Growth and Physiological Traits of Blueberry Seedlings in Response to Different Nitrogen Forms
Abstract
1. Introduction
2. Results
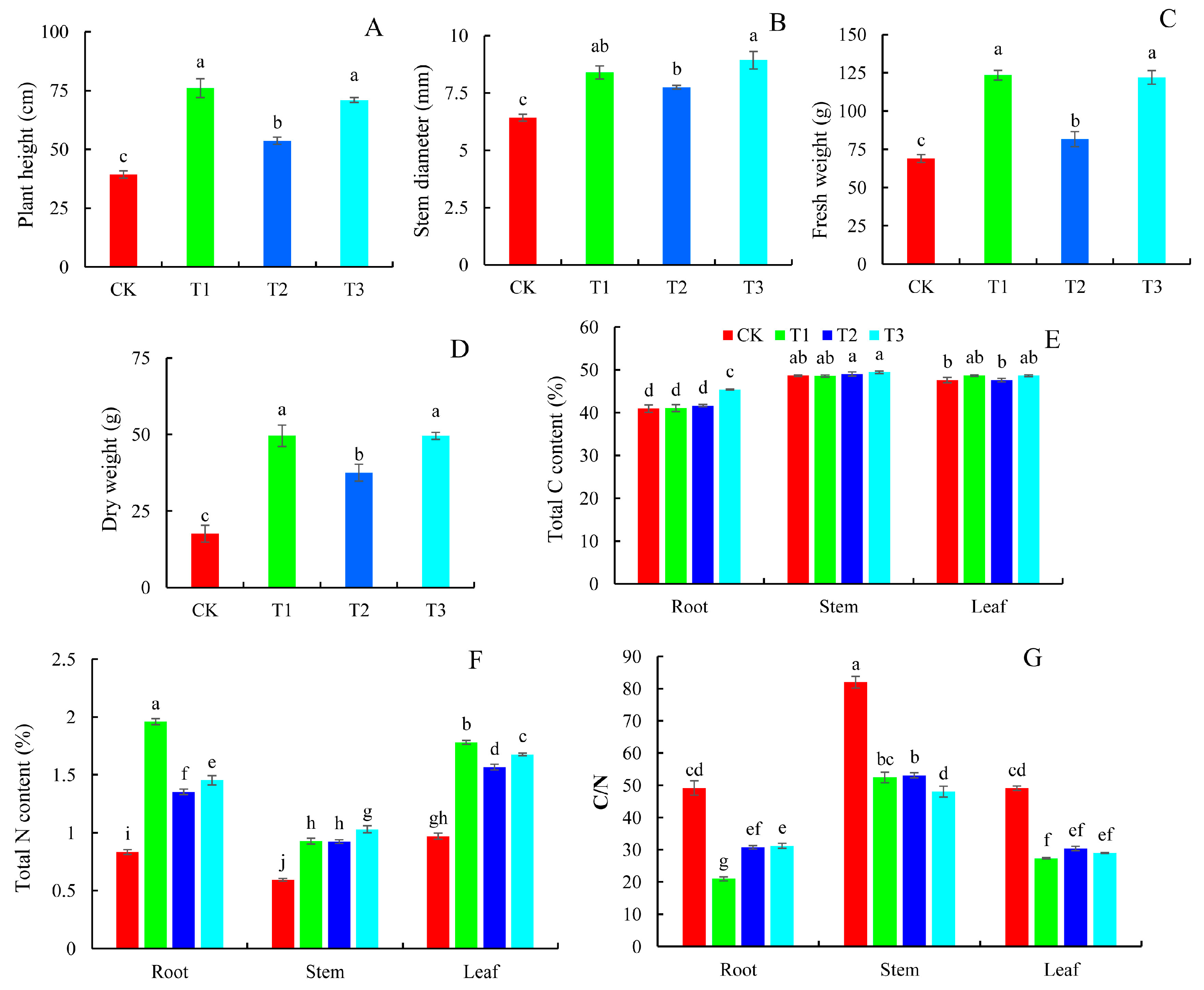
2.1. Plant Growth Indicators and Carbon (C) and N Content
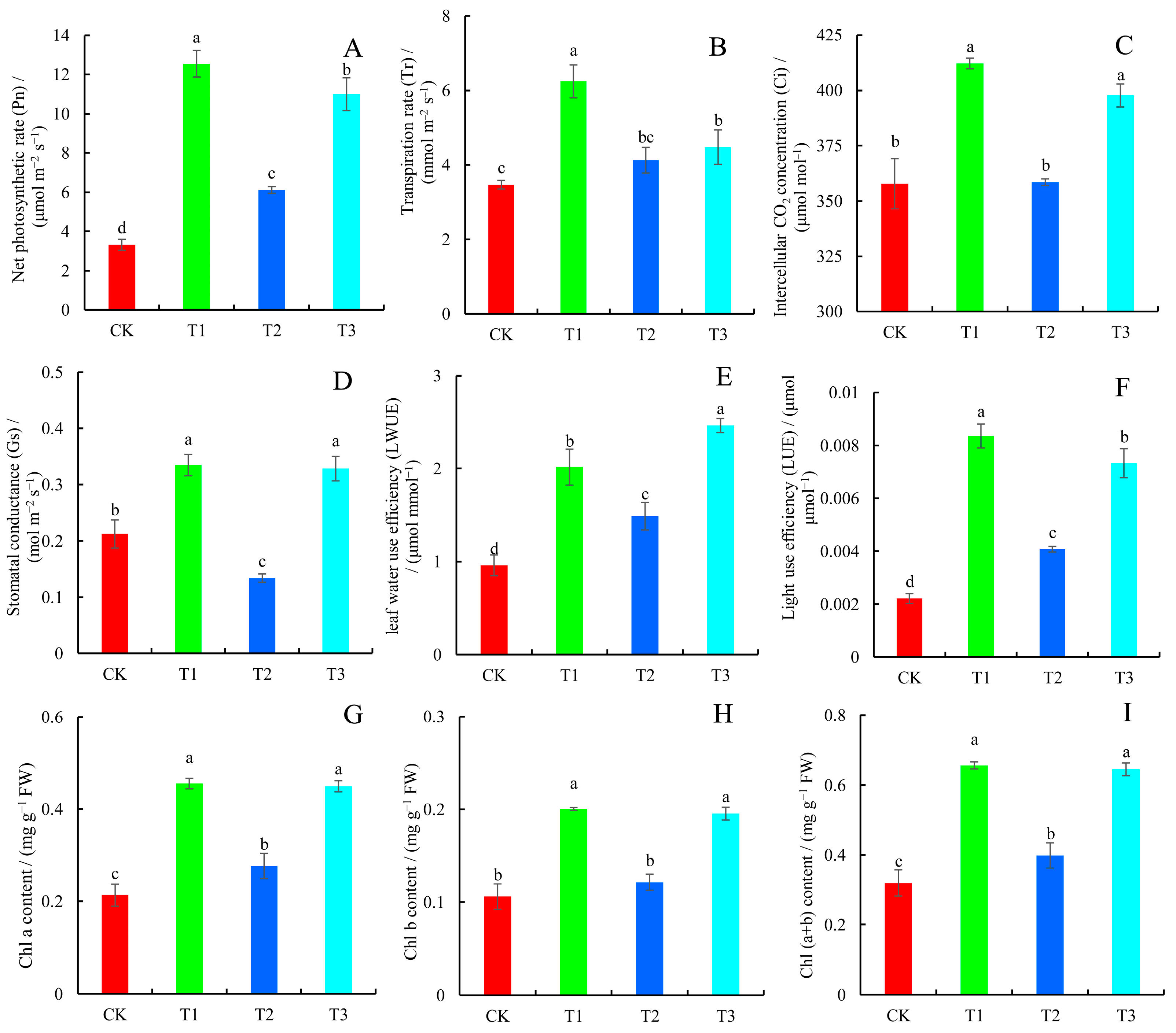
2.2. Photosynthetic Parameters and Chlorophyll Content
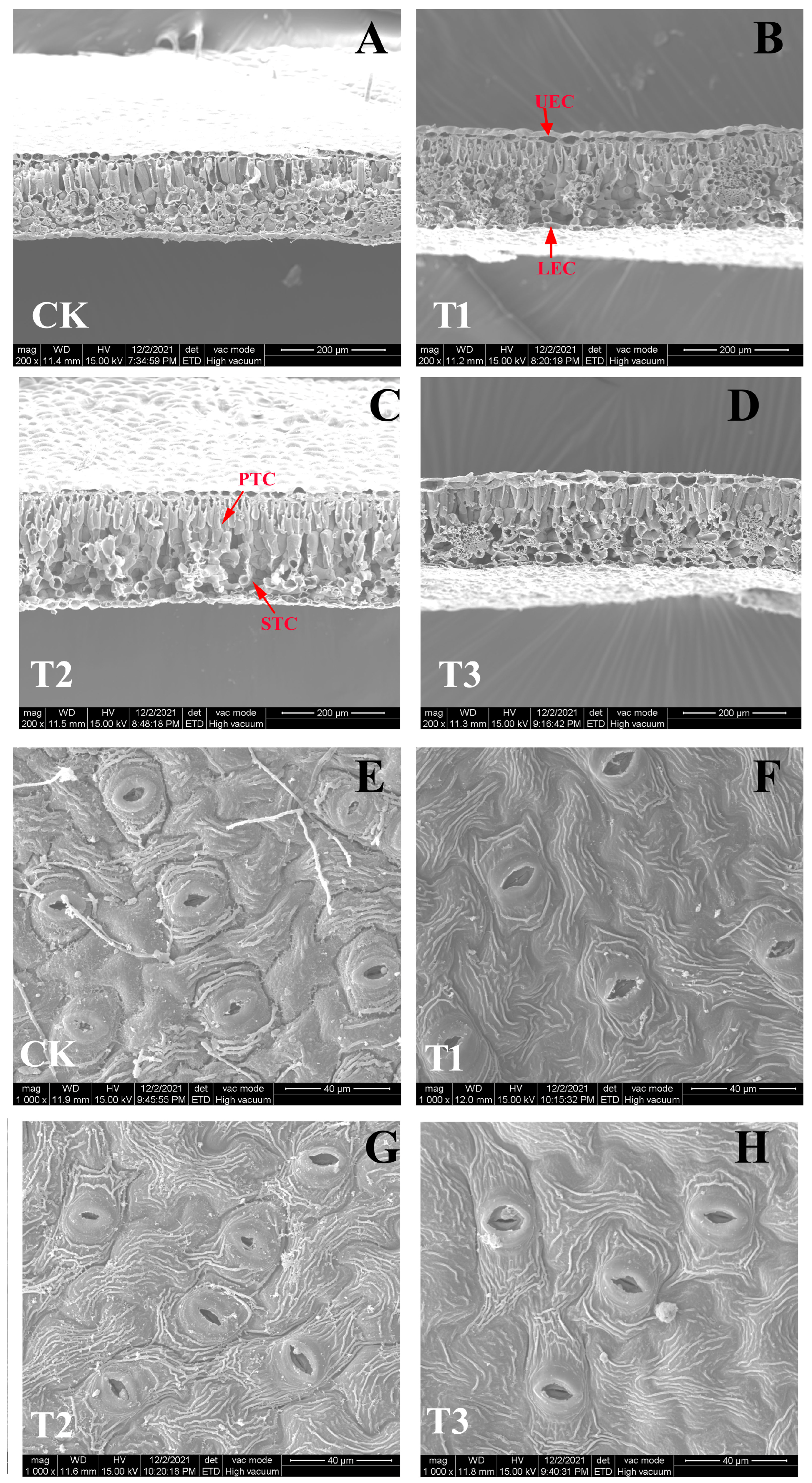
2.3. Leaf Ultrastructure
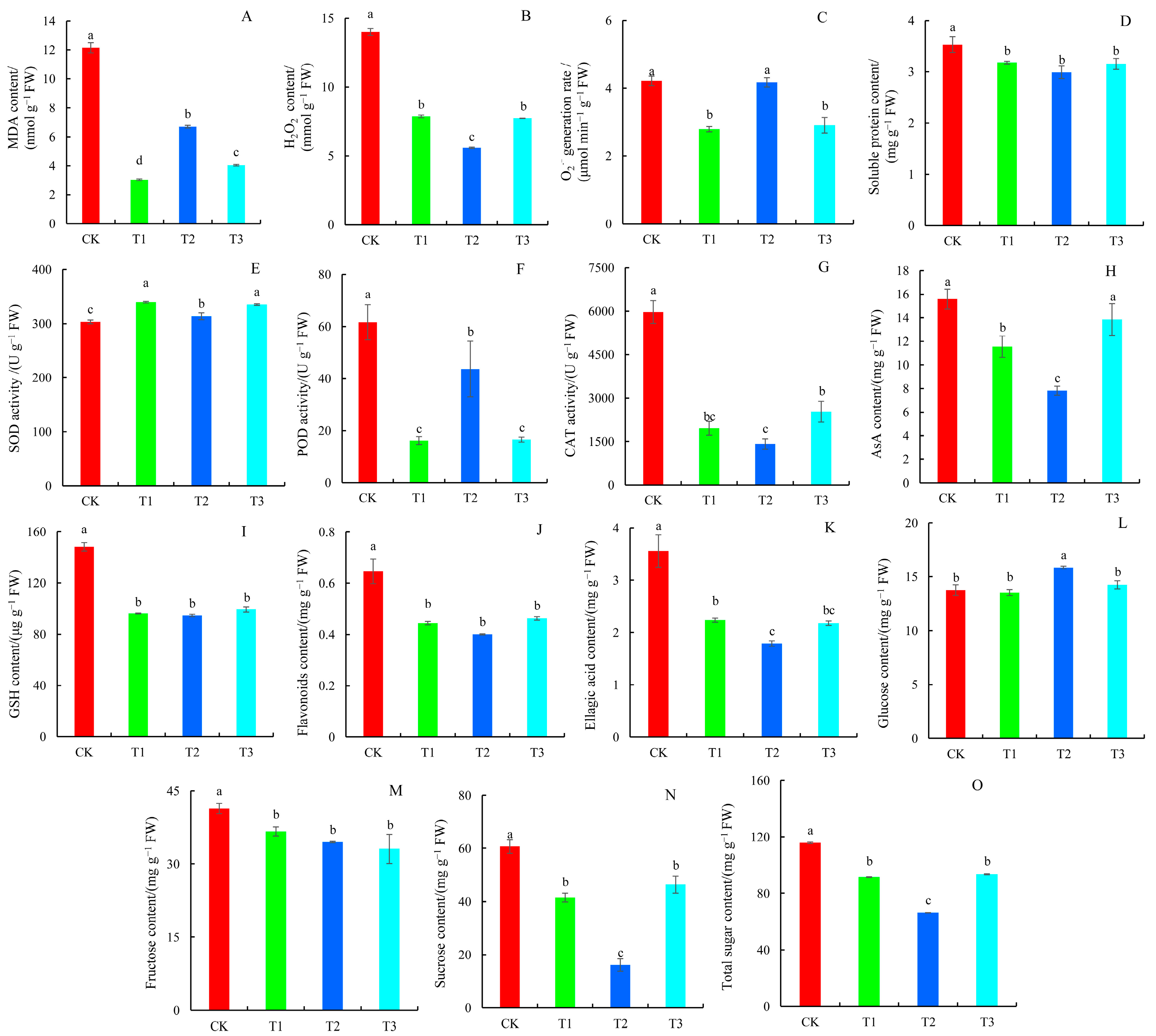
2.4. Antioxidant System, Flavonoids, Ellagic Acid, and Sugar Content
2.5. Free Amino Acid Content
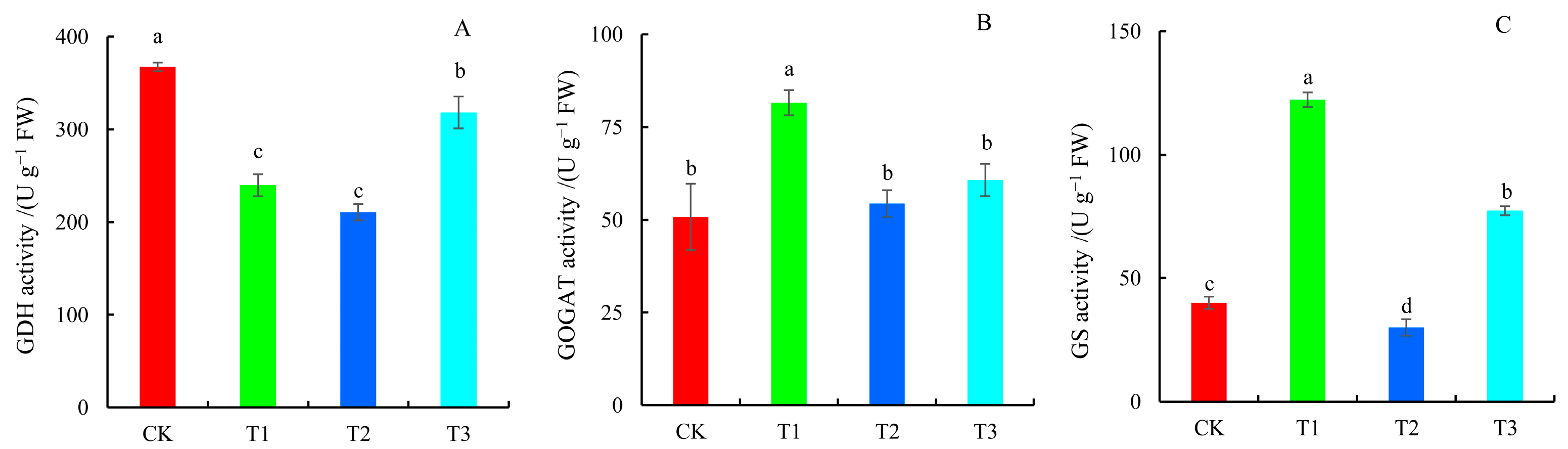
2.6. Key Enzyme Activities Involved in N Metabolism
2.7. Physicochemical Properties of Blueberry Cultivation Substrate
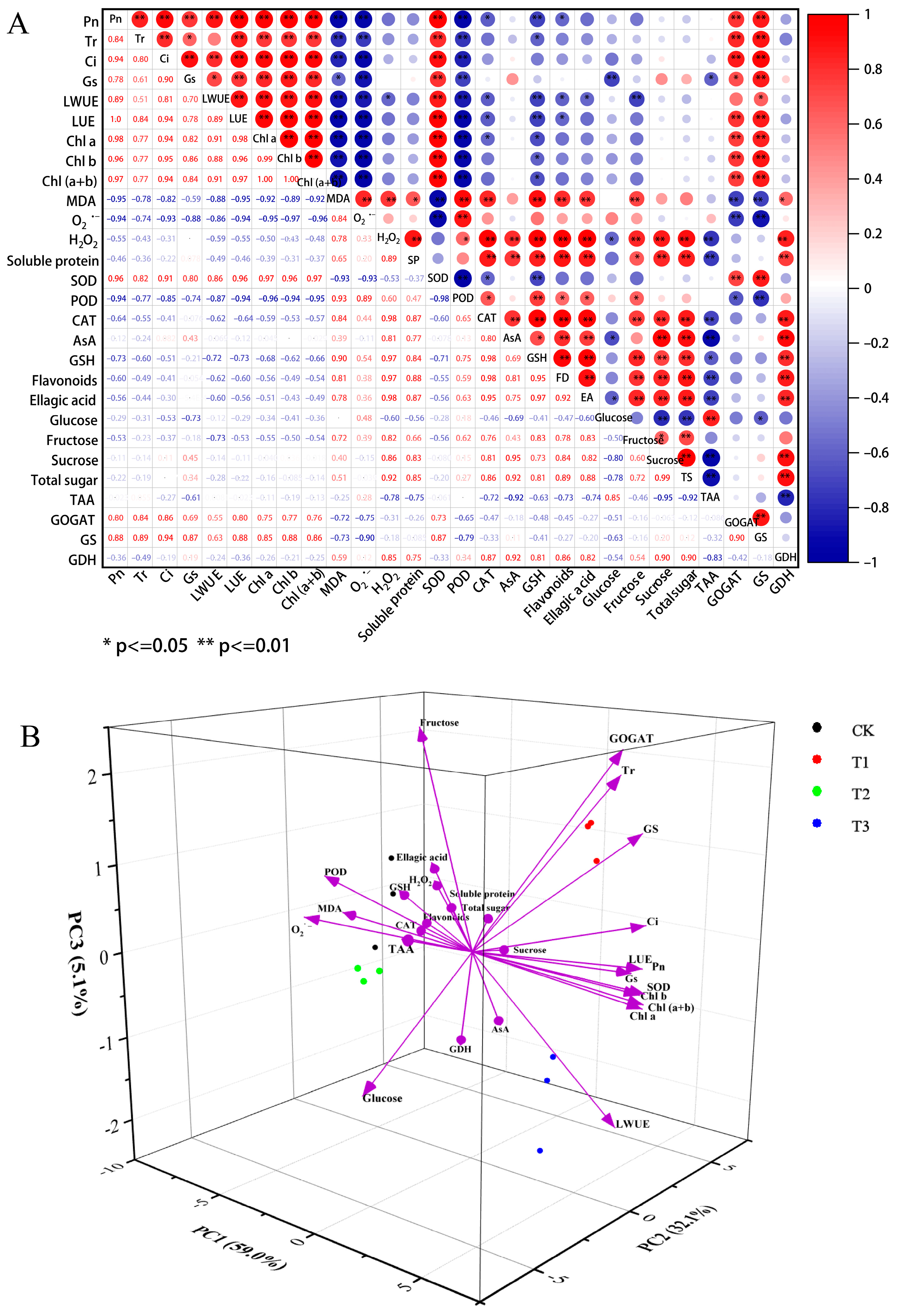
2.8. Correlation and Principal Component Analysis (PCA) of Physiological Parameters
3. Discussion
3.1. Plant Nitrogen Form Preference
3.2. Growth Status of Blueberries in Response to Different N Forms
3.3. Photosynthetic Performance and Antioxidant Capacity of Blueberries in Response to Different N Forms
3.4. Free Amino Acids Changes in Blueberries in Response to Different N Forms
3.5. Enzymes Involved in N Metabolism of Blueberries in Response to Different N Forms
3.6. Growth Substrate Effect
4. Materials and Methods
4.1. Plant Material
4.2. Measurement of Plant Material Growth Indexes, Total C and Total N Content
4.3. Measurement of Photosynthetic Parameters and Chlorophyll Content
4.4. Analysis of Antioxidant System Indexes
4.5. Determination of Sucrose, Fructose and Glucose Content
4.6. Determination of Flavonoids and Ellagic Acid Content
4.7. Determination of Free Amino Acid Content
4.8. Determination of Glutamate Dehydrogenase (GDH), Glutamine Synthetase (GS), and Glutamate Synthetase (GOGAT) Activities
4.9. Micromorphology Observation of Blueberry Leaf
4.10. Measurement of the PH and Conductivity (EC) Values of the Substrate
4.11. Measurement of Organic Matter and Alkali-Hydrolysable Nitrogen Content
4.12. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Ferruzzi, M.G.; Maiz, M.; Cladis, D.P.; Nakatsu, C.H.; McCabe, G.P.; Lila, M.A. Crop, host, and gut microbiome variation influence precision nutrition: An example of blueberries. Antioxidants 2023, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Liu, C.J.; Yuan, Y.H.; Liu, J.J.; Wang, H.L.; Ma, Q.; Zhou, Y.F.; Liu, C.; Gong, X.W.; Feng, B.L. Comparative transcriptome and physiological analysis unravel proso millet (Panicum miliaceum L.) source leaf adaptation to nitrogen deficiency with high nitrogen use efficiency. Environ. Exp. Bot. 2022, 199, 104891. [Google Scholar] [CrossRef]
- Wei, Z.W.; Yang, H.Y.; Duan, Y.K.; Fan, S.F.; Wu, W.L.; Lyu, L.F.; Li, W.L. Growth and physiological responses of blackberry seedlings to different NH4+: NO3− ratios. J. Soil Sci. Plant Nutr. 2024, 24, 4549–4564. [Google Scholar] [CrossRef]
- Hachiya, T.; Inaba, J.; Wakazaki, M.; Sato, M.; Toyooka, K.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Nakagawa, T.; Kiba, T.; et al. Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 2021, 12, 4944. [Google Scholar] [CrossRef]
- Witte, P.C. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Doyle, J.W.; Nambeesan, S.U.; Malladi, A. Physiology of nitrogen and calcium nutrition in blueberry (Vaccinium sp.). Agronomy 2021, 11, 765. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wei, Z.W.; Duan, Y.K.; Wu, Y.Q.; Zhang, C.H.; Wu, W.L.; Lyu, L.F.; Li, W.L. Transcriptomic and metabolomic investigation of the adaptation mechanisms of blueberries to nitrogen deficiency stress. Sci. Hortic. 2023, 321, 112376. [Google Scholar] [CrossRef]
- Merhaut, D.J.; Darnell, R.L. Ammonium and nitrate accumulation in containerized southern highbush blueberry plants. HortScience 1995, 30, 1378–1381. [Google Scholar] [CrossRef]
- Bryla, D.R.; Strik, B.C. Nutrient requirements, leaf tissue standards, and new options for fertigation of northern highbush blueberry. HortTechnology 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Tamir, G.; Afik, G.; Zilkah, S.; Dai, N.; Bar-Tal, A. The use of increasing proportions of N-NH4+ among the total applied inorganic N to improve acidification and the nutritional status and performance of blueberry plants in soilless culture. Sci. Hortic. 2021, 276, 109754. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Coleto, I.; Vega-Mas, I.; Glauser, G.; González-Moro, M.B.; Marino, D.; Ariz, I. New insights on Arabidopsis thaliana root adaption to ammonium nutrition by the use of a quantitative proteomic approach. Int. J. Mol. Sci. 2019, 20, 814. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Xiao, Z.L.; Zhang, X.Y.; Zhao, C.Y.; Li, Y.Y.; Che, T.; Li, Y.J.; Zang, X.Y.; Jiang, S.Y.; Li, H.X.; et al. The nutrient preferences of rice and wheat influence fluoranthene uptake. Front. Environ. Sci. 2022, 10, 987743. [Google Scholar] [CrossRef]
- Mao, L.L.; Wei, B.H.; Tie, J.Z.; Gao, X.Q.; Wang, X.H.; Yang, J.J.; Dawuda, M.M.; Yu, J.H.; Hu, L.L. Yield and nutritional quality analysis in Brassica pekinensis: Regulation of different nitrogen forms. Food Chem. X 2024, 24, 102024. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, H.; Yang, H.; Wei, Z.; Che, J.; Wu, W.; Lyu, L.; Li, W. Physiological and morphological responses of blackberry seedlings to different nitrogen forms. Plants 2023, 12, 1480. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.M.; Lu, Y.T.; Qiu, Q.L.; Fan, D.M.; Wang, X.C.; Zheng, X.Q. Influence of different nitrogen sources on carbon and nitrogen metabolism and gene expression in tea plants (Camellia sinensis L.). Plant Physiol. Bioch. 2021, 167, 561–566. [Google Scholar] [CrossRef]
- Carr, N.F.; Boaretto, R.M.; Mattos, D. Coffee seedlings growth under varied NO3−: NH4+ ratio: Consequences for nitrogen metabolism, amino acids profile, and regulation of plasma membrane H+-ATPase. Plant Physiol. Bioch. 2020, 154, 11–20. [Google Scholar] [CrossRef]
- Rosen, C.J.; Allan, D.L.; Luby, J.J. Nitrogen form and solution pH influence growth and nutrition of two Vaccinium clones. J. Am. Soc. Hortic. Sci. 1990, 115, 83–89. [Google Scholar] [CrossRef]
- Alt, D.S.; Doyle, J.W.; Malladi, A. Nitrogen-source preference in blueberry (Vaccinium sp.): Enhanced shoot nitrogen assimilation in response to direct supply of nitrate. J. Plant Physiol. 2017, 216, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Meng, H.; Teng, K.; Fan, X.; Zhang, H.; Teng, W.; Yue, Y.; Wu, J. Effects of nitrogen forms on the growth and nitrogen accumulation in Buchloe dactyloides seedlings. Plants 2022, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Oswald, O.; Graham, I.A. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: Nitrogen availability. Plant Physiol. 2002, 128, 472–481. [Google Scholar] [CrossRef]
- Araya, T.; Noguchi, K.; Terashima, I. Effect of nitrogen nutrition on the carbohydrate repression of photosynthesis in leaves of Phaseolus vulgaris L. J. Plant Res. 2010, 123, 371–379. [Google Scholar] [CrossRef]
- Urban, A.; Rogowski, P.; Wasilewska-Dębowska, W.; Romanowska, E. Understanding maize response to nitrogen limitation in different light conditions for the improvement of photosynthesis. Plants 2021, 10, 1932. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, Y.; Yang, Y.; Liu, T.; Wang, M.; Li, Y.; Guo, S. Variation of mesophyll conductance mediated by nitrogen form is related to changes in cell wall property and chloroplast number. Hortic. Res. 2024, 11, uhae112. [Google Scholar] [CrossRef]
- Shohag, M.J.I.; Tian, S.F.; Sriti, N.; Liu, G.D. Enhancing Bok Choy growth through synergistic effects of hydrogel and different nitrogen fertilizer forms. Sci. Hortic. 2024, 336, 113400. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting chlorophyll metabolism with accumulation of the photosynthetic apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulffqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hessini, K.; Ben Hamed, K.; Gandour, M.; Mejri, M.; Abdelly, C.; Cruz, C. Ammonium nutrition in the halophyte Spartina alterniflora under salt stress: Evidence for a priming effect of ammonium? Plant Soil 2013, 370, 163–173. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, H.; Wei, Z.; Yang, H.; Fan, S.; Wu, W.; Lyu, L.; Li, W. Effects of different nitrogen forms on blackberry fruit quality. Foods 2023, 12, 2318. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Hajibarat, Z.; Saidi, A.; Ghazvini, H.; Hajibarat, Z. Investigation of morpho-physiolgical traits and gene expression in barley under nitrogen deficiency. Sci. Rep. 2024, 14, 8875. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Xu, M.; Cheng, Z.; Yang, L.T. Effects of nitrogen deficiency on the photosynthesis, chlorophyll a fluorescence, antioxidant system, and sulfur compounds in Oryza sativa. Int. J. Mol. Sci. 2024, 25, 10409. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Repčák, M.; Kron, I. Nitrogen deficiency induced changes of free amino acids and coumarin contents in the leaves of Matricaria chamomilla. Acta Physiol. Plant. 2006, 28, 159–164. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chen, F.; Cheng, Z.; Wang, Y.; Yang, P.; Zhang, Y.; Chan, Z. Manipulation of arginase expression modulates abiotic stress tolerance in arabidopsis: Effect on arginine metabolism and ROS accumulation. J. Exp. Bot. 2013, 64, 1367–1379. [Google Scholar] [CrossRef]
- Le, X.H.; Lee, C.P.; Millar, A.H. The mitochondrial pyruvate carrier (MPC) complex mediates one of three pyruvate-supplying pathways that sustain Arabidopsis respiratory metabolism. Plant Cell 2021, 33, 2776–2793. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Zhang, M.; Chen, J.; Liu, J.; Han, H.; Hua, X. Arabidopsis AMINO ACID PERMEASE1 contributes to salt stress-induced proline uptake from exogenous sources. Front. Plant Sci. 2017, 8, 2182. [Google Scholar] [CrossRef]
- Mercure, S.A.; Daoust, B.; Samson, G. Causal relationship between growth inhibition, accumulation of phenolic metabolites, and changes of UV-induced fluorescences in nitrogen-deficient barley plants. Can. J. Bot. 2004, 82, 815–821. [Google Scholar] [CrossRef]
- Ji, H.F.; Qi, Y.X.; Zhang, X.; Yang, G.P. Effect of L-histidine on photosynthesis of maize under salt stress. Appl. Ecol. Env. Res. 2024, 22, 3233–3248. [Google Scholar] [CrossRef]
- Ma, C.; Ban, T.T.; Yu, H.J.; Li, Q.; Li, X.H.; Jiang, W.J.; Xie, J.M. Urea addition promotes the metabolism and utilization of nitrogen in cucumber. Agronomy 2019, 9, 262. [Google Scholar] [CrossRef]
- Cun, Z.; Li, X.; Zhang, J.Y.; Hong, J.; Gao, L.L.; Yang, J.; Ma, S.Y.; Chen, J.W. Identification of candidate genes and residues for improving nitrogen use efficiency in the N-sensitive medicinal plant Panax notoginseng. BMC Plant Biol. 2024, 24, 105. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Good, A.G. Glutamate deamination by glutamate dehydrogenase plays a central role in amino acid catabolism in plants. Plant Signal. Behav. 2008, 3, 842–843. [Google Scholar] [CrossRef]
- Tang, D.D.; Jiao, Z.X.; Zhang, Q.F.; Liu, M.; Ruan, J.Y. Glutamate dehydrogenase isogenes CsGDHs cooperate with glutamine synthetase isogenes CsGSs to assimilate ammonium in tea plant (Camellia sinensis L.). Plant Sci. 2021, 312, 111031. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, F.E.B.; Ayadi, S.; Giorgia Capasso, G.; Landi, S.; Paradisone, V.; Jallouli, S.; Hammami, Z.; Chamekh, Z.; Zouari, I.; Trifa, Y.; et al. Salt stress induces differentiated nitrogen uptake and antioxidant responses in two contrasting barley landraces from MENA region. Agronomy 2020, 10, 1426. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Song, X.; Wang, Q.; Luo, J.; Guo, D.; Ma, Y. Effect of fertilizer application on watermelon growth, structure of dissolved organic matter and microbial functional diversity in organic substrates. Sustainability 2022, 14, 6951. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms: A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wu, Y.Q.; Duan, Y.K.; Zhang, C.H.; Huang, Z.J.; Wu, W.L.; Lyu, L.F.; Li, W.L. Metabolomics combined with physiological and transcriptomic analyses reveal regulatory features associated with blueberry growth in different soilless substrates. Sci. Hortic. 2022, 302, 111145. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Wang, A.G.; Luo, G.H. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol. Commun. 1990, 6, 55–57. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C. Plant peroxidase. Methods Enzymol. 1955, 2, 801–813. [Google Scholar]
- Li, H.X.; Xiao, Y.; Cao, L.L.; Yan, X.; Li, C.; Shi, H.Y.; Wang, J.W.; Ye, Y.H. Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS ONE 2013, 8, e73380. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Maas, J.L.; Wang, S.Y.; Galletta, G.J. Evaluation of strawberry cultivars for ellagic acid content. HortScience 1991, 26, 66–68. [Google Scholar] [CrossRef]
- Zhou, M.; Hua, T.; Ma, X.; Sun, H.; Xu, L. Protein content and amino acids profile in 10 cultivars of ginkgo (Ginkgo biloba L.) nut from China. R. Soc. Open Sci. 2019, 6, 181571. [Google Scholar] [CrossRef]
- Dong, S.S.; Yang, H.Y.; Wu, W.L.; Li, W.L.; Lyu, L.F. Physiological and morphological responses of blueberry to manganese stress in soil. Braz. J. Bot. 2020, 43, 419–427. [Google Scholar] [CrossRef]
| Amino Acid (mg(100 g)−1 DW) | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| Asp | 1.74 ± 0.06 c | 5.03 ± 0.06 a | 3.61 ± 0.42 b | 3.35 ± 0.82 b |
| Ala | 7.16 ± 0.24 b | 11.43 ± 0.34 a | 12.14 ± 0.96 a | 7.67 ± 1.91 b |
| Arg | 0.66 ± 0.25 b | 3.02 ± 0.08 b | 79.40 ± 5.99 a | 3.30 ± 0.88 b |
| Cys | 0.22 ± 0.04 c | 0.46 ± 0.18 bc | 1.00 ± 0.08 a | 0.80 ± 0.18 ab |
| Glu | 2.73 ± 0.16 a | 1.95 ± 0.90 a | 2.03 ± 0.32 a | 1.64 ± 0.58 a |
| Gly | 0.34 ± 0.02 b | 0.62 ± 0.07 b | 0.98 ± 0.06 a | 0.61 ± 0.16 b |
| His | 2.32 ± 0.12 d | 7.48 ± 0.16 b | 13.86 ± 1.09 a | 5.04 ± 0.50 c |
| Ile | 1.81 ± 0.29 b | 3.02 ± 0.11 a | 2.18 ± 0.16 ab | 3.17 ± 0.78 a |
| Leu | 1.70 ± 0.01 b | 3.39 ± 0.18 a | 2.19 ± 0.17 ab | 3.62 ± 0.89 a |
| Lys | 1.34 ± 0.05 c | 3.10 ± 0.12 b | 6.19 ± 0.50 a | 2.69 ± 0.65 b |
| Met | 0.03 ± 0.0046 bc | 0.01 ± 0.0003 c | 0.06 ± 0.0061 a | 0.04 ± 0.0163 ab |
| Phe | 2.57 ± 0.14 b | 8.46 ± 0.64 a | 7.96 ± 0.34 a | 6.69 ± 1.70 a |
| Pro | 3.66 ± 0.36 b | 6.58 ± 0.80 ab | 8.71 ± 0.71 a | 6.57 ± 2.16 ab |
| Ser | 2.73 ± 0.42 c | 8.22 ± 0.56 a | 6.29 ± 1.24 ab | 5.38 ± 1.64 bc |
| Tyr | 1.92 ± 0.08 c | 5.29 ± 0.08 a | 2.64 ± 0.30 bc | 3.41 ± 0.84 b |
| Thr | 1.18 ± 0.13 b | 2.23 ± 0.10 b | 8.75 ± 1.03 a | 2.00 ± 0.52 b |
| Val | 2.39 ± 0.09 b | 4.62 ± 0.06 a | 3.76 ± 0.27 ab | 4.55 ± 1.09 a |
| ∑EAA | 12.76 ± 1.00 c | 32.30 ± 1.25 b | 44.94 ± 3.48 a | 26.10 ± 7.31 b |
| ∑NEAA | 20.83 ± 0.86 c | 42.60 ± 1.92 b | 116.81 ± 8.07 a | 32.73 ± 6.82 bc |
| ∑TAA | 33.59 ± 1.84 c | 74.90 ± 3.11 b | 161.75 ± 11.47 a | 58.83 ± 14.12 b |
| Treatment | pH | EC (mS cm−1) | Organic Matter (%) | Organic Carbon (%) | Alkali-Hydrolyzable N (AHN) (mg kg−1 DW) |
|---|---|---|---|---|---|
| CK | 5.49 ± 0.06 b | 0.97 ± 0.04 d | 73.02 ± 0.75 c | 42.35 ± 0.44 c | 368.67 ± 15.42 c |
| T1 | 4.42 ± 0.0 d | 3.37 ± 0.02 a | 74.94 ± 0.70 b | 43.47 ± 0.40 b | 1585.73 ± 30.84 a |
| T2 | 5.97 ± 0.01 a | 2.12 ± 0.02 c | 73.56 ± 1.15 bc | 42.67 ± 0.67 bc | 483.47 ± 4.28 b |
| T3 | 4.57 ± 0.02c | 2.29 ± 0.01 b | 79.84 ± 0.52 a | 46.31 ± 0.30 a | 516.13 ± 11.66 b |
| Parameter | Component | |
|---|---|---|
| 1 | 2 | |
| Pn | 0.940 ** | 0.317 |
| Tr | 0.794 ** | 0.257 |
| Ci | 0.818 ** | 0.536 |
| Gs | 0.578 | 0.787 ** |
| LWUE | 0.869 ** | 0.210 |
| LUE | 0.940 ** | 0.317 |
| Chl a | 0.914 ** | 0.376 |
| Chl b | 0.881 ** | 0.449 |
| Chl (a + b) | 0.906 ** | 0.397 |
| MDA | −0.999 ** | −0.020 |
| O2˙− | −0.818 ** | −0.535 |
| H2O2 | −0.788 ** | 0.607 |
| Soluble protein | −0.668 ** | 0.611 |
| SOD | 0.918 ** | 0.336 |
| POD | −0.924 ** | −0.250 |
| CAT | −0.850 ** | 0.506 |
| AsA | −0.417 | 0.829 ** |
| GSH | −0.909 ** | 0.404 |
| Flavonoids | −0.823 ** | 0.534 |
| Ellagic acid | −0.785 ** | 0.575 |
| Glucose | 0.000 | −0.944 ** |
| Fructose | −0.714 ** | 0.377 |
| Sucrose | −0.424 | 0.893 ** |
| Total sugar | −0.525 | 0.839 ** |
| TAA | 0.277 | −0.935 ** |
| GOGAT | 0.734 ** | 0.360 |
| GS | 0.730 ** | 0.602 |
| GDH | −0.616 | 0.665 ** |
| Total | 16.53 | 8.99 |
| % of variance | 59.03 | 32.10 |
| Cumulative % | 59.02 | 91.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wu, Y.; Zhang, C.; Lyu, L.; Wu, W.; Huang, Z.; Li, W. Growth and Physiological Traits of Blueberry Seedlings in Response to Different Nitrogen Forms. Plants 2025, 14, 1444. https://doi.org/10.3390/plants14101444
Yang H, Wu Y, Zhang C, Lyu L, Wu W, Huang Z, Li W. Growth and Physiological Traits of Blueberry Seedlings in Response to Different Nitrogen Forms. Plants. 2025; 14(10):1444. https://doi.org/10.3390/plants14101444
Chicago/Turabian StyleYang, Haiyan, Yaqiong Wu, Chunhong Zhang, Lianfei Lyu, Wenlong Wu, Zhengjin Huang, and Weilin Li. 2025. "Growth and Physiological Traits of Blueberry Seedlings in Response to Different Nitrogen Forms" Plants 14, no. 10: 1444. https://doi.org/10.3390/plants14101444
APA StyleYang, H., Wu, Y., Zhang, C., Lyu, L., Wu, W., Huang, Z., & Li, W. (2025). Growth and Physiological Traits of Blueberry Seedlings in Response to Different Nitrogen Forms. Plants, 14(10), 1444. https://doi.org/10.3390/plants14101444






