Stilbene Glycosides in Pinus cembra L. Bark: Isolation, Characterization, and Assessment of Antioxidant Potential and Antitumor Activity on HeLa Cells
Abstract
1. Introduction
2. Results
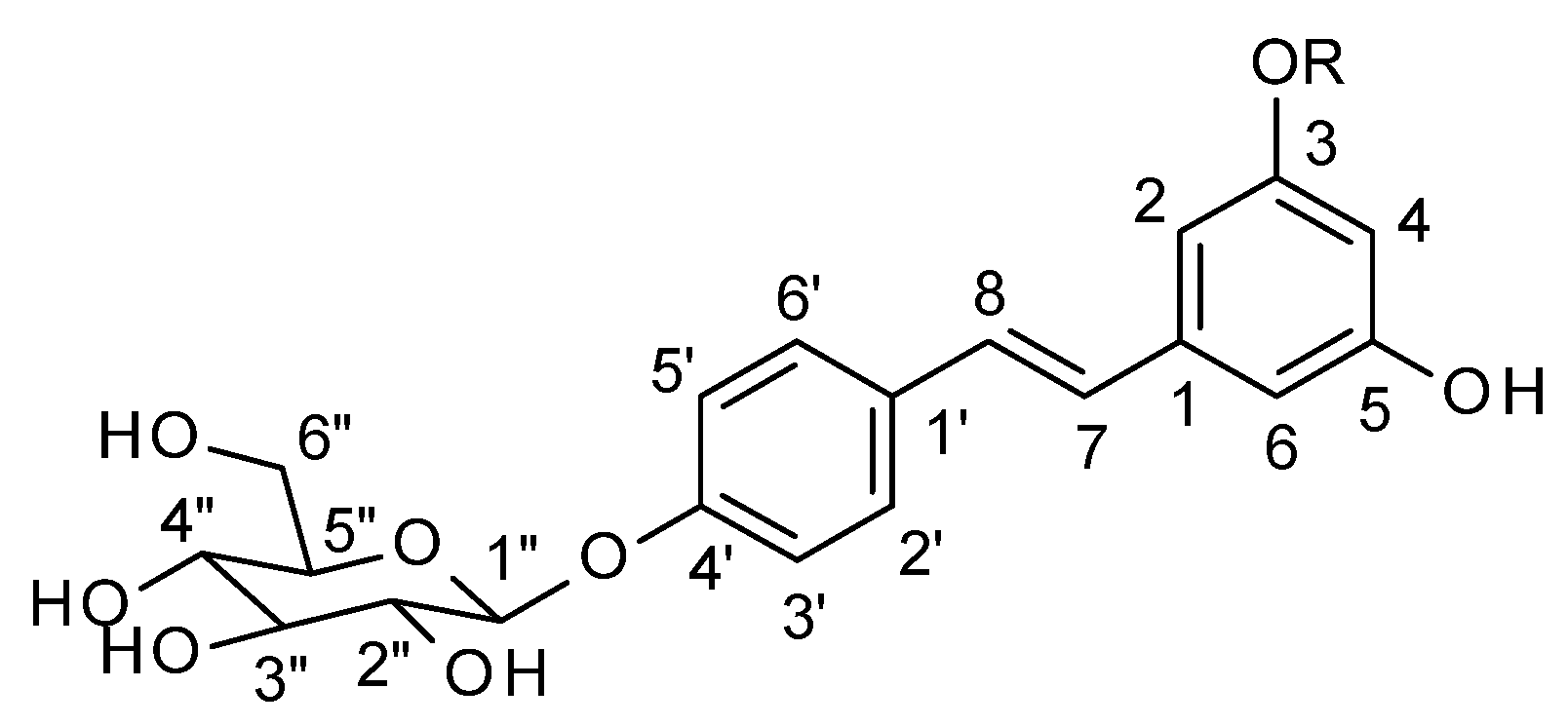
2.1. Structure Elucidation of Compounds 1 and 2
2.2. Antioxidant Activity
2.3. Cytototoxic Activity on HeLa Cells
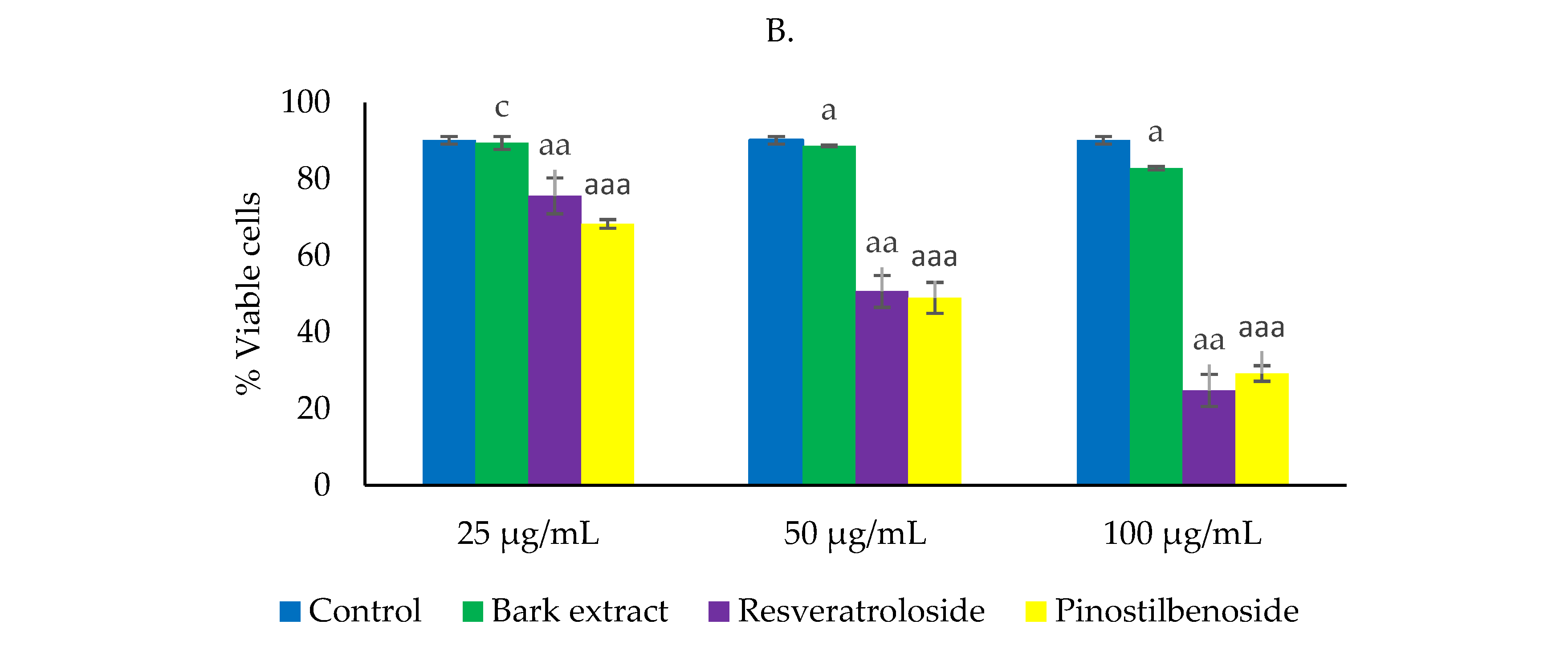
2.3.1. Effects on Cell Viability
2.3.2. Effects on Apoptosis
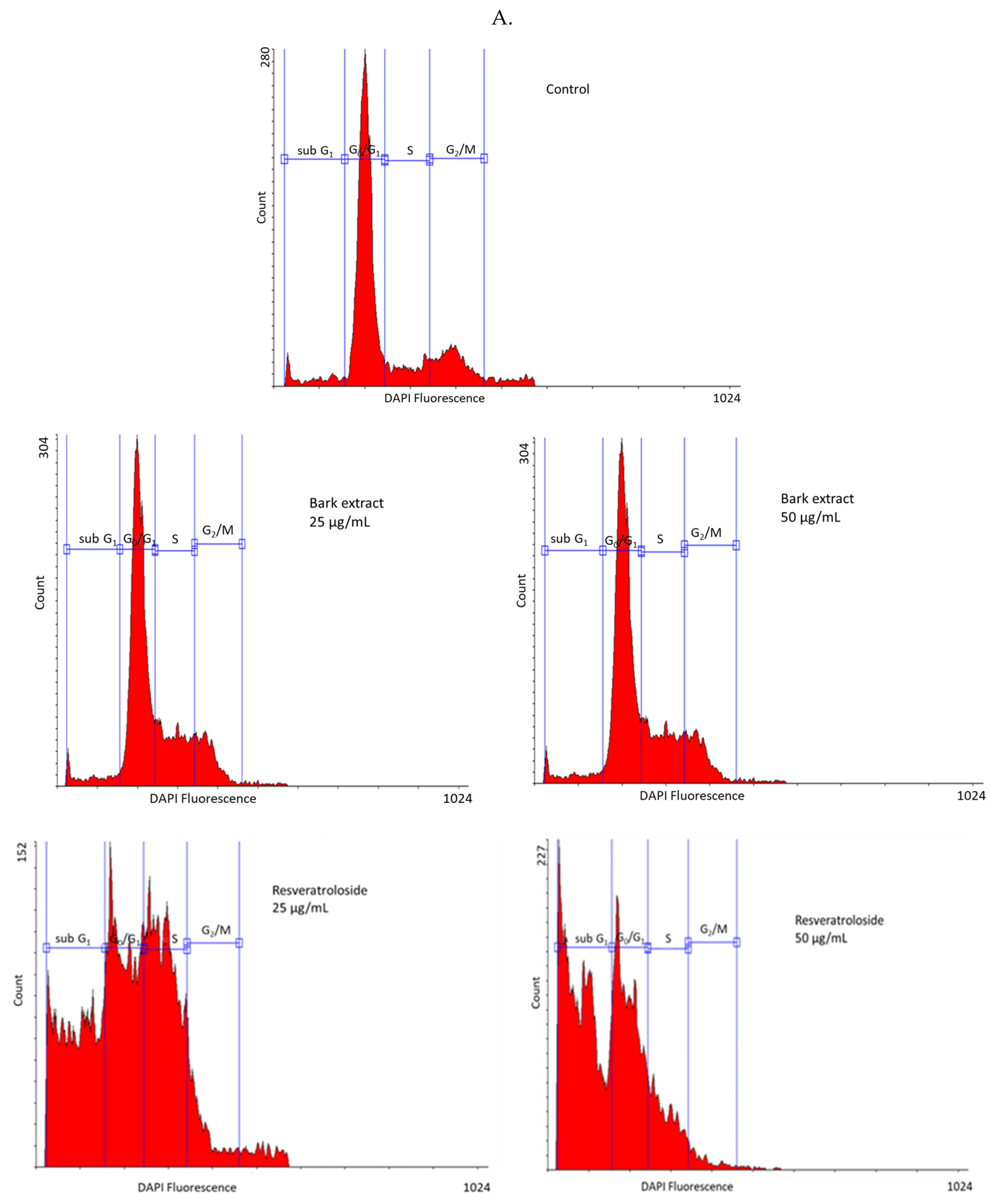
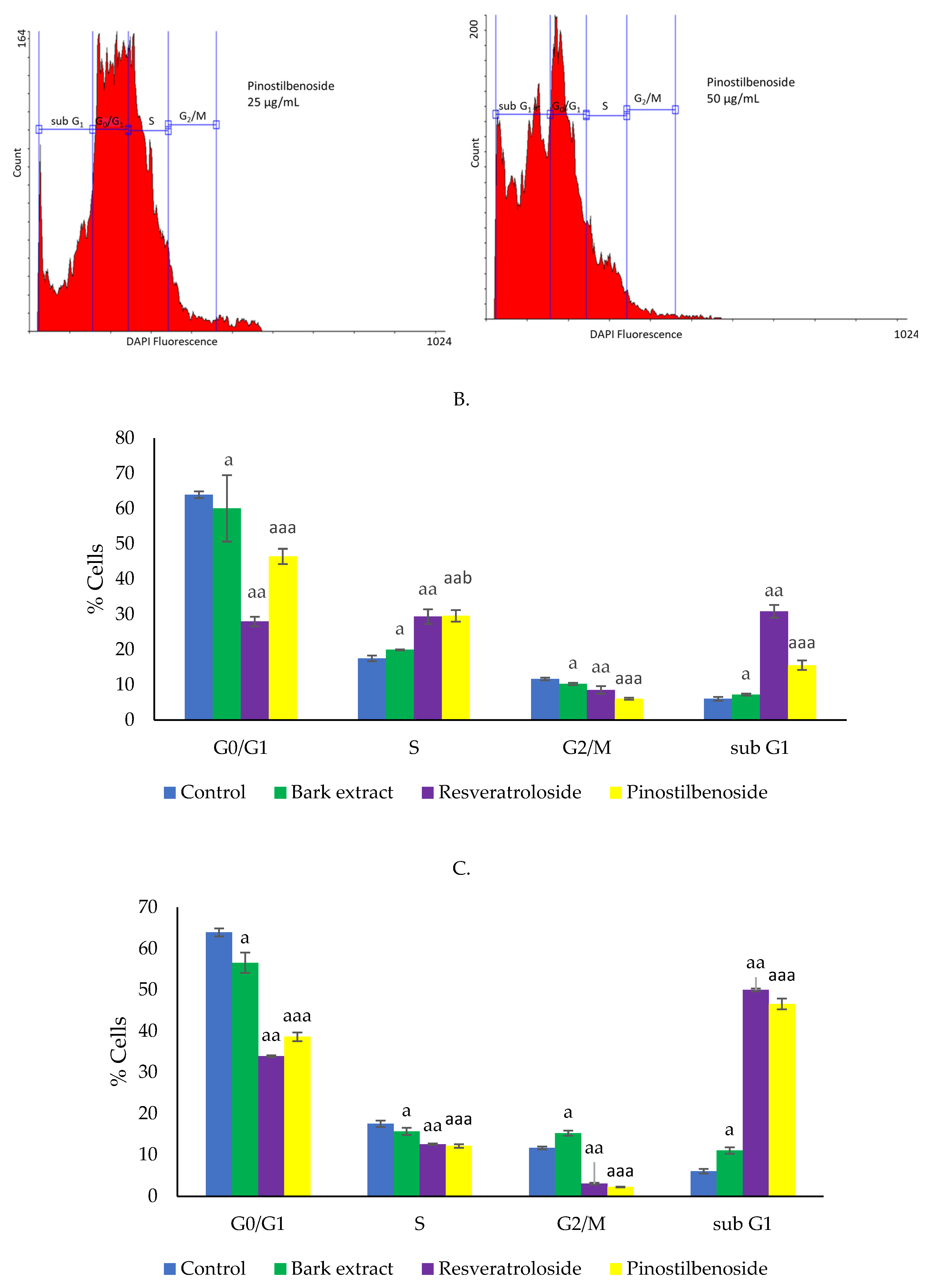
2.3.3. Effects on Cell Cycle
2.3.4. Effects on Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Isolation of Stilbene Glycosides
4.4. Structure Elucidation
4.5. Antioxidant Activity
4.5.1. DPPH Radical Scavenging Assay
4.5.2. Reducing Power Assay
4.6. Cytotoxic Activity on HeLa Cells
4.6.1. Cell Culture
4.6.2. Cell Viability Assay
4.6.3. Apoptosis Assay
4.6.4. Cell Cycle Assay
4.6.5. Cell Proliferation Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, E.L.S.S.; Xavier, J.A.; Fragoso, M.B.T.; Silva, M.O.; Escodro, P.B.; Oliveira, A.C.M.; Tucci, P.; Saso, L.; Goulart, M.O.F. E-Stilbenes: General Chemical and Biological Aspects, Potential Pharmacological Activity Based on the Nrf2 Pathway. Pharmaceuticals 2024, 17, 232. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell. Int. 2021, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Liu, P.; Tang, W.; Xiang, K.; Li, G. Pterostilbene in the treatment of inflammatory and oncological diseases. Front. Pharmacol. 2024, 14, 1323377. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Shakya, A.K.; Al-Qudah, M.A.; Barhoumi, L.M.; Abu-Sal, H.E.; Hasan, H.S.; Al-Bataineh, N.; Abu-Orabi, S.; Mubarak, M.S. Piceatannol, a comprehensive review of health perspectives and pharmacological aspects. Arab. J. Chem. 2024, 17, 105939. [Google Scholar] [CrossRef]
- Bakrim, S.; Machate, H.; Benali, T.; Sahib, N.; Jaouadi, I.; Omari, N.E.; Aboulaghras, S.; Bangar, S.P.; Lorenzo, J.M.; Zengin, G.; et al. Natural Sources and Pharmacological Properties of Pinosylvin. Plants 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Piekarski, J.; Merecz-Sadowska, A.; Muskała, M.; Sitarek, P. Investigation of the molecular mechanisms underlying the anti-inflammatory and antitumour effects of isorhapontigenin: Insights from in vitro and in vivo studies. Biomed. Pharmacother. 2024, 180, 117479. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Joshi, V.C.; Wang, Y.H.; Khan, I.A. Simultaneous identification and quantification of anthraquinones, polydatin, and resveratrol in Polygonum multiflorum, various Polygonum species, and dietary supplements by liquid chromatography and microscopic study of Polygonum species. J. AOAC Int. 2007, 90, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Suprun, A.R.; Dubrovina, A.S.; Grigorchuk, V.P.; Kiselev, K.V. Stilbene Content and Expression of Stilbene Synthase Genes in Korean Pine Pinus koraiensis Siebold & Zucc. Forests 2023, 14, 1239. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.F.; Mérillon, J.M. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Jyske, T.; Brännström, H.; Halmemies, E.; Laakso, T.; Kilpeläinen, P.; Hyvönen, J.; Kärkkäinen, K.; Saranpää, P. Stilbenoids of Norway spruce bark: Does the variability caused by raw-material processing offset the biological variability. Biomass Convers. Biorefin. 2024, 14, 5085–5099. [Google Scholar] [CrossRef]
- Francezon, N.; Meda, N.-S.-B.R.; Stevanovic, T. Optimization of Bioactive Polyphenols Extraction from Picea mariana Bark. Molecules 2017, 22, 2118. [Google Scholar] [CrossRef]
- Kwon, D.J.; Young-Soo, B. Stilbenoids of Korean Pine (Pinus koraiensis) Inner Bark. Mokchae Konghak 2009, 37, 474–479. [Google Scholar]
- Celimene, C.; Micales, J.; Ferge, L.; Young, R. Efficacy of Pinosylvins against White-Rot and Brown-Rot Fungi. Holzforschung 1999, 53, 491–497. [Google Scholar] [CrossRef]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef]
- Alperth, F.; Schneebauer, A.; Kunert, O.; Bucar, F. Phytochemical Analysis of Pinus cembra Heartwood—UHPLC-DAD-ESI-MSn with Focus on Flavonoids, Stilbenes, Bibenzyls and Improved HPLC Separation. Plants 2023, 12, 3388. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Jayasuriya, H.; Lee, E.S.; Koonchanok, N.M.; Geahlen, R.L.; Ashendel, C.L.; McLaughlin, J.L.; Chang, C.J. Kinase inhibitors from Polygonum cuspidatum. J. Nat. Prod. 1993, 56, 1805–1810. [Google Scholar] [CrossRef]
- Vastano, B.C.; Chen, Y.; Zhu, N.; Ho, C.T.; Zhou, Z.; Rosen, R.T. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J. Agric. Food Chem. 2000, 48, 253–256. [Google Scholar] [CrossRef]
- Makong, Y.S.; Mouthé Happi, G.; Djouaka Bavoua, J.L.; Wansi, J.D.; Nahar, L.; Kamdem Waffo, A.F.; Martin, C.; Sewald, N.; Sarker, S.D. Cytotoxic Stilbenes and Canthinone Alkaloids from Brucea antidysenterica (Simaroubaceae). Molecules 2019, 24, 4412. [Google Scholar] [CrossRef] [PubMed]
- Segun, P.A.; Ogbole, O.O.; Ismail, F.M.D.; Nahar, L.; Evans, A.R.; Ajaiyeoba, E.O.; Sarker, S.D. Resveratrol derivatives from Commiphora africana (A. Rich.) Endl. display cytotoxicity and selectivity against several human cancer cell lines. Phytother. Res. 2019, 33, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, Antioxidant and Antimicrobial Investigations of Pinus cembra L. Bark and Needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, Pycnogenol. Free Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Silva, A.S.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.A.; Dhanjal, D.S.; Nicoletti, M.; et al. Conifers Phytochemicals: A Valuable Forest with Therapeutic Potential. Molecules 2021, 26, 3005. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Pereira, C.; Gomes, A.P.; Neto, R.T.; Almeida, A.; Santos, S.A.O.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Characterisation, Antioxidant and Antibacterial Activities of Pinus pinaster Ait. and Pinus pinea L. Bark Polar Extracts: Prospecting Forestry By-Products as Renewable Sources of Bioactive Compounds. Appl. Sci. 2022, 12, 784. [Google Scholar] [CrossRef]
- Lim, W.X.J.; Gammon, C.S.; von Hurst, P.; Chepulis, L.; Page, R.A. The Inhibitory Effects of New Zealand Pine Bark (Enzogenol®) on α-Amylase, α-Glucosidase, and Dipeptidyl Peptidase-4 (DPP-4) Enzymes. Nutrients 2022, 14, 1596. [Google Scholar] [CrossRef]
- Benković, E.T.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Štrukelj, B. Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crops Prod. 2014, 52, 23–28. [Google Scholar] [CrossRef]
- Wieser, G.; Manning, W.J.; Tausz, M.; Bytnerowicz, A. Evidence for potential impacts of ozone on Pinus cembra L. at mountain sites in Europe: An overview. Environ. Pollut. 2006, 139, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Santos, J.; Ramos, C.; Teixeira, J.A.; Rocha, C.M.R. Unravelling the Biological Potential of Pinus pinaster bark extracts. Antioxidants 2020, 9, 334. [Google Scholar] [CrossRef]
- Cretu, E.; Miron, S.D.; Miron, A. Bioactivity screening of Pinus brutia bark extracts: Superoxide dismutase-like and nitric oxide scavenging effects. Rev. Med. Chir. Soc. Med. Nat. Iasi 2013, 117, 551–557. [Google Scholar] [PubMed]
- Cretu, E.; Salminen, J.-P.; Karonen, M.; Miron, A.; Charalambous, C.; Constantinou, A.I.; Aprotosoaie, A.C. In vitro antioxidant activity and phenolic content of Cedrus brevifolia bark. Nat. Prod. Commun. 2014, 9, 481–482. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, W.; Shen, T.; Wang, M.-H. Antioxidant activity and protection from DNA damage by water extract from pine (Pinus densiflora) bark. Prev. Nutr. Food Sci. 2012, 17, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Cretu, E.; Karonen, M.; Salminen, J.-P.; Mircea, C.; Trifan, A.; Charalambous, C.; Constantinou, A.I.; Miron, A. In vitro study on the antioxidant activity of a polyphenol-rich extract from Pinus brutia bark and its fractions. J. Med. Food 2013, 16, 984–991. [Google Scholar] [CrossRef]
- Kiss, A.; Baksa, V.; Bege, M.; Tálas, L.; Borbás, A.; Bereczki, I.; Bánfalvi, G.; Szemán-Nagy, G. MTT test and time-lapse microscopy to evaluate the antitumor potential of nucleoside analogues. Anticancer Res. 2021, 41, 137–149. [Google Scholar] [CrossRef]
- Ma, H.; Lai, F.; Xie, H.; Wang, J.; Wang, H. Involvement of the Bcl-2 family members in Pinus massoniana bark extract induced apoptosis in HeLa cells. Phytother. Res. 2008, 22, 1472–1476. [Google Scholar] [CrossRef]
- Parveen, S.; Varalakshmi, K.N. Accumulation of cells in sub-G1 phase and apoptosis induction by a bioactive fraction from the seaweed Gelidiella acerosa. Biosc. Biotech. Res. Comm. 2020, 13, 1184–1190. [Google Scholar] [CrossRef]
- Wu, D.-C.; Li, S.; Yang, D.-Q.; Cui, Y.-Y. Effects of Pinus massoniana bark extract on the adhesion and migration capabilities of HeLa cells. Fitoterapia 2011, 82, 1202–1205. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Feng, J.; Zhang, X.-L.; Li, M.-Q.; Cui, Y.-Y. Effects of Pinus massoniana bark extract on the invasion capability of HeLa cells. J. Funct. Foods 2016, 24, 520–526. [Google Scholar] [CrossRef]
- Mihailescu Amalinei, R.L.; Trifan, A.; Cioanca, O.; Miron, S.D.; Mihai, C.T.; Rotinberg, P.; Miron, A. Polyphenol-rich extract from Pinus sylvestris L. bark—Chemical and antitumor studies. Rev. Med. Chir. Soc. Med. Nat. Iasi 2014, 118, 551–557. [Google Scholar] [PubMed]
- Achmad, A.B.; Proboningrat, A.; Ansori, A.N.M.; Fadholly, A.; Rochmi, S.E.; Samsudin, R.R.; Hidayatik, N.; Hendarti, G.A.; Jayanti, S. Stem bark ethanolic extract of Pinus merkusii induces caspase-9 mediated apoptosis in HeLa cells. Open Vet. J. 2024, 14, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Q.; Zhang, T.; Han, Z.; Li, J.; Liu, Z.; Zheng, F. Procyanidins from Pinus koraiensis bark inhibits HeLa cell growth by inducing apoptosis and reducing surviving protein expression. Afr. J. Biotechnol. 2011, 10, 7766–7771. [Google Scholar] [CrossRef]
- Gromova, A.S.; Tyukavkina, N.A.; Lutskii, V.I.; Kalabin, G.A.; Kushnarev, D.F. Hydroxystilbenes of the inner bark of Pinus sibirica. Chem. Nat. Compd. 1975, 11, 715–719. [Google Scholar] [CrossRef]
- Dar, B.A.; Lone, S.H.; Shah, W.A.; Bhat, K.A. LC-MS guided isolation of bioactive principles from Iris hookeriana and bioevaluation of isolates for antimicrobial and antioxidant activities. Drug Res. 2016, 66, 427–431. [Google Scholar] [CrossRef]
- Uesugi, D.; Hamada, H.; Shimoda, K.; Kubota, N.; Ozaki, S.; Nagatani, N. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci. Biotechnol. Biochem. 2017, 81, 226–230. [Google Scholar] [CrossRef]
- Al-Mamary, M.A.; Moussa, Z. Antioxidant Activity: The Presence and Impact of Hydroxyl Groups in Small Molecules of Natural and Synthetic Origin. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; She, G.; Zheng, X.; Shao, L.; Wang, P.; Pang, M.; Xie, S.; Sun, Y. Resveratrol induces cervical cancer HeLa cell apoptosis through the activation and nuclear translocation promotion of FOXO3a. Pharmazie 2020, 75, 250–254. [Google Scholar] [CrossRef]
- Li, L.; Qiu, R.-L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.-J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef]
- Nadile, M.; Retsidou, M.I.; Gioti, K.; Beloukas, A.; Tsiani, E. Resveratrol against Cervical Cancer: Evidence from In Vitro and In Vivo Studies. Nutrients 2022, 14, 5273. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.H.; Lee, Y.-J.; Choi, H.; Song, K.-S.; Lee, K.J.; Nam, J.-W. Evaluating the anticancer potential of Polygonum multiflorum-root derived stilbenes against H2452 malignant pleural mesothelioma cells. Fitoterapia 2024, 177, 106135. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Wang, X.; Hua, P.; He, C.; Chen, M. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm. Sin. B 2022, 12, 3567–3593. [Google Scholar] [CrossRef] [PubMed]
- Scifo, C.; Cardile, V.; Russo, A.; Consoli, R.; Vancheri, C.; Capasso, F.; Vanella, A.; Renis, M. Resveratrol and Propolis as Necrosis or Apoptosis Inducers in Human Prostate Carcinoma Cells. Oncol. Res. 2004, 14, 415–426. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef]
- Wawszczyk, J.; Jesse, K.; Smolik, S.; Kapral, M. Mechanism of Pterostilbene-Induced Cell Death in HT-29 Colon Cancer Cells. Molecules 2022, 27, 369. [Google Scholar] [CrossRef]
- Horsman, M.R.; Murata, R.; Breidahl, T.; Nielsen, F.U.; Maxwell, R.J.; Stødkiled-Jørgensen, H.; Overgaard, J. Combretastatins Novel Vascular Targeting Drugs for Improving Anticancer Therapy. In Angiogenesis. Advances in Experimental Medicine and Biology; Maragoudakis, M.E., Ed.; Springer: Boston, MA, USA, 2000; Volume 476, pp. 311–323. [Google Scholar] [CrossRef]
- Almalki, S.G. The pathophysiology of the cell cycle in cancer and treatment strategies using various cell cycle checkpoint inhibitors. Pathol. Res. Pract. 2023, 251, 154854. [Google Scholar] [CrossRef]
- Chalal, M.; Delmas, D.; Meunier, P.; Latruffe, N.; Vervandier-Fasseur, D. Inhibition of Cancer Derived Cell Lines Proliferation by Synthesized Hydroxylated Stilbenes and New Ferrocenyl-Stilbene Analogs. Comparison with Resveratrol. Molecules 2014, 19, 7850–7868. [Google Scholar] [CrossRef]
- Wang, D.; Guo, H.; Yang, H.; Wang, D.; Gao, P.; Wei, W. Pterostilbene, an Active Constituent of Blueberries, Suppresses Proliferation Potential of Human Cholangiocarcinoma via Enhancing the Autophagic Flux. Front. Pharmacol. 2019, 10, 1238. [Google Scholar] [CrossRef]
- Wawszczyk, J.; Jesse, K.; Kapral, M. Pterostilbene-Mediated Inhibition of Cell Proliferation and Cell Death Induction in Amelanotic and Melanotic Melanoma. Int. J. Mol. Sci. 2023, 24, 1115. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef]
- Healy, E.; Dempsey, M.; Lally, C.; Ryan, M.P. Apoptosis and necrosis: Mechanisms of cell death induced by cyclosporine A in a renal proximal tubular cell line. Kidney Int. 1998, 54, 1955–1966. [Google Scholar] [CrossRef]
- Higuchi, Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem. Pharmacol. 2003, 66, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, R.; Araki, S.; Furukawa, M.; Furukawa, Y.; Ebara, S.; Shiokawa, D.; Hayashi, K.; Tanuma, S.; Kitamura, D. DNase γ Is the Effector Endonuclease for Internucleosomal DNA Fragmentation in Necrosis. PLoS ONE 2013, 8, e80223. [Google Scholar] [CrossRef]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M.E.; Xiao, D.; Weinstein, I.B. Resveratrol Induces Growth Inhibition, S-phase Arrest, Apoptosis, and Changes in Biomarker Expression in Several Human Cancer Cell Lines. Clin. Cancer Res. 2002, 8, 893–903. [Google Scholar] [PubMed]
- Chen, Y.; Tseng, S.H.; Lai, H.-S.; Chen, W.J. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery 2004, 136, 57–66. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L. The grape and wine constituent piceatannol inhibits proliferation of human bladder cancer cells via blocking cell cycle progression and inducing Fas/membrane bound Fas-ligand mediated apoptotic pathway. Mol. Nutr. Food Res. 2008, 52, 408–418. [Google Scholar] [CrossRef]
- Siedlecka-Kroplewska, K.; Jozwik, A.; Kaszubowska, L.; Kowalczyk, A.; Boguslawski, W. Pterostilbene induces cell cycle arrest and apoptosis in MOLT4 human leukemia cells. Folia Histochem. Cytobiol. 2012, 50, 574–580. [Google Scholar] [CrossRef]
- Chang, G.; Xiao, W.; Xu, Z.; Yu, D.; Li, B.; Zhang, Y.; Sun, X.; Xie, Y.; Chang, S.; Gao, L.; et al. Pterostilbene Induces Cell Apoptosis and Cell Cycle Arrest in T-Cell Leukemia/Lymphoma by Suppressing the ERK1/2 Pathway. BioMed Res. Int. 2017, 2017, 9872073. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, G.; Xu, Z.; Yang, G.; Li, B.; Wu, X.; Xiao, W.; Xie, B.; Hu, L.; Sun, X.; et al. Pterostilbene induces apoptosis and cell cycle arrest in diffuse large B-cell lymphoma cells. Sci. Rep. 2016, 6, 37417. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chang, Y.H.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food Chem. 2007, 55, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef]
- Lepak, A.; Gutmann, A.; Kulmer, S.T.; Nidetzky, B. Creating a water-soluble resveratrol-based antioxidant by site-selective enzymatic glucosylation. ChemBioChem 2015, 16, 1870–1874. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules 2020, 25, 1437. [Google Scholar] [CrossRef]
- Imtiyaz, K.; Shafi, M.; Fakhri, K.U.; Uroog, L.; Zeya, B.; Anwer, S.T.; Rizvi, S.T.A. Polydatin: A natural compound with multifaceted anticancer properties. J. Tradit. Complement. Med. 2024; in press, corrected proof. [Google Scholar] [CrossRef]
- Malterud, K.E.; Farbrot, T.L.; Huse, A.E.; Sund, R.B. Antioxidant and radical scavenging effects of anthraquinones and anthrones. Pharmacology 1993, 47, 77–85. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of Radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef]
- Singh, N.; Rajini, P.S. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Doyle, A.; Griffiths, J.B. (Eds.) Cell and Tissue Culture: Laboratory Procedures in Biotechnology; John Wiley & Sons: Chichester, UK, 1998; pp. 219–290. [Google Scholar]
- Shapiro, H.M. Practical Flow Cytometry, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 273–410. [Google Scholar]
- Marin, L.; Minguela, A.; Torio, A.; Moya-Quiles, M.R.; Muro, M.; Montes-Ares, O.; Parrado, A.; Álvarez-López, M.R.; Garcia-Alonso, A.M. Flow Cytometric Quantification of Apoptosis and Proliferation in Mixed Lymphocyte Culture. Cytom. Part A 2003, 51A, 107–118. [Google Scholar] [CrossRef]
- McCarthy, D.A. Cell Preparation. In Flow Cytometry: Principles and Applications; Macey, M.G., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 17–53. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meyer, N. Annexin V/7-AAD staining in keratinocytes. In Mammalian Cell Viability. Methods in Molecular Biology; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 740, pp. 57–63. [Google Scholar] [CrossRef]
- Nechita, A.; Cotea, V.; Nechita, C.B.; Pincu, R.; Mihai, C.T.; Colibaba, C.L. Study of cytostatic and cytototoxic activity of several polyphenolic extracts obtained from Vitis vinifera. Not. Bot. Horti Agrobot. 2012, 40, 216–221. [Google Scholar] [CrossRef]
- Otto, F. DAPI Staining of Fixed Cells for High-Resolution Flow Cytometry of Nuclear DNA. In Methods in Cell Biology; Darzynkiewicz, Z., Crissman, H., Eds.; Academic Press: Oxford, UK, 1990; Volume 33, pp. 105–110. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. CFSE dilution to study human T and NK cell proliferation in vitro. In Methods in Enzymology; Galluzzi, L., Rudqvist, N.-P., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 631, pp. 239–255. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, C.; Mihai, C.-T.; Vochita, G.; Gherghel, D.; Mangalagiu, I.I.; Gafton, M.; Miron, S.-D.; Iurciuc Tincu, C.-E.; Nahar, L.; Sarker, S.D.; et al. Stilbene Glycosides in Pinus cembra L. Bark: Isolation, Characterization, and Assessment of Antioxidant Potential and Antitumor Activity on HeLa Cells. Plants 2025, 14, 1459. https://doi.org/10.3390/plants14101459
Lungu C, Mihai C-T, Vochita G, Gherghel D, Mangalagiu II, Gafton M, Miron S-D, Iurciuc Tincu C-E, Nahar L, Sarker SD, et al. Stilbene Glycosides in Pinus cembra L. Bark: Isolation, Characterization, and Assessment of Antioxidant Potential and Antitumor Activity on HeLa Cells. Plants. 2025; 14(10):1459. https://doi.org/10.3390/plants14101459
Chicago/Turabian StyleLungu, Cristina, Cosmin-Teodor Mihai, Gabriela Vochita, Daniela Gherghel, Ionel I. Mangalagiu, Mihaela Gafton, Sorin-Dan Miron, Camelia-Elena Iurciuc Tincu, Lutfun Nahar, Satyajit D. Sarker, and et al. 2025. "Stilbene Glycosides in Pinus cembra L. Bark: Isolation, Characterization, and Assessment of Antioxidant Potential and Antitumor Activity on HeLa Cells" Plants 14, no. 10: 1459. https://doi.org/10.3390/plants14101459
APA StyleLungu, C., Mihai, C.-T., Vochita, G., Gherghel, D., Mangalagiu, I. I., Gafton, M., Miron, S.-D., Iurciuc Tincu, C.-E., Nahar, L., Sarker, S. D., & Miron, A. (2025). Stilbene Glycosides in Pinus cembra L. Bark: Isolation, Characterization, and Assessment of Antioxidant Potential and Antitumor Activity on HeLa Cells. Plants, 14(10), 1459. https://doi.org/10.3390/plants14101459





.png)


