Glucosinolates from Broccoli By-Products Obtained by Pressurized Liquid Extraction Exert Anti-Inflammatory Activity on Non-Malignant Colonic Myofibroblasts
Abstract
1. Introduction
2. Results
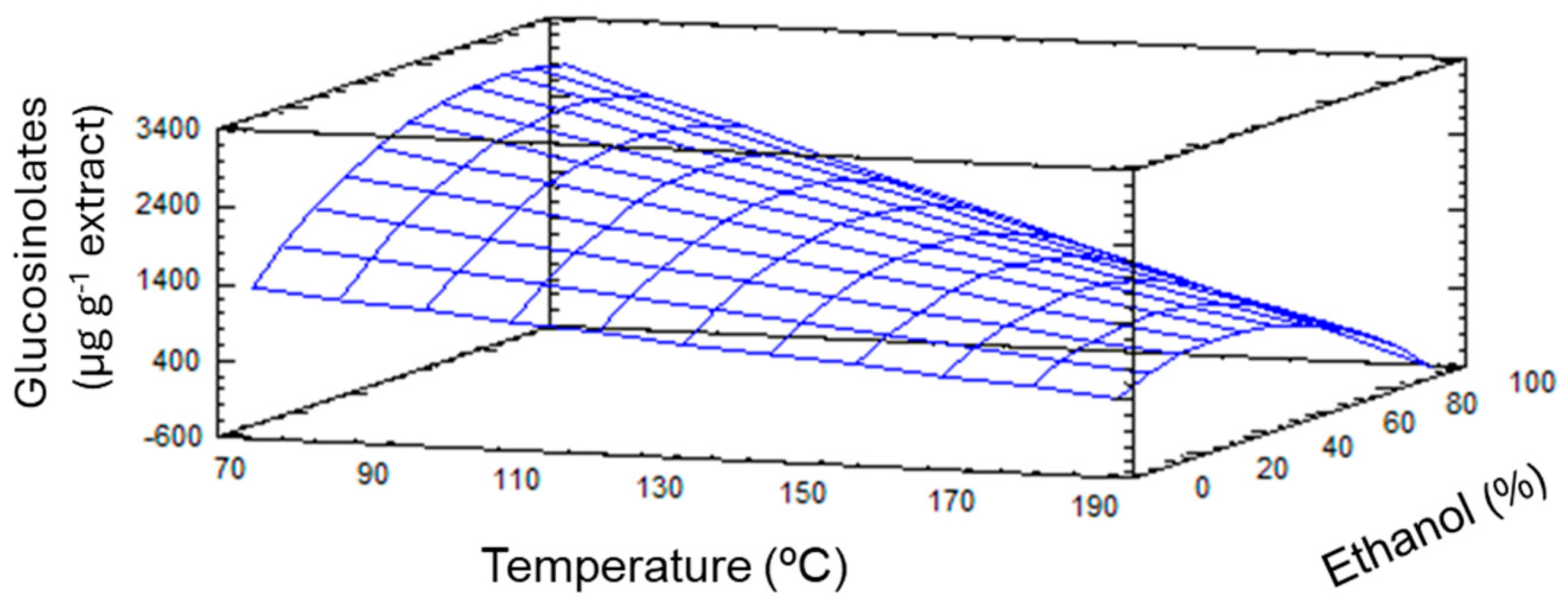
2.1. Optimization of the PLE Conditions to Obtain GSLs from Broccoli By-Products
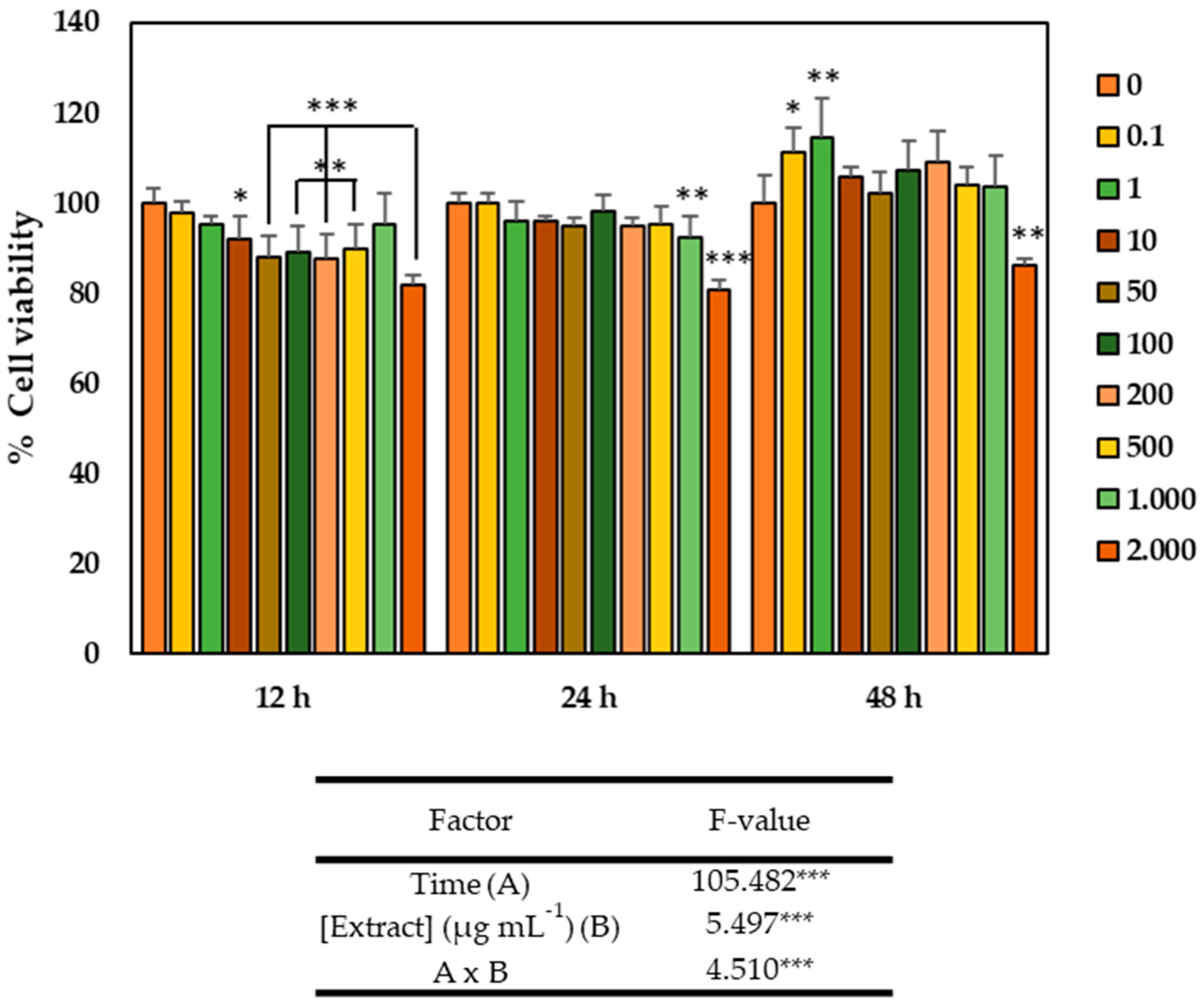
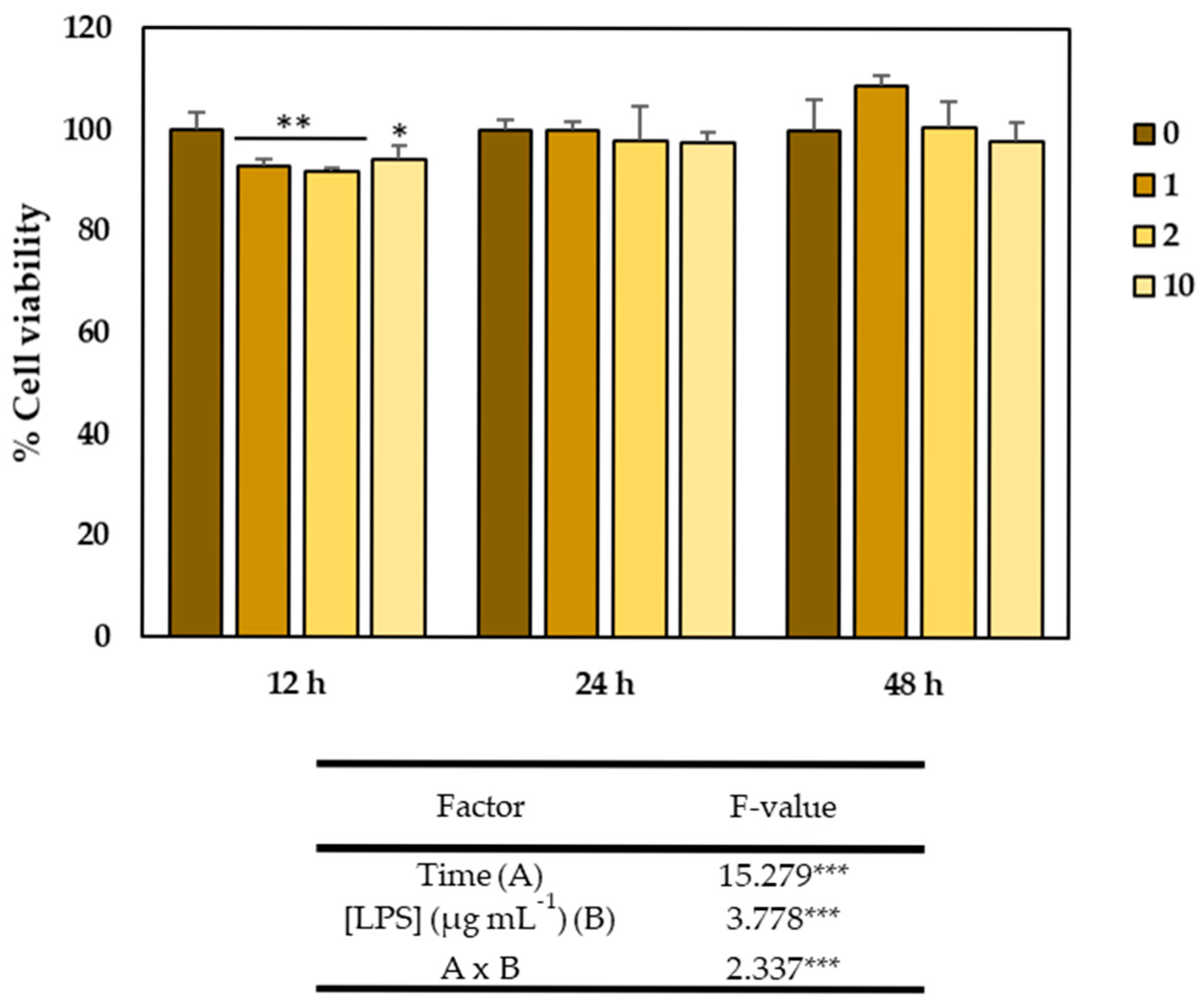
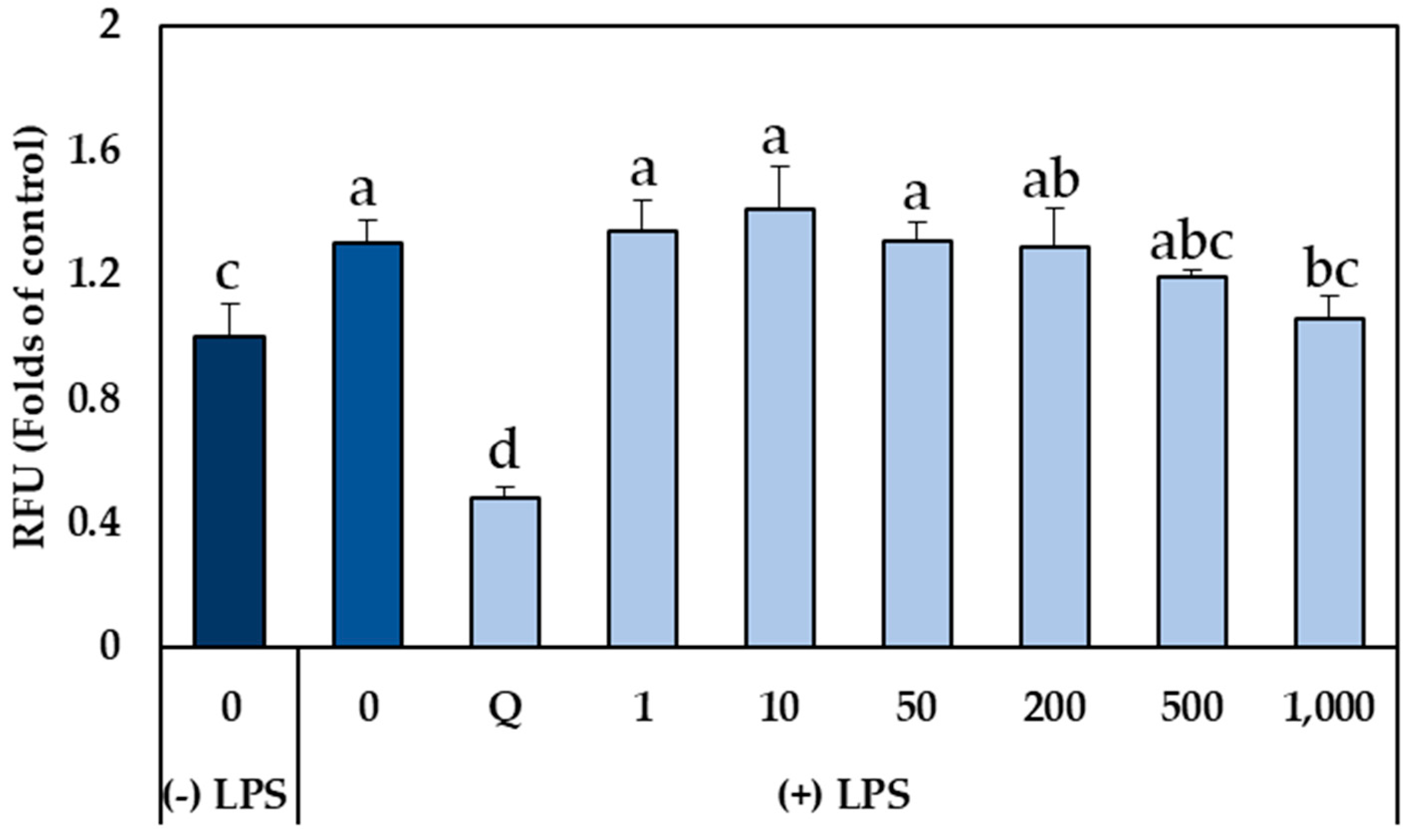
2.2. Cytotoxicity of the GSLs-Enriched Extract and LPS on the CCD-18Co Cell Line
2.3. Effect of GSLs-Enriched Extracts on Intracellular ROS Production in LPS-Stimulated CCD-18Co Myofibroblasts
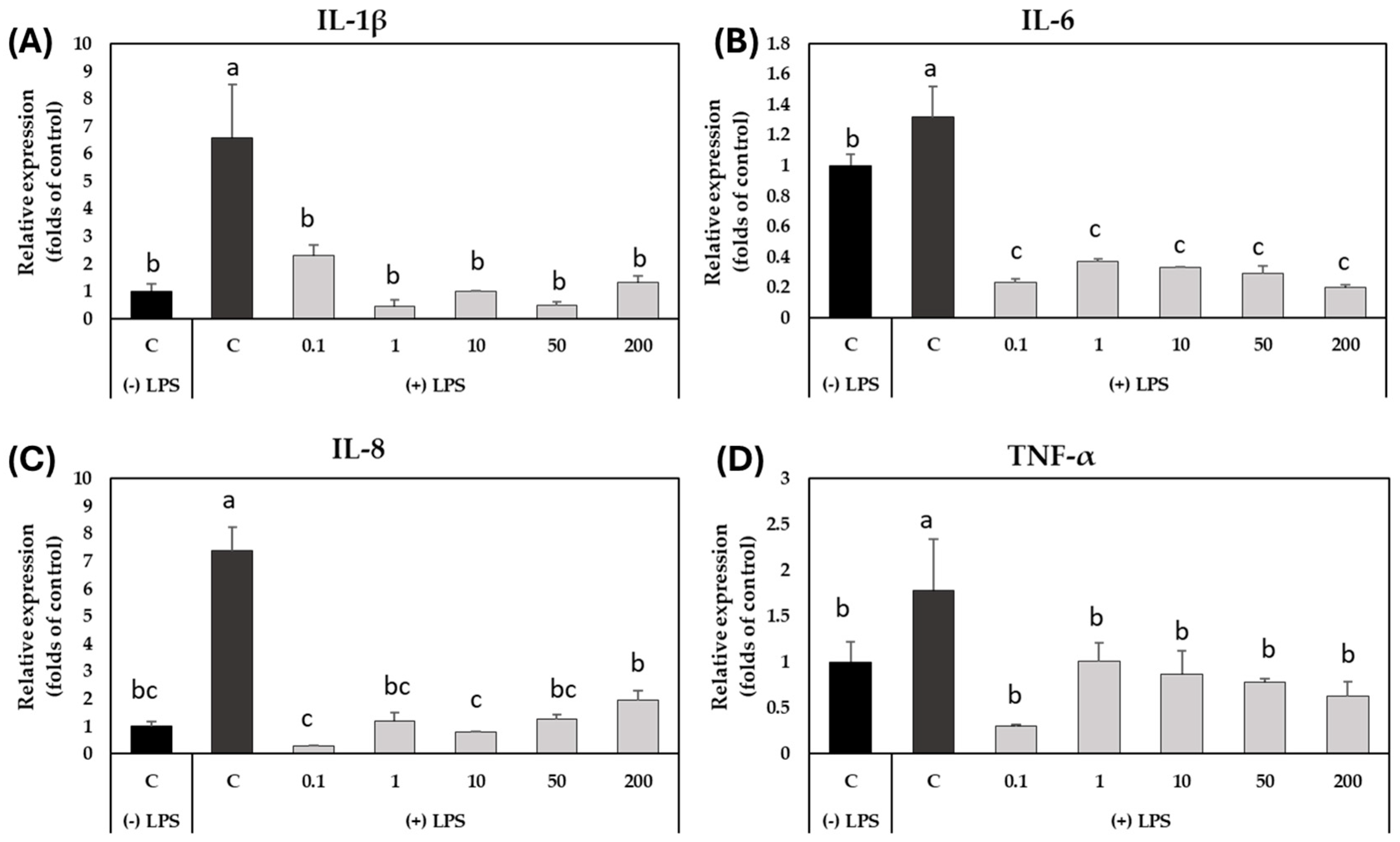
2.4. Effect of GSLs-Enriched Extract on the Expression of Inflammation-Related Genes in LPS-Stimulated CCD-18Co Colon Myofibroblasts
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. Extraction of GSLs Using Pressurized Liquids
4.4. Conventional Extraction of GSLs
4.5. Quantification of GSLs in PLE and CE Extracts
4.6. CCD-18Co Cell Culture and Anti-Inflammatory Assays
4.6.1. Cell Viability Assay
4.6.2. In Vitro Antioxidant Activities
4.6.3. Determination of Intracellular ROS Production
4.6.4. RNA Isolation and Quantitative Real-Time RT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Angel-Morales, G.; Noratto, G.; Mertens-Talcott, S. Red Wine Polyphenolics Reduce the Expression of Inflammation Markers in Human Colon-Derived CCD-18Co Myofibroblast Cells: Potential Role of MicroRNA-126. Food Funct. 2012, 3, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Natural Products as Dietary Agents for the Prevention and Mitigation of Oxidative Damage and Inflammation in the Intestinal Barrier. Antioxidants 2024, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Liu, P.; Zhong, J.; Hu, Y.; Fan, Y.; Zhuang, S.; Liu, Z. Kaempferol Inhibits Multiple Pathways Involved in the Secretion of Inflammatory Mediators from LPS-Induced Rat Intestinal Microvascular Endothelial Cells. Mol. Med. Rep. 2019, 19, 1958–1964. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Li, F.; Lei, X.; Ge, L.; Li, H.; Zhao, N.; Ming, J. The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review. Foods 2024, 13, 3507. [Google Scholar] [CrossRef]
- Ochar, K.; Iwar, K.; Nair, V.; Chung, Y.; Ha, B.; Kim, S. The Potential of Glucosinolates and Their Hydrolysis Products as Inhibitors of Cytokine Storms. Molecules 2024, 29, 4826. [Google Scholar] [CrossRef]
- Kojima, M.; Morisaki, T.; Izuhara, K.; Uchiyama, A.; Matsunari, Y.; Katano, M.; Tanaka, M. Lipopolysaccharide Increases Cyclo-Oxygenase-2 Expression in a Colon Carcinoma Cell Line through Nuclear Factor-ΚB Activation. Oncogene 2000, 19, 1225–1231. [Google Scholar] [CrossRef]
- Mayeux, P.R. Pathobiology of Lipopolysaccharide. J. Toxicol. Environ. Health 1997, 51, 415–435. [Google Scholar] [CrossRef]
- Dos Santos Dias, M.M.; Martino, H.S.D.; Noratto, G.; Roque-Andrade, A.; Stringheta, P.C.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Anti-Inflammatory Activity of Polyphenolics from Açai (Euterpe Oleracea Martius) in Intestinal Myofibroblasts CCD-18Co Cells. Food Funct. 2015, 6, 3249–3256. [Google Scholar] [CrossRef]
- Wu, X.-X.; Huang, X.-L.; Chen, R.-R.; Li, T.; Ye, H.-J.; Xie, W.; Huang, Z.-M.; Cao, G.-Z. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 2019, 42, 2215–2225. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; de Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Mol. Nutr. Food Res. 2009, 53, S219–S265. [Google Scholar] [CrossRef] [PubMed]
- Gudiño, I.; Casquete, R.; Martín, A.; Wu, Y.; Benito, M. Comprehensive Analysis of Bioactive Compounds, Functional Properties, and Applications of Broccoli by-Products. Foods 2024, 13, 3918. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Zhuang, W.; Wu, C.; Rao, Z.; Du, L.; Zhou, Y. Cruciferous Vegetable and Isothiocyanate Intake and Multiple Health Outcomes. Food Chem. 2022, 375, 131816. [Google Scholar] [CrossRef] [PubMed]
- Syed, R.U.; Moni, S.S.; Khaled Bin Break, M.; Khojali, W.M.A.; Jafar, M.; Alshammari, M.D.; Abdelsalam, K.; Taymour, S.; Saad, K.; Alreshidi, M.; et al. Broccoli: A Multi-Faceted Vegetable for Health: An in-Depth Review of Its Nutritional Attributes, Antimicrobial Abilities, and Anti-Inflammatory Properties. Antibiotics 2023, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Xu, Z.; Sun, Q.; Iniguez, A.; Du, M.; Zhu, M. Broccoli-Derived Glucoraphanin Activates AMPK/PGC1α/NRF2 Pathway and Ameliorates Dextran-Sulphate-Sodium-Induced Colitis in Mice. Antioxidants 2022, 11, 2404. [Google Scholar] [CrossRef]
- Gupta, P.; Ray, J.; Aggarwal, B.K.; Goyal, P. Food Processing Residue Analysis and its Functional Components as Related to Human Health: Recent Developments. Austin J. Nutr. Food Sci. 2015, 3, 01–07. [Google Scholar]
- Monllor, P.; Romero, G.; Muelas, R.; Sandoval-Castro, C.A.; Sendra, E.; Ramón Díaz, J. Ensiling Process in Commercial Bales of Horticultural By-Products from Artichoke and Broccoli. Animals 2020, 10, 831. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food Waste Generation And Industrial Uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives From Fruit Processing Wastes: Green Approaches To Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Borja-Martínez, M.; Lozano-Sánchez, J.; Borrás-Linares, I.; Pedreño, M.A.; Sabater-Jara, A.B. Revalorization of Broccoli By-Products for Cosmetic Uses Using Supercritical Fluid Extraction. Antioxidants 2020, 9, 1195. [Google Scholar] [CrossRef]
- Gil, K.A.; Tuberoso, C.I.G. Crucial Challenges in the Development of Green Extraction Technologies to Obtain Antioxidant Bioactive Compounds from Agro-Industrial by-Products. Chem. Biochem. Eng. Q. 2021, 35, 105–138. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Domínguez, H. Challenges in the Extraction of Antiinflammatory and Antioxidant Compounds from New Plant Sources. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Hernández-Ledesma, B., Martínez-Villaluenga, C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 427–446. ISBN 9780128234822. [Google Scholar]
- Ares, A.M.; Bernal, J.; Nozal, M.J.; Turner, C.; Plaza, M. Fast Determination of Intact Glucosinolates in Broccoli Leaf by Pressurized Liquid Extraction and Ultra High Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry. Food Res. Int. 2015, 76, 498–505. [Google Scholar] [CrossRef] [PubMed]
- del Pilar Sánchez-Camargo, A.; Ibáñez, E.; Cifuentes, A.; Herrero, M. Bioactives Obtained from Plants, Seaweeds, Microalgae and Food by-Products Using Pressurized Liquid Extraction and Supercritical Fluid Extraction. Compr. Anal. Chem. 2017, 76, 27–51. [Google Scholar] [CrossRef]
- Pagliari, S.; Domínguez-Rodríguez, G.; Cifuentes, A.; Ibáñez, E.; Labra, M.; Campone, L. Pressurized Liquid Extraction of Glucosinolates from Camelina sativa (L.) Crantz by-Products: Process Optimization and Biological Activities of Green Extract. Food Chem. X 2024, 22, 101324. [Google Scholar] [CrossRef]
- Bojorquez-Rodríguez, E.M.; Guajardo-Flores, D.; Jacobo-Velázquezl, D.A.; Serna-Saldívar, S.O. Evaluation of the Effects of Process Conditions on the Extraction of Glucosinolates from Broccoli Sprouts. Horticulturae 2022, 8, 1090. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of Extraction of Phenolic Compounds from Wheat Using Response Surface Methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Campos, D.; Chirinos, R.; Barreto, O.; Noratto, G.; Pedreschi, R. Optimized Methodology for the Simultaneous Extraction of Glucosinolates, Phenolic Compounds and Antioxidant Capacity from Maca (Lepidium meyenii). Ind. Crops Prod. 2013, 49, 747–754. [Google Scholar] [CrossRef]
- Geronikaki, A.A.; Gavalas, A.M. Antioxidants and Inflammatory Disease: Synthetic and Natural Antioxidants with Anti-Inflammatory Activity. Comb. Chem. High. Throughput Screen. 2006, 9, 425–442. [Google Scholar] [CrossRef]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced Raw 264.7 Macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef]
- Samuel, L.M.; As, F.; Khiong, K. Effect of Steamed Broccoli Juice (Brassica oleraceae L. Var. Italica) to the Serum Interleukin 8 Level in Colitis Murine Model. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2015, 16, 148–152. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Analysis of the Tumoral Cytotoxicity of Green Tea-Infusions Enriched with Broccoli. Food Chem. 2012, 132, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jeffery, E.H.; Miller, M.J.; Wallig, M.A.; Wu, Y. Lightly Cooked Broccoli Is as Effective as Raw Broccoli in Mitigating Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2018, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Q. Sulforaphane Protects Intestinal Epithelial Cells Against Lipopolysaccharide-Induced Injury By Activating The AMPK/SIRT1/PGC-1ɑ Pathway. Bioengineered 2021, 12, 4349–4360. [Google Scholar] [CrossRef]
- Peng, C.; Wu, C.; Xu, X.; Pan, L.; Lou, Z.; Zhao, Y.; Jiang, H.; He, Z.; Ruan, B. Indole-3-Carbinol Ameliorates Necroptosis and Inflammation of Intestinal Epithelial Cells in Mice with Ulcerative Colitis by Activating Aryl Hydrocarbon Receptor. Exp. Cell Res. 2021, 404, 112638. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological Effects of Broccoli Consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic Modification of Nrf2 by Sulforaphane Increases the Antioxidative and Anti-Inflammatory Capacity in a Cellular Model of Alzheimer’s Disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef]
- Bessler, H.; Djaldetti, M. Broccoli and Human Health: Immunomodulatory Effect of Sulforaphane in a Model of Colon Cancer. Int. J. Food Sci. Nutr. 2018, 69, 946–953. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane Inhibits Extracellular, Intracellular, and Antibiotic-Resistant Strains of Helicobacter pylori and Prevents Benzo[a]Pyrene-Induced Stomach Tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Bertl, E.; Bartsch, H.; Gerhäuser, C. Inhibition of Angiogenesis and Endothelial Cell Functions Are Novel Sulforaphane-Mediated Mechanisms in Chemoprevention. Mol. Cancer Ther. 2006, 5, 575–585. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Yan, L.; Shui, S.; Wang, L.; Zheng, W.; Liu, S.; Liu, C.; Zheng, L. Sulforaphane Attenuates 5-Fluorouracil Induced Intestinal Injury in Mice. J. Funct. Foods 2020, 69, 103965. [Google Scholar] [CrossRef]
- García-Ibañez, P.; Ben-Romdhane, O.; Moreno, D.A. Glucosinolates and Their Bioactive Metabolites as Functional Compounds Modulating Inflammation. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Hernández-Ledesma, B., Martínez-Villaluenga, C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 189–204. ISBN 9780128234822. [Google Scholar]
- Sánchez-Pujante, P.J.; Gionfriddo, M.; Sabater-Jara, A.B.; Almagro, L.; Pedreño, M.A.; Diaz-Vivancos, P. Enhanced Bioactive Compound Production in Broccoli Cells Due to Coronatine and Methyl Jasmonate Is Linked to Antioxidative Metabolism. J. Plant Physiol. 2020, 248, 153136. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Hernandez, V.; Fenoll, J.; Hellin, P. Pre-harvest application of ozonated water on broccoli crops: Effect on head quality. J. Food Compos. Anal. 2019, 83, 103260. [Google Scholar] [CrossRef]
- Tian, Q.; Rosselot, R.A.; Schwartz, S.J. Quantitative Determination Of Intact Glucosinolates In Broccoli, Broccoli Sprouts, Brussels Sprouts, And Cauliflower By High-Performance Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry. Anal. Biochem. 2005, 343, 93–99. [Google Scholar] [CrossRef]
- Escribano, J. Characterization of the Antiradical Activity of Betalains from Beta vulgaris L. Roots. Phytochem. Anal. 1998, 9, 124–127. [Google Scholar] [CrossRef]
- Borja-Martínez, M.; Pedreño, M.; Sabater-Jara, A. Broccoli Byproduct Extracts Attenuate the Expression of UVB-Induced Proinflammatory Cytokines in HaCaT Keratinocytes. Antioxidants 2024, 13, 1479. [Google Scholar] [CrossRef]
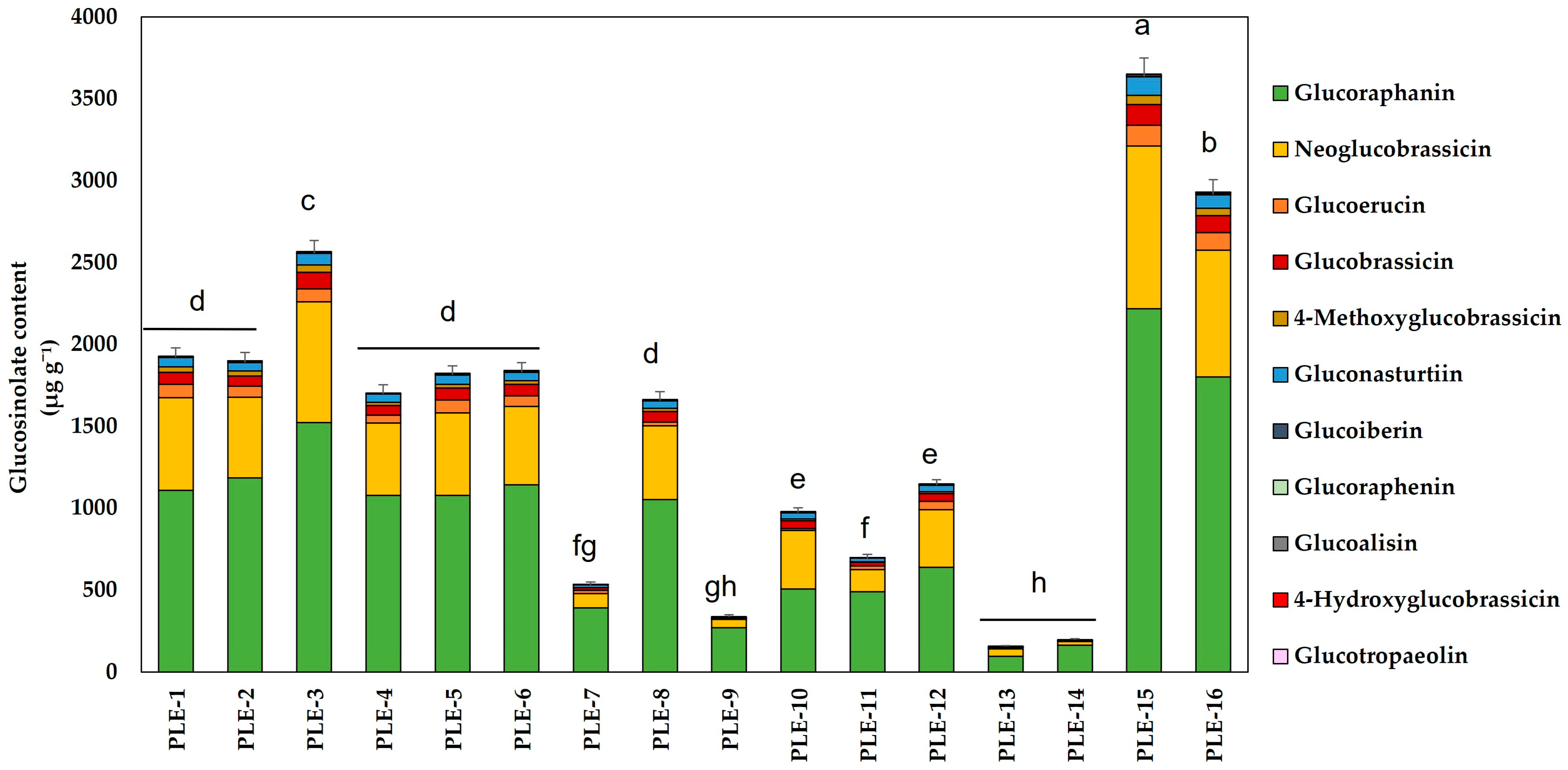
| Condition | Temperature (°C) | Ethanol (%) | Time (min) | Response Variable * (µg GSLs g−1 Extract) | EY (%) |
|---|---|---|---|---|---|
| PLE-1 | 70 | 15 | 20 | 1926 ± 59 | 37 |
| PLE-2 | 128 | 50 | 22 | 1896 ± 62 | 38 |
| PLE-3 | 70 | 85 | 5 | 2565 ± 81 | 27 |
| PLE-4 | 128 | 50 | 12.5 | 1701 ± 56 | 37 |
| PLE-5 | 70 | 15 | 5 | 1820 ± 57 | 38 |
| PLE-6 | 128 | 50 | 12.5 | 1839 ± 59 | 36 |
| PLE-7 | 185 | 15 | 5 | 531 ± 20 | 56 |
| PLE-8 | 128 | 50 | 3 | 1661 ± 55 | 36 |
| PLE-9 | 200 | 50 | 12.5 | 335 ± 14 | 40 |
| PLE-10 | 128 | 95 | 12.5 | 977 ± 29 | 22 |
| PLE-11 | 185 | 15 | 20 | 696 ± 25 | 52 |
| PLE-12 | 128 | 5 | 12.5 | 1145 ± 35 | 45 |
| PLE-13 | 185 | 85 | 5 | 155 ± 5 | 38 |
| PLE-14 | 185 | 85 | 20 | 194 ± 8 | 41 |
| PLE-15 | 70 | 85 | 20 | 3649 ± 116 | 27 |
| PLE-16 | 53 | 50 | 12.5 | 2930 ± 94 | 32 |
| Source | Y = Glucosinolate Content | ||||
|---|---|---|---|---|---|
| SS | Df | MS | F-Ratio | p-Value | |
| X1: Temperature | 1.21 × 107 | 1 | 1.21 × 107 | 131.88 | 0.0000 * |
| X2: Ethanol | 166,424 | 1 | 166,424 | 1.81 | 0.2209 |
| X1 X2 | 1.40 × 106 | 1 | 1.40 × 106 | 15.19 | 0.0059 * |
| X2 X2 | 680,824 | 1 | 680,824 | 7.39 | 0.0298 * |
| Lack-of-fit | 234,659 | 4 | 58,664.7 | 0.64 | 0.6527 |
| Pure error | 644,881 | 7 | 92,125.8 | ||
| Total (corr.) | 1.53 × 107 | 15 | |||
| R2 | 0.942 | ||||
| Condition | Theoretical | Experimental | CV (%) |
|---|---|---|---|
| PLE-1 | 1895 | 1926 ± 59 | 0.6 |
| PLE-2 | 1741 | 1896 ± 62 | 3.0 |
| PLE-3 | 2974 | 2565 ± 81 | 5.2 |
| PLE-4 | 1741 | 1701 ± 56 | 0.8 |
| PLE-5 | 1895 | 1820 ± 57 | 1.4 |
| PLE-6 | 1741 | 1839 ± 59 | 1.9 |
| PLE-7 | 659 | 531 ± 20 | 7.6 |
| PLE-8 | 1741 | 1661 ± 55 | 1.7 |
| PLE-9 | 444 | 335 ± 14 | 9.8 |
| PLE-10 | 1310 | 977 ± 29 | 10.3 |
| PLE-11 | 659 | 696 ± 25 | 1.9 |
| PLE-12 | 1008 | 1145 ± 35 | 4.5 |
| PLE-13 | 65 | 155 ± 5 | 28.9 |
| PLE-14 | 65 | 194 ± 8 | 35.3 |
| PLE-15 | 2974 | 3649 ± 116 | 7.2 |
| PLE-16 | 3093 | 2930 ± 94 | 1.9 |
| Optimal conditions | 3405 | 4530 ± 132 | 10 |
| Glucosinolates | PLE-OP (μg g−1 Extract) | CE (μg g−1 Extract) | |
|---|---|---|---|
| Alifatic | Glucoiberin | 6.9 ± 0.4 | 8 ± 1 |
| Glucoraphanin | 2144 ± 101 | 4888 ± 961 | |
| Gluconapin | 0.6 ± 0.04 | n.d. | |
| Glucoalisin | 14 ± 1 | 27 ± 5 | |
| Glucoerucin | 380 ± 10 | 919 ± 177 | |
| Glucoraphenin | n.d. | 4 ± 2 | |
| Indolic | Glucobrassicin | 216 ± 12 | 471 ± 88 |
| Neoglucobrassicin | 1497 ± 141 | 2116 ± 370 | |
| 4-Hydroxyglucobrassicin | 22 ± 1 | 82 ± 17 | |
| 4-Metoxyglucobrasicin | 130 ± 11 | 258 ± 39 | |
| Aromatic | Glucotropaeolin | n.d. | 8 ± 1 |
| Gluconasturtiin | 120 ± 12 | 234 ± 50 | |
| TOTAL | 4530 ± 132 | 9015 ± 449 | |
| EY (%) | 21 | 31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borja-Martínez, M.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Almagro, L.; Pedreño, M.A.; Sabater-Jara, A.B. Glucosinolates from Broccoli By-Products Obtained by Pressurized Liquid Extraction Exert Anti-Inflammatory Activity on Non-Malignant Colonic Myofibroblasts. Plants 2025, 14, 1700. https://doi.org/10.3390/plants14111700
Borja-Martínez M, Lozano-Sánchez J, Quirantes-Piné R, Almagro L, Pedreño MA, Sabater-Jara AB. Glucosinolates from Broccoli By-Products Obtained by Pressurized Liquid Extraction Exert Anti-Inflammatory Activity on Non-Malignant Colonic Myofibroblasts. Plants. 2025; 14(11):1700. https://doi.org/10.3390/plants14111700
Chicago/Turabian StyleBorja-Martínez, María, Jesús Lozano-Sánchez, Rosa Quirantes-Piné, Lorena Almagro, María A Pedreño, and Ana B. Sabater-Jara. 2025. "Glucosinolates from Broccoli By-Products Obtained by Pressurized Liquid Extraction Exert Anti-Inflammatory Activity on Non-Malignant Colonic Myofibroblasts" Plants 14, no. 11: 1700. https://doi.org/10.3390/plants14111700
APA StyleBorja-Martínez, M., Lozano-Sánchez, J., Quirantes-Piné, R., Almagro, L., Pedreño, M. A., & Sabater-Jara, A. B. (2025). Glucosinolates from Broccoli By-Products Obtained by Pressurized Liquid Extraction Exert Anti-Inflammatory Activity on Non-Malignant Colonic Myofibroblasts. Plants, 14(11), 1700. https://doi.org/10.3390/plants14111700







