Populus × euramericana Accumulates More Organic Pollutants (PAHs and PCBs), While P. nigra ‘Italica’ Absorbs More Heavy Metals
Abstract
1. Introduction
2. Results and Discussion
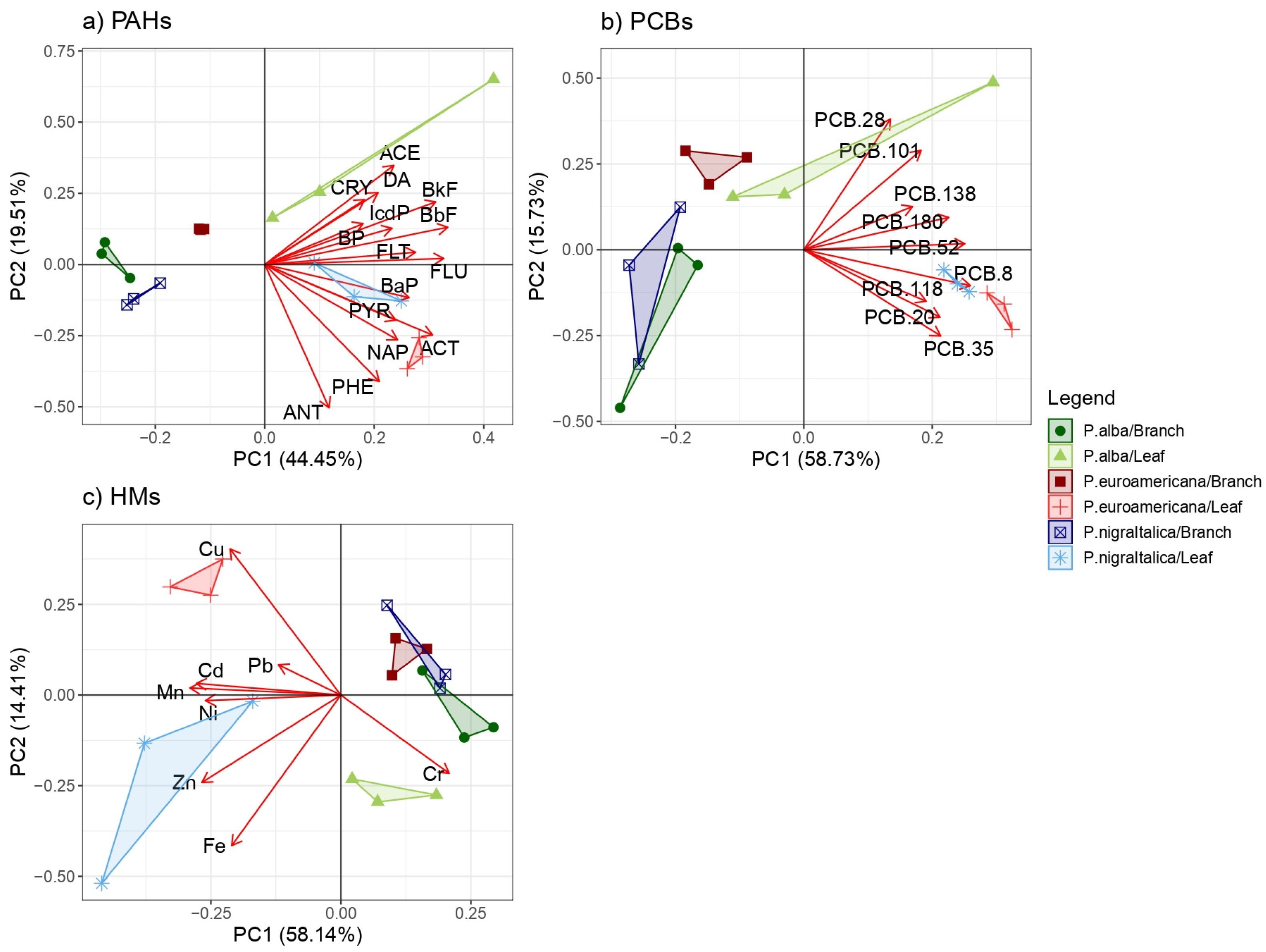
2.1. Polycyclic Aromatic Hydrocarbon (PAH) Phytoaccumulation
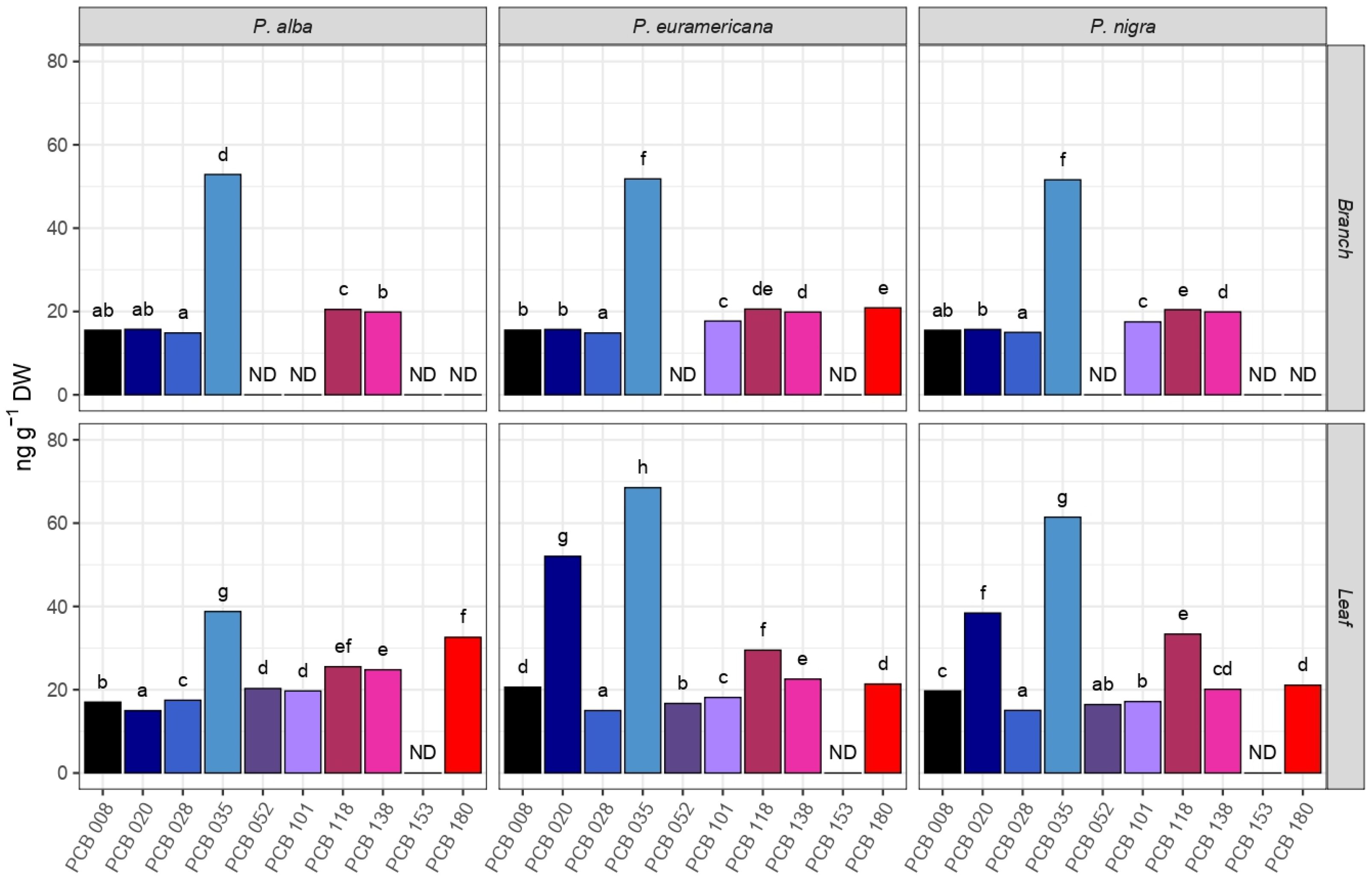
2.2. PCB Phytoaccumulation
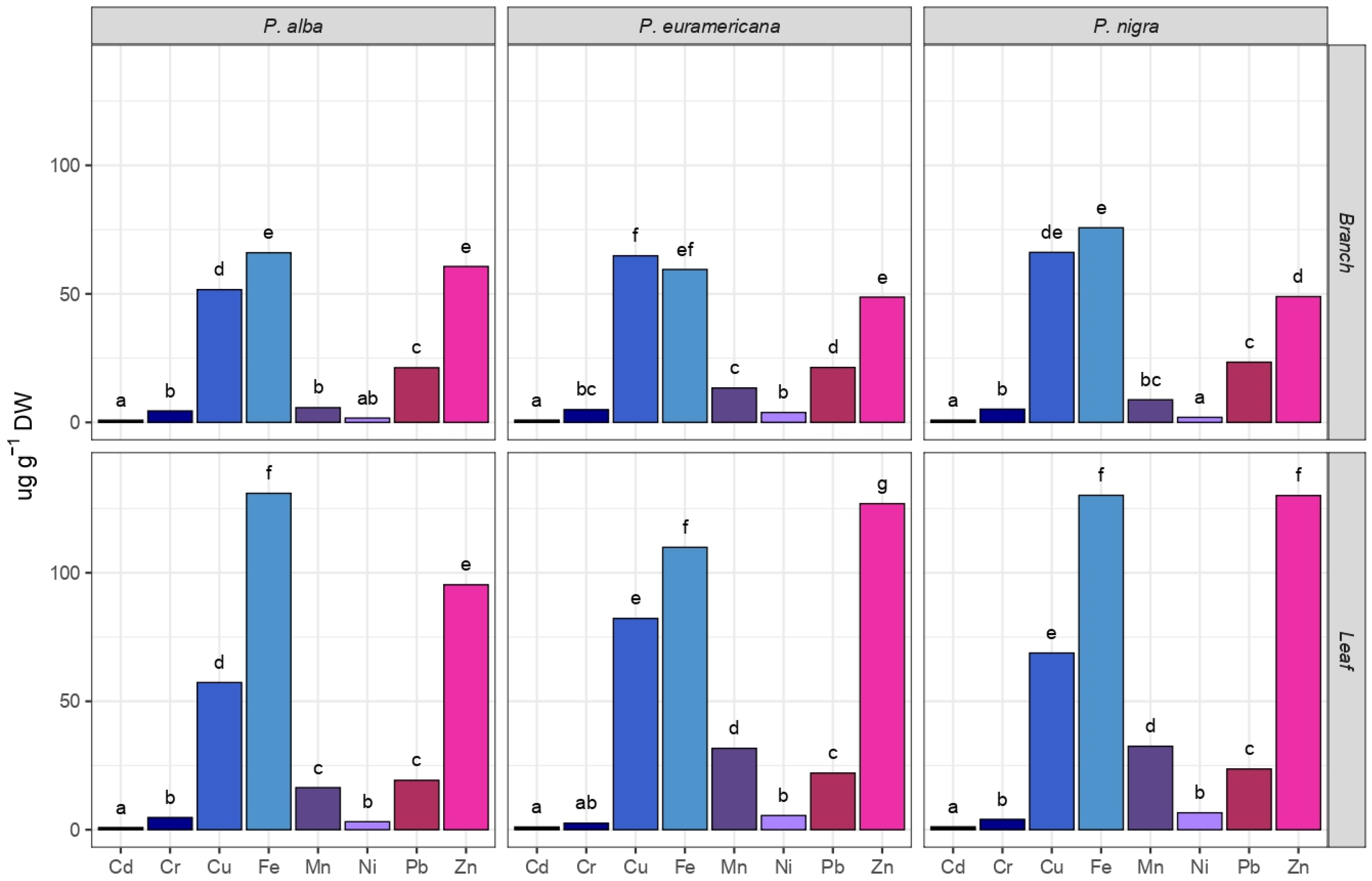
2.3. Heavy Metal Phytoaccumulation
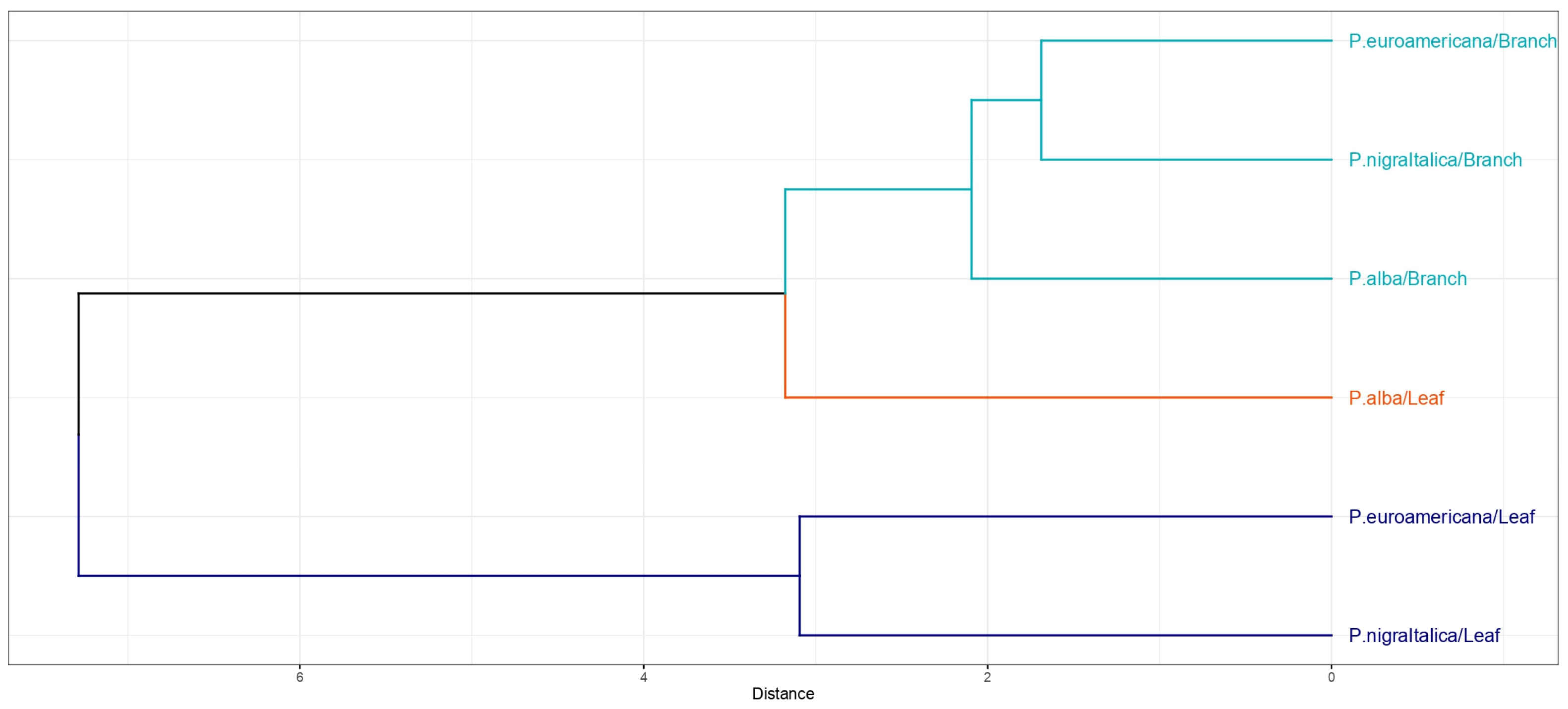
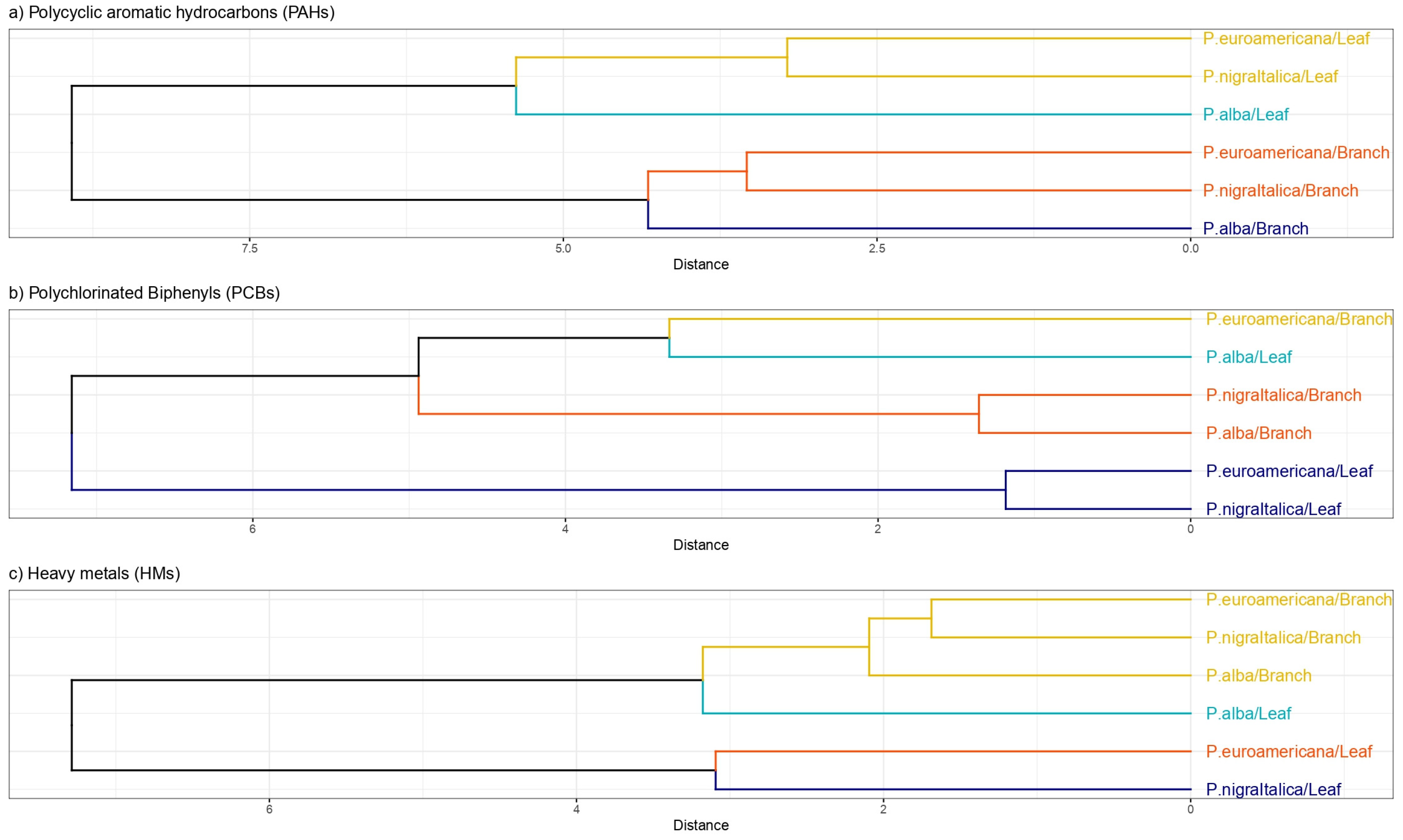
2.4. Species and Organ Capacity of PAH, PCB, and HM Uptake
2.5. Poplars as a PAH, PCB, and HM Remediation Tool in an Urban Environment
3. Materials and Methods
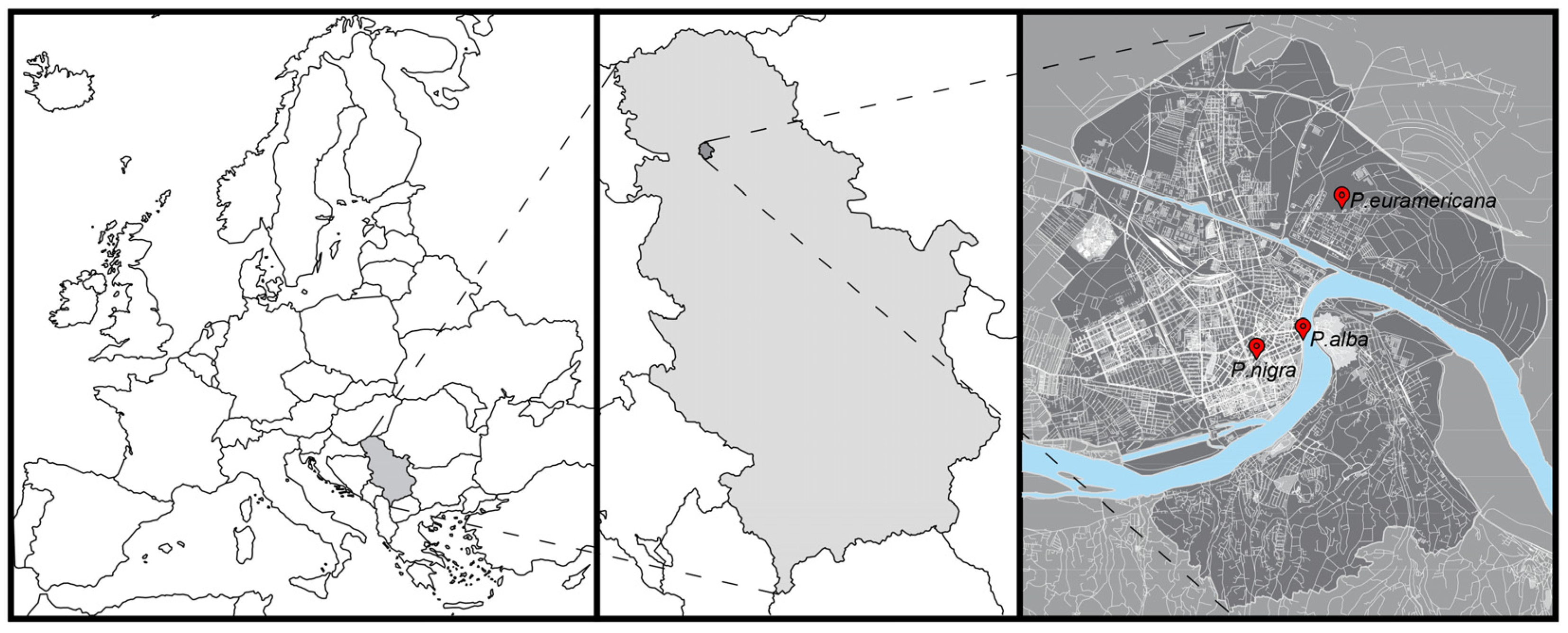
3.1. Study Area and Site Descriptions
3.2. Data Collection and Preparation
3.3. Standards and Reagents
3.4. Instrumental Analysis
3.4.1. PAH and PCB Quantification: Extraction and Clean-Up Procedures and GC/MS Analysis
3.4.2. Heavy Metal: Digestion Procedure and AAS Measurements
3.5. Statistical Analyzes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Melnyk, A.; Dettlaff, A.; Kuklińska, K.; Namieśnik, J.; Wolska, L. Concentration and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyls (PCBs) in Surface Soil near a Municipal Solid Waste (MSW) Landfill. Sci. Total Environ. 2015, 530–531, 18–27. [Google Scholar] [CrossRef] [PubMed]
- EEA (European Environmental Agency). Air Quality in Europe—2019 Report; No. 10/2019; Publications Office of the European Union: Copenhagen, Denmark, 2019.
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef]
- Tőzsér, D.; Horváth, R.; Simon, E.; Magura, T. Heavy Metal Uptake by Plant Parts of Populus Species: A Meta-Analysis. Environ. Sci. Pollut. Res. 2023, 30, 69416–69430. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Reynolds, C.M. Phytodegradation of Organic Compounds. Curr. Opin. Biotechnol. 2004, 15, 225–230. [Google Scholar] [CrossRef]
- McCutcheon, S.C.; Susarla, S.; Medina, V.F. Phytoremediation: An Ecological Solution to Organic Chemical Contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar]
- Wijekoon, W.; Priyashantha, H.; Gajanayake, P.; Manage, P.; Liyanage, C.; Jayarathna, S.; Kumarasinghe, U. Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation. Sustainability 2025, 17, 822. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mitra, A.; Datta, S.; Veer, V. Phytoremediation Protocols: An Overview. In Plant-Based Remediation Processes; Gupta, D., Ed.; Soil Biology Book Series; Springer: Berlin/Heidelberg, Germany, 2013; Volume 35, pp. 1–18. [Google Scholar] [CrossRef]
- Jakovljević, T.; Bubalo, M.C.; Orlović, S.; Sedak, M.; Bilandžić, N.; Brozinčević, I.; Redovniković, I.R. Adaptive Response of Poplar (Populus nigra L.) after Prolonged Cd Exposure Period. Environ. Sci. Pollut. Res. 2014, 21, 3792–3802. [Google Scholar] [CrossRef]
- Ancona, V.; Barra Caracciolo, A.; Grenni, P.; Di Lenola, M.; Campanale, C.; Calabrese, A.; Uricchio, V.F.; Mascolo, G.; Massacci, A. Plant-Assisted Bioremediation of a Historically PCB and Heavy Metal-Contaminated Area in Southern Italy. N. Biotechnol. 2017, 38, 65–73. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Vuksanović, V.; Gavranović Markić, A.; Kiprovski, B.; Zorić, M.; Orlović, S. Metal- and Organ-Specific Response to Heavy Metal-Induced Stress Mediated by Antioxidant Enzymes’ Activities, Polyamines, and Plant Hormones Levels in Populus Deltoides. Plants 2022, 11, 3246. [Google Scholar] [CrossRef]
- Kesić, L.; Kovačević, B.; Milović, M.; Poljaković-Pajnik, L.; Pekeč, S.; Višacki, V.; Orlović, S. Physiological Responses of Poplar and Willow Clones Grown in Pot Trials on Soil from Landfills. Topola 2024, 82, 55–63. [Google Scholar] [CrossRef]
- Singh, O.V.; Jain, R.K. Phytoremediation of Toxic Aromatic Pollutants from Soil. Appl. Microbiol. Biotechnol. 2003, 63, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Pajevic, S.; Borisev, M.; Nikolic, N.; Krstic, B.; Pilipovic, A.; Orlovic, S. Phytoremediation Capacity of Poplar (Populus Spp.) and Willow (Salix Spp.) Clonesin Relation to Photosynthesis. Arch. Biol. Sci. 2009, 61, 239–247. [Google Scholar] [CrossRef]
- Trudić, B.; Kebert, M.; Popović, M.B.; Štajner, D.; Orlović, S.; Galović, V.; Pilipović, A. The Effect of Heavy Metal Pollution in Soil on Serbian Poplar Clones. Sumar. List 2013, 137, 287–296. [Google Scholar]
- Chroma, L.; Mackova, M.; Kucerova, P.; In Der Wiesche, C.; Burkhard, J.; Macek, T. Enzymes in Plant Metabolism of PCBs and PAHs. Acta Biotechnol. 2002, 22, 35–41. [Google Scholar] [CrossRef]
- Pilipović, A.; Orlović, S.; Kovačević, B.; Poljaković-Pajnik, L.; Popović, J.; Raković, D.; Jovanović, A. Biomass Production of Euramerican Poplars (Populus x Euramericana (Dode) Guinier) in Short Rotation Plantations with Different Spacing. Topola 2023, 952, 33–41. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef]
- Hazrat, A.; Ezzat, K.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Manoli, E.; Kouras, A.; Karagkiozidou, O.; Argyropoulos, G.; Voutsa, D.; Samara, C. Polycyclic Aromatic Hydrocarbons (PAHs) at Traffic and Urban Background Sites of Northern Greece: Source Apportionment of Ambient PAH Levels and PAH-Induced Lung Cancer Risk. Environ. Sci. Pollut. Res. 2016, 23, 3556–3568. [Google Scholar] [CrossRef]
- Alexandrino, K.; Sánchez, N.E.; Viteri, F. Levels and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) near Hospitals and Schools Using Leaves and Barks of Sambucus Nigra and Acacia Melanoxylon. Environ. Geochem. Health 2024, 46, 32. [Google Scholar] [CrossRef]
- Niu, X.; Yu, J.; Zhang, N.; Sun, J.; Ho, K.F.; Shen, Z.; Huang, Y.; Cao, J. Human Pulmonary Cytotoxicity of Vehicular Derived PM2.5: A Study from Three Characterized Tunnels. Atmos. Environ. 2024, 326, 120481. [Google Scholar] [CrossRef]
- Zhu, R.; Wei, Y.; He, L.; Wang, M.; Hu, J.; Li, Z.; Lai, Y.; Su, S. Particulate Matter Emissions from Light-Duty Gasoline Vehicles under Different Ambient Temperatures: Physical Properties and Chemical Compositions. Sci. Total Environ. 2024, 926, 171791. [Google Scholar] [CrossRef]
- Li, F.; Gu, J.; Xin, J.; Schnelle-Kreis, J.; Wang, Y.; Liu, Z.; Shen, R.; Michalke, B.; Abbaszade, G.; Zimmermann, R. Characteristics of Chemical Profile, Sources and PAH Toxicity of PM2.5 in Beijing in Autumn-Winter Transit Season with Regard to Domestic Heating, Pollution Control Measures and Meteorology. Chemosphere 2021, 276, 130143. [Google Scholar] [CrossRef]
- Jakovljević, I.; Dvoršćak, M.; Jagić, K.; Klinčić, D. Polycyclic aromatic hydrocarbons—Levels, pollution sources, and risk assessment. Arh. Za Hig. Rada I Toksikol. Zagreb 2023, 74, A47. [Google Scholar]
- Bi, S.; Cao, H.; Zhang, B.; Dong, H.; Gao, Y.; Zhou, X.; Jiang, Y.; Jiang, W. PM2.5-Bound PAHs near a Typical Industrial Park: Determining Health Risks Associated with Specific Industrial Sources. Atmos. Environ. 2023, 302, 119715. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.; Nassani, A.A.; Haffar, M.; Khan, H.U.R.; Zaman, K. Access to Sustainable Healthcare Infrastructure: A Review of Industrial Emissions, Coal Fires, and Particulate Matter. Environ. Sci. Pollut. Res. 2023, 30, 69080–69095. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z. Investigating Industrial PAH Air Pollution in Relation to Population Exposure in Major Countries: A Scoring Approach. J. Environ. Manag. 2023, 338, 117801. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAH); EPA/600/R-93/089 (NTIS PB94116571); USEPA (United States Environmental Protection Agency): Washington, DC, USA, 1993.
- Purcaro, G.; Moret, S.; Conte, L.S. Rapid SPE-HPLC Determination of the 16European Priority Polycyclic Aromatic Hydrocarbons in Olive Oils. J. Sep. Sci. 2008, 31, 3936–3944. [Google Scholar] [CrossRef]
- Khadhar, S.; Higashi, T.; Hamdi, H.; Matsuyama, S.; Charef, A. Distribution of 16 EPA-Priority Polycyclic Aromatic Hydrocarbons (PAHs) in Sludges Collected from Nine Tunisian Wastewater Treatment Plants. J. Hazard. Mater. 2010, 183, 98–102. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A Review of Airborne Polycyclic Aromatic Hydrocarbons (PAHs) and Their Human Health Effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S. Polychlorinated Biphenyls (PCBs). In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Ross, G. The Public Health Implications of Polychlorinated Biphenyls (PCBs) in the Environment. Ecotoxicol. Environ. Saf. 2004, 59, 275–291. [Google Scholar] [CrossRef]
- Safe, S.H. Polychlorinated Biphenyls (PCBs): Environmental Impact, Biochemical and Toxic Responses, and Implications for Risk Assessment. Crit. Rev. Toxicol. 1994, 24, 87–149. [Google Scholar] [CrossRef]
- Ododo, M.M.; Wabal, B.K. Polychlorinated Biphenyls (PCBs) and Their Impacts on Human Health: A Review. J. Environ. Pollut. Hum. Health 2019, 7, 73–77. [Google Scholar]
- Jaikanlaya, C.; Settachan, D.; Denison, M.S.; Ruchirawat, M.; van den Berg, M. PCBs Contamination in Seafood Species at the Eastern Coast of Thailand. Chemosphere 2009, 76, 239–249. [Google Scholar] [CrossRef]
- Ulbrich, B.; Stahlmann, R. Developmental Toxicity of Polychlorinated Biphenyls (PCBs): A Systematic Review of Experimental Data. Arch. Toxicol. 2004, 78, 252–268. [Google Scholar] [CrossRef]
- Kravkaz Kuşçu, İ.S.; Kılıç Bayraktar, M.; Tunçer, B. Determination of Heavy Metal (Cr, Co, and Ni) Accumulation in Selected Vegetables Depending on Traffic Density. Water. Air. Soil Pollut. 2022, 233, 224. [Google Scholar] [CrossRef]
- Cobbett, C.S.; Goldsbrough, P.B. Phytochelatins and Metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Srivastava, N. Role of Phytochelatins in Phytoremediation of Heavy Metals Contaminated Soils. Phytoremediation Manag. Environ. Contam. 2016, 3, 393–419. [Google Scholar] [CrossRef]
- Rodriguez, J.H.; Wannaz, E.D.; Salazar, M.J.; Pignata, M.L.; Fangmeier, A.; Franzaring, J. Accumulation of Polycyclic Aromatic Hydrocarbons and Heavy Metals in the Tree Foliage of Eucalyptus Rostrata, Pinus Radiata and Populus Hybridus in the Vicinity of a Large Aluminium Smelter in Argentina. Atmos. Environ. 2012, 55, 35–42. [Google Scholar] [CrossRef]
- Widdowson, M.A.; Shearer, S.; Andersen, R.G.; Novak, J.T. Remediation of Polycyclic Aromatic Hydrocarbon Compounds in Groundwater Using Poplar Trees. Environ. Sci. Technol. 2005, 39, 1598–1605. [Google Scholar] [CrossRef]
- Kostić, S.; Kebert, M.; Teslić, N.; Stojanović, D.B.; Zorić, M.; Kovačević, B.; Orlović, S. Polycyclic Aromatic Hydrocarbon (PAH) Phytoaccumulation in Urban Areas by Platanus × Acerifolia, Celtis Australis, and Tilia Grandifolia Leaves and Branches. Environ. Sci. Pollut. Res. 2024, 31, 31273–31286. [Google Scholar] [CrossRef]
- Liang, J.; Fang, H.; Zhang, T.; Wang, X. Polycyclic Aromatic Hydrocarbons in the Leaves of Twelve Plant Species along an Urbanization Gradient in Shanghai, China. Environ. Sci. Pollut. Res. 2017, 24, 9361–9369. [Google Scholar] [CrossRef]
- Gréau, L.; Blaudez, D.; Heintz, D.; Zumsteg, J.; Billet, D.; Cébron, A. Response of Poplar and Associated Fungal Endophytic Communities to a PAH Contamination Gradient. Int. J. Mol. Sci. 2022, 23, 5909. [Google Scholar] [CrossRef]
- Wittig, R.; Ballach, H.J.; Kuhn, A. Exposure of the Roots of Populus nigra L. Cv. Loenen to PAHs and Its Effect on Growth and Water Balance. Environ. Sci. Pollut. Res. 2003, 10, 235–244. [Google Scholar] [CrossRef]
- Liu, J.; Schnoor, J.L. Uptake and Translocation of Lesser-Chlorinated Polychlorinated Biphenyls (PCBs) in Whole Hybrid Poplar Plants after Hydroponic Exposure. Chemosphere 2008, 73, 1608–1616. [Google Scholar] [CrossRef]
- Zhai, G.; Hu, D.; Lehmler, H.J.; Schnoor, J.L. Enantioselective Biotransformation of Chiral PCBs in Whole Poplar Plants. Environ. Sci. Technol. 2011, 45, 2308–2316. [Google Scholar] [CrossRef]
- Ancona, V.; Rascio, I.; Aimola, G.; Campanale, C.; Grenni, P.; di Lenola, M.; Garbini, G.L.; Uricchio, V.F.; Caracciolo, A.B. Poplar-Assisted Bioremediation for Recovering a PCB and Heavy-Metal-Contaminated Area. Agriculture 2021, 11, 689. [Google Scholar] [CrossRef]
- Baldantoni, D.; Cicatelli, A.; Bellino, A.; Castiglione, S. Different Behaviours in Phytoremediation Capacity of Two Heavy Metal Tolerant Poplar Clones in Relation to Iron and Other Trace Elements. J. Environ. Manag. 2014, 146, 94–99. [Google Scholar] [CrossRef]
- Kovačević, B.; Milović, M.; Kesić, L.; Pajnik, L.P.; Pekeč, S.; Stanković, D.; Orlović, S. Interclonal Variation in Heavy Metal Accumulation Among Poplar and Willow Clones: Implications for Phytoremediation of Contaminated Landfill Soils. Plants 2025, 14, 567. [Google Scholar] [CrossRef]
- Pilipovic, A.; Orlovic, S.; Roncevic, S.; Nikolic, N.; Zupunski, M.; Spasojevic, J. Results of Selection of Poplars and Willows for Water and Sediment Phytoremediation. J. Agric. For. 2015, 61, 205–211. [Google Scholar] [CrossRef]
- Padoan, E.; Passarella, I.; Prati, M.; Bergante, S.; Facciotto, G.; Ajmone-Marsan, F. The Suitability of Short Rotation Coppice Crops for Phytoremediation of Urban Soils. Appl. Sci. 2020, 10, 307. [Google Scholar] [CrossRef]
- Wong, C.S.C.; Li, X.; Thornton, I. Urban Environmental Geochemistry of Trace Metals. Environ. Pollut. 2006, 142, 1–16. [Google Scholar] [CrossRef]
- Tanić, M.N.; Dinić, D.; Kartalović, B.; Mihaljev, Ž.; Stupar, S.; Ćujić, M.; Onjia, A. Occurrence, Source Apportionment, and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Soil of Urban Parks in a Mid-Sized City. Water Air Soil Pollut. 2023, 234, 484. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. N. Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Prasse, C.; Zech, W.; Itanna, F.; Glaser, B. Contamination and Source Assessment of Metals, Polychlorinated Biphenyls, and Polycyclic Aromatic Hydrocarbons in Urban Soils from Addis Ababa, Ethiopia. Toxicol. Environ. Chem. 2012, 94, 1954–1979. [Google Scholar] [CrossRef]
- Levei, L.; Cadar, O.; Babalau-Fuss, V.; Kovacs, E.; Torok, A.I.; Levei, E.A.; Ozunu, A. Use of Black Poplar Leaves for the Biomonitoring of Air Pollution in an Urban Agglomeration. Plants 2021, 10, 548. [Google Scholar] [CrossRef]
- Galal, T.M.; Shehata, H.S. Bioaccumulation and Translocation of Heavy Metals by Plantago major L. Grown in Contaminated Soils under the Effect of Traffic Pollution. Ecol. Indic. 2015, 48, 244–251. [Google Scholar] [CrossRef]
- Capuana, M. A Review of the Performance of Woody and Herbaceous Ornamental Plants for Phytoremediation in Urban Areas. IForest 2020, 13, 139–151. [Google Scholar] [CrossRef]
- Pilipović, A.; Zalesny, R.S., Jr.; Orlović, S.; Drekić, M.; Pekeč, S.; Katanić, M.; Poljaković-Pajnik, L. Growth and Physiological Responses of Three Poplar Clones Grown on Soils Artificially Contaminated with Heavy Metals, Diesel Fuel, and Herbicides. Int. J. Phytoremediation 2020, 22, 436–450. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Headlee, W.L.; Gopalakrishnan, G.; Bauer, E.O.; Hall, R.B.; Hazel, D.W.; Isebrands, J.G.; Licht, L.A.; Negri, M.C.; Nichols, E.G.; et al. Ecosystem Services of Poplar at Long-term Phytoremediation Sites in the Midwest and Southeast, United States. Wiley Interdiscip. Rev. Energy Environ. 2019, 8, e349. [Google Scholar] [CrossRef]
- Liang, Z.; Zeng, H.; Kong, J. Contrasting Responses and Phytoremediation Potential of Two Poplar Species to Combined Strontium and Diesel Oil Stress. Plants 2023, 12, 2145. [Google Scholar] [CrossRef]
- Kostić, S.; Čukanović, J.; Orlović, S.; Ljubojević, M.; Mladenović, E. Allometric Relations of Sycamore Maple (Acer pseudoplatanus) and Its Red Leaf Cultivar (A. pseudoplatanus “Atropurpureum”) in Street and Park Habitats of Novi Sad (Serbia, Europe). J. For. 2019, 117, 114–127. [Google Scholar] [CrossRef]
- JP “Urbanizam” (Javno Preduzeće “Urbanizam”). Merenje Podzemnih Voda u Novom Sadu u 2023. Godini. Available online: https://www.nsurbanizam.rs/sites/default/files/PODZEMNE_VODE_2023.pdf (accessed on 6 May 2024).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Koppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Bechtel, B.; Alexander, P.J.; Böhner, J.; Ching, J.; Conrad, O.; Feddema, J.; Mills, G.; See, L.; Stewart, I. Mapping Local Climate Zones for a Worldwide Database of the Form and Function of Cities. ISPRS Int. J. Geo-Inf. 2015, 4, 199–219. [Google Scholar] [CrossRef]
- Feng, S.; Hom, B.J. Sensitive and Reproducible Detection of PAHs Usinf the Agilent 5977A Series GC/MSD; Agilent Technologies: Santa Clara, CA, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2016, 8, 474. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2021. Available online: https://cran.r-project.org/web/packages/rstatix/rstatix.pdf (accessed on 15 October 2024).

| Compounds | Abbreviations | Species | Organ | Species × Organ | |||
|---|---|---|---|---|---|---|---|
| F-Test | P | F-Test | p | F-Test | p | ||
| Polycyclic aromatic hydrocarbons (PAHs) | |||||||
| Naphthalene | NAP | 8.539 | 5.00 × 10−3 | 38.616 | 4.49 × 10−5 | 8.255 | 6.00 × 10−3 |
| Acenaphthylene | ACE | 1.344 | 0.297 | 4.448 | 0.048 | 1.149 | 0.349 |
| Acenaphthene | ACT | 6.831 | 1.00 × 10−2 | 44.747 | 2.23 × 10−5 | 9.113 | 4.00 × 10−3 |
| Fluorene | FLU | 8.098 | 0.012 | 32.680 | / | / | / |
| Phenanthrene | PHE | 12.780 | 1.00 × 10−3 | 30.130 | 1.39 × 10−4 | 22.739 | 8.28 × 10−5 |
| Anthracene | ANT | 17.227 | 2.97 × 10−4 | 6.378 | 0.270 | 21.239 | 1.14 × 10−4 |
| Fluoranthene | FLT | 0.375 | 0.697 | 2.715 | 0.130 | 0.171 | 0.688 |
| Pyrene | PYR | 0.828 | 0.460 | 6.465 | 0.026 | 0.915 | 0.427 |
| Chrysene | CRY | 0.832 | 0.461 | 2.645 | 0.132 | 0.788 | 0.479 |
| Benzo[b]fluoranthene | BbF | 0.222 | 0.804 | 31.717 | 1.10 × 10−4 | 0.343 | 0.716 |
| Benzo[k]fluoranthene | BkF | 0.469 | 0.636 | 16.199 | 0.002 | 0.466 | 0.638 |
| Benzo[a]pyrene | BaP | 1.139 | 3.53 × 10−1 | 44.517 | 2.29 × 10−5 | 0.552 | 0.591 |
| Indeno[1,2,3-cd]pyrene | IcdP | 0.765 | 0.493 | 0.787 | 0.398 | 0.298 | 0.750 |
| Dibenzo[a,h]anthracene | DA | 11.456 | 0.003 | 16.836 | 0.002 | 9.430 | 0.012 |
| Benzo[g,h,i]perylene | BP | 1.172 | 0.346 | 0.691 | 0.424 | 1.057 | 0.380 |
| Polychlorinated biphenyls (PCBs) | |||||||
| PCB 8 | 6.522 | 1.20 × 10−2 | 72.674 | 1.95 × 10−6 | 6.436 | 1.30 × 10−2 | |
| PCB 20 | 4.581 | 0.047 | 8.200 | 0.021 | 3.031 | 0.105 | |
| PCB 28 | 0.891 | 0.441 | 0.804 | 0.391 | 0.681 | 0.528 | |
| PCB 35 | 19.865 | 6.35 × 10−8 | 50.271 | 3.66 × 10−9 | 20.609 | 5.13 × 10−8 | |
| PCB 52 | / | / | / | / | / | / | |
| PCB 101 | 0.787 | 0.487 | 0.012 | 0.914 | 0.047 | 0.826 | |
| PCB 118 | 0.863 | 0.454 | 8.790 | 0.016 | 0.710 | 0.517 | |
| PCB 138 | 0.511 | 0.618 | 1.253 | 0.295 | 0.237 | 0.795 | |
| PCB 153 | / | / | / | / | / | / | |
| PCB 180 | 36.376 | 3.85 × 10−4 | 2.025 | 0.014 | / | / | |
| Heavy metals (HMs) | |||||||
| Cu | 38.201 | 6.26 × 10−6 | 22.214 | 5.03 × 10−4 | 6.145 | 0.015 | |
| Cd | 5.879 | 0.017 | 14.960 | 0.002 | 4.033 | 0.046 | |
| Cr | 3.61 | 0.059 | 12.701 | 4.00 × 10−4 | 6.914 | 0.010 | |
| Fe | 3.914 | 4.8 × 10−4 | 40.468 | 3.6 × 10−5 | 2.063 | 0.170 | |
| Pb | 3.00 | 0.088 | 0.119 | 0.074 | 0.582 | 0.574 | |
| Zn | 3.765 | 0.054 | 21.209 | 6.10 × 10−4 | 4.582 | 0.033 | |
| Mn | 21.822 | 1.01 × 10−4 | 132.994 | 7.52 × 10−8 | 6.175 | 0.014 | |
| Ni | 3.965 | 0.048 | 11.616 | 0.005 | 1.848 | 0.200 | |
| Species | Location | Coordinates | Type of Local Climate Zone | Street Orientation | Degree of Urbanization (%) | Mean Street Canyon Width (m) | Mean Street Canyon Height (m) | Height/Width Ratio | Altitude (m a.s.l.) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | |||||||||
| Populus alba | Sunčani kej | 45.251668 | 19.855855 | Open midrise | N-S | 30 | 35.97 | 32.40 | 0.90 | 74.69 |
| Populus nigra ‘Italica’ | Dimitrija Tucovića | 45.247900 | 19.842148 | Compact midrise | NE-SW | 56 | 17.20 | 15.17 | 0.88 | 76.05 |
| Populus × euramericana cl. I-214 | Šajkaškog odreda | 45.280335 | 19.854746 | Sparsely built | E-W | 21 | 25.82 | 23.20 | 0.90 | 75.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalozi, O.; Kebert, M.; Orlović, S.; Ilić, M.; Kostić, S. Populus × euramericana Accumulates More Organic Pollutants (PAHs and PCBs), While P. nigra ‘Italica’ Absorbs More Heavy Metals. Plants 2025, 14, 1445. https://doi.org/10.3390/plants14101445
Kalozi O, Kebert M, Orlović S, Ilić M, Kostić S. Populus × euramericana Accumulates More Organic Pollutants (PAHs and PCBs), While P. nigra ‘Italica’ Absorbs More Heavy Metals. Plants. 2025; 14(10):1445. https://doi.org/10.3390/plants14101445
Chicago/Turabian StyleKalozi, Olivera, Marko Kebert, Saša Orlović, Marko Ilić, and Saša Kostić. 2025. "Populus × euramericana Accumulates More Organic Pollutants (PAHs and PCBs), While P. nigra ‘Italica’ Absorbs More Heavy Metals" Plants 14, no. 10: 1445. https://doi.org/10.3390/plants14101445
APA StyleKalozi, O., Kebert, M., Orlović, S., Ilić, M., & Kostić, S. (2025). Populus × euramericana Accumulates More Organic Pollutants (PAHs and PCBs), While P. nigra ‘Italica’ Absorbs More Heavy Metals. Plants, 14(10), 1445. https://doi.org/10.3390/plants14101445











