 | 1.21 | 2.19 |
| α-Terpinene | 0.99 | 0.45 | - |
| Limonene | 0.45 | 2.22 | - |
| Terpinolene | 0.41 | 0.53 | - |
| β-Ocimene | 0.42 | - | - |
| β-Pinene | 0.13 | 4.1 | 2.66 |
| Sabinene | 0.13 | - | - |
| α-Pinene | - | 28.0 | 2.66 |
| Linalyl Acetate | - | 1.12 | - |
| Crisantenone | - | - | 12.87 |
| Camphene | - | 7.00 | 9.26 |
| Subtotal | 9.53 | 47.53 | 29.64 |
| Oxygenated monoterpenes | |||
| Linalool | 0.32 | 3.45 | - |
| Camphor | 0.27 | 6.20 | 21.91 |
| Terpinen-4-ol | 0.18 | - | - |
| 1,8-Cineole (Eucalyptol) | - | 11.00 | 27.67 |
| Borneol | - | 7.72 | 2.59 |
| β-Thujone | - | 0.73 | - |
| α-Thujone | - | - | 5.32 |
| Bornyl Acetate | - | - | 1.45 |
| 4-Caranol | - | - | 0.09 |
| 4-Terpineol | 0.31 | - | 0.54 |
| α-Terpineol | - | 4.40 | - |
| α-Thujene | 0.23 | - | - |
| α-Phellandrene | 0.60 | - | - |
| Subtotal | 1.91 | 33.37 | 59.57 |
| Sesquiterpene hydrocarbons | |||
| β-Caryophyllene | 1.07 | 6.64 | 1.46 |
| α-Humulene | 0.70 | 0.75 | - |
| Germacrene | 0.07 | - | - |
| β-Bisabolene | 0.63 | - | - |
| Farnesene | 0.40 | - | - |
| α-Caryophyllene | - | - | 0.89 |
| Alloaromadendrene | - | - | 0.24 |
| α-Gurjunene | - | - | 0.13 |
| Carophyllene oxide | 0.88 | ||
| Subtotal | 2.87 | 8.27 | 2.72 |
| Oxygenated sesquiterpenes | |||
| Viridiflor | - | - | 1.22 |
| Palustrol | - | - | 0.75 |
| Ledol | - | - | 0.53 |
| Spathulenol | - | - | 0.31 |
| Subtotal | - | - | 2.81 |
| Other compounds | |||
| Camphol | 0.70 | - | - |
| 1-Octen-3-ol | 0.49 | - | - |
| 3-Octanone | 0.36 | - | - |
| Carvacrol Methyl Ether | 0.03 | - | - |
| Maool | - | - | 0.62 |
| Diethyl Phthalate | - | - | 0.53 |
| Naphthalene | - | - | 0.28 |
| Subtotal | 1.58 | - | 1.43 |
| Concentrations | Cumulative Mortality (%) (Mean ± SE) | Survival Time (Days) | Abbott’s Corrected Mortality | Toxicity Class | ||||
|---|---|---|---|---|---|---|---|---|
| Plant Extracts | (µL L−1) | Day 1 | Day 2 | Day 3 | Day 4 | (Mean ± SE) | (%) | * |
| O. vulgare | 5000 | 66.00 ± 7.92 | 74.00 ± 8.46 | 80.00 ± 7.30 | 90.00 ± 4.47 a | 0.90 ± 0.203 a | 89.13 | 4 |
| 2500 | 14.00 ± 4.27 | 20.00 ± 2.98 | 34.00 ± 6.00 | 36.00 ± 7.77 bc | 2.96 ± 0.216 bcd | 30.43 | 2 | |
| 1280 | 0.00 ± 0.00 | 4.00 ± 2.67 | 12.00 ± 4.42 | 22.00 ± 5.54 efg | 3.62 ± 0.114 ed | 15.22 | 1 | |
| 640 | 2.00 ± 2.00 | 4.00 ± 2.67 | 12.00 ± 3.27 | 20.00 ± 5.16 ef | 3.62 ± 0.124 ed | 13.04 | 1 | |
| 320 | 2.00 ± 2.00 | 6.00 ± 3.06 | 10.00 ± 3.33 | 16.00 ± 4.00 efg | 3.66 ± 0.127 ed | 8.70 | 1 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.00 ± 3.27 fg | 3.92 ± 0.038 e | 0.00 | - | |
| S. rosmarinus | 5000 | 16.00 ± 6.53 | 36.00 ± 9.33 | 42.00 ± 9.17 | 46.00 ± 10.3 b | 2.60 ± 0.234 b | 46.00 | 2 |
| 2500 | 12.00 ± 4.42 | 24.00 ± 4.99 | 34.00 ± 4.27 | 40.00 ± 5.16 bc | 2.90 ± 0.214 bcd | 40.00 | 2 | |
| 1280 | 2.00 ± 2.00 | 12.00 ± 4.42 | 20.00 ± 5.16 | 26.00 ± 3.06 de | 3.40 ± 0.159 cde | 26.00 | 2 | |
| 640 | 2.00 ± 2.00 | 16.00 ± 4.99 | 16.00 ± 4.99 | 22.00 ± 6.96 e | 3.44 ± 0.165 cde | 22.00 | 1 | |
| 320 | 4.00 ± 2.67 | 6.00 ± 3.06 | 6.00 ± 3.06 | 12.00 ± 4.42 efg | 3.72 ± 0.128 cd | 12.00 | 1 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00±0.00 g | 4.00 ± 0.00 e | 0.00 | - | |
| S. officinalis | 5000 | 12.00 ± 5.33 | 32.00 ± 9.52 | 42.00 ± 9.17 | 42.00 ± 9.17 bc | 2.72 ± 0.225 bc | 39.58 | 2 |
| 2500 | 12.00 ± 6.80 | 32.00 ± 6.80 | 40.00 ± 7.30 | 54.00 ± 8.46 b | 2.62 ± 0.216 bc | 52.08 | 3 | |
| 1280 | 10.00 ± 4.47 | 28.00 ± 6.11 | 32.00 ± 8.00 | 34.00 ± 6.70 bcd | 2.96 ± 0.218 bcd | 31.25 | 2 | |
| 640 | 0.00 ± 0.00 | 14.00 ± 4.27 | 22.00 ± 5.54 | 22.00 ± 5.54 cde | 3.42 ± 0.159 cde | 18.75 | 1 | |
| 320 | 4.00 ± 2.67 | 16.00 ± 4.00 | 18.00 ± 4.67 | 28.00 ± 3.27 bcde | 3.34 ± 0.173 bcde | 25.00 | 1 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.00 ± 2.67 e | 3.96 ± 0.028 e | 0.00 | - | |
| Essential Oils | LC10 mL L−1 (95% CI) | LC30 mL L−1 (95% CI) | LC50 mL L−1 (95% CI) | LC90 mL L−1 (95% CI) | LC95 mL L−1 (95% CI) | Intercept ± SE | Slope ± SE | Goodness of fit χ2 (d.f.) |
|---|---|---|---|---|---|---|---|---|
| Origanum vulgare | 0.369 (0.209–0.530) | 1.068 (0.800–1.362) | 2.229 (1.740–3.051) | 13.452 (8.015–31.805) | 22.392 (12.085–63.218) | −5.49 ± 0.71 | 1.64 ± 0.22 | 21.62 (3) p = 0.000 |
| Salvia rosmarinus | 0.207 (0.034–0.428) | 1.489 (0.908–2.452) | 5.835 (3.281–22.289) | 164.369 (35.154–10573.530) | 423.448 (67.507–61877.107) | −3.32 ± 0.67 | 0.88 ± 0.21 | 0.53 (3) p = 0.911 |
| Salvia officinalis | 0.027 (0.000002–0.146) | 0.655 (0.080–1.292) | 6.014 (2.630–261.607) | 1358.595 (74.342–20,379,803,862.871) | 6315.387 (184.714–3651,741,272,548.380) | −2.05 ± 0.61 | 0.54 ± 0.19 | 5.73 (3) p = 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, T.; Cerasa, G.; Marotta, I.; Conte, M.; Orlando, S.; Salamone, A.; Mammano, M.M.; Greco, C.; Tsolakis, H. Toxicity of Essential Oils of Origanum vulgare, Salvia rosmarinus, and Salvia officinalis Against Aculops lycopersici. Plants 2025, 14, 1462. https://doi.org/10.3390/plants14101462
Giordano T, Cerasa G, Marotta I, Conte M, Orlando S, Salamone A, Mammano MM, Greco C, Tsolakis H. Toxicity of Essential Oils of Origanum vulgare, Salvia rosmarinus, and Salvia officinalis Against Aculops lycopersici. Plants. 2025; 14(10):1462. https://doi.org/10.3390/plants14101462
Chicago/Turabian StyleGiordano, Thomas, Giuliano Cerasa, Ilaria Marotta, Mauro Conte, Santo Orlando, Adele Salamone, Michele Massimo Mammano, Carlo Greco, and Haralabos Tsolakis. 2025. "Toxicity of Essential Oils of Origanum vulgare, Salvia rosmarinus, and Salvia officinalis Against Aculops lycopersici" Plants 14, no. 10: 1462. https://doi.org/10.3390/plants14101462
APA StyleGiordano, T., Cerasa, G., Marotta, I., Conte, M., Orlando, S., Salamone, A., Mammano, M. M., Greco, C., & Tsolakis, H. (2025). Toxicity of Essential Oils of Origanum vulgare, Salvia rosmarinus, and Salvia officinalis Against Aculops lycopersici. Plants, 14(10), 1462. https://doi.org/10.3390/plants14101462










