Impacts of Climate Change on the Spatial Distribution and Habitat Suitability of Nitraria tangutorum
Abstract
1. Introduction
2. Materials and Methods
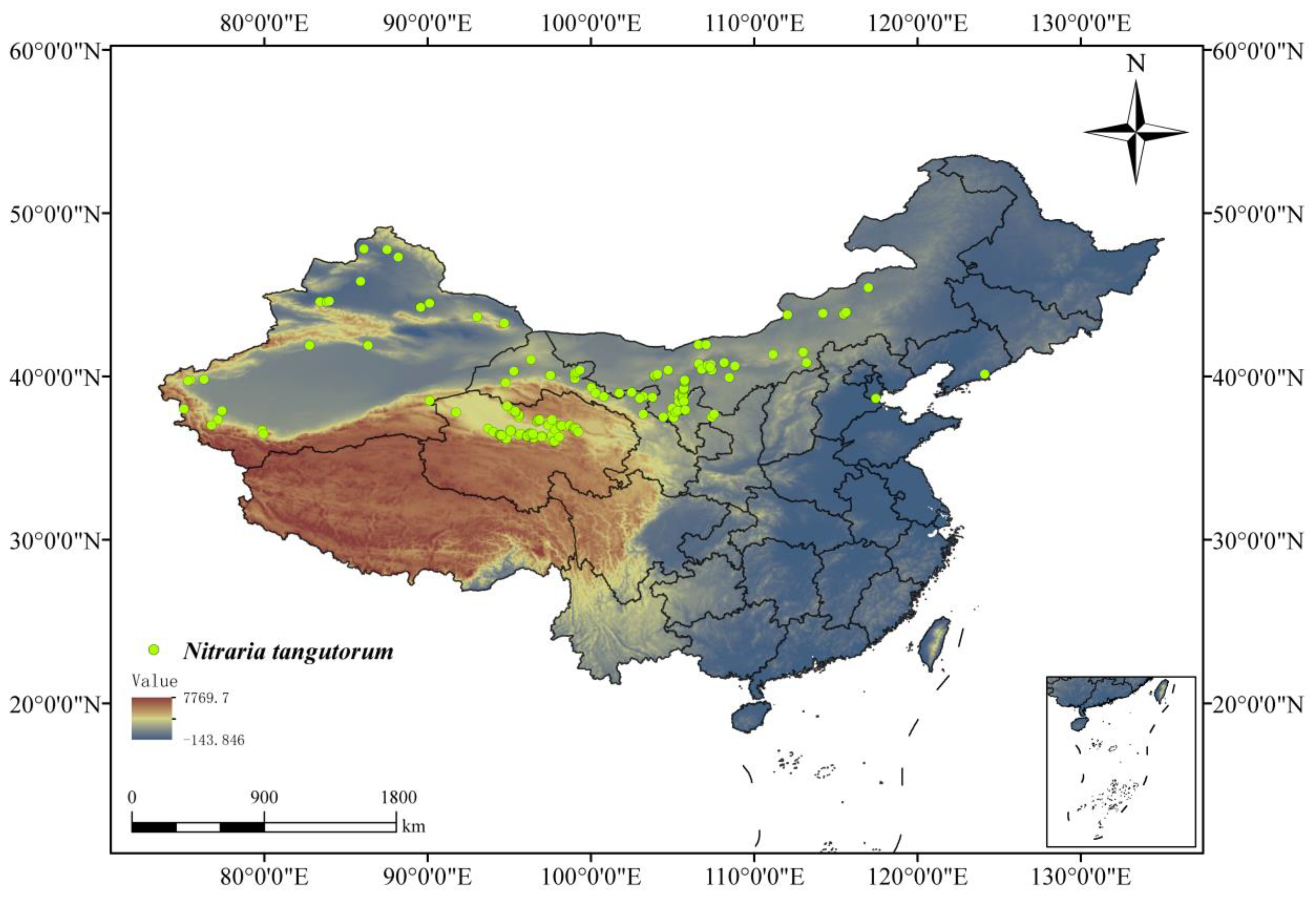
2.1. N. tangutorum Distribution Point Collection
2.2. Environmental Variable Screening and Processing
2.3. Establishment, Optimization, and Evaluation of the MaxEnt Model
2.4. Division of Potential Suitable Habitats for N. tangutorum
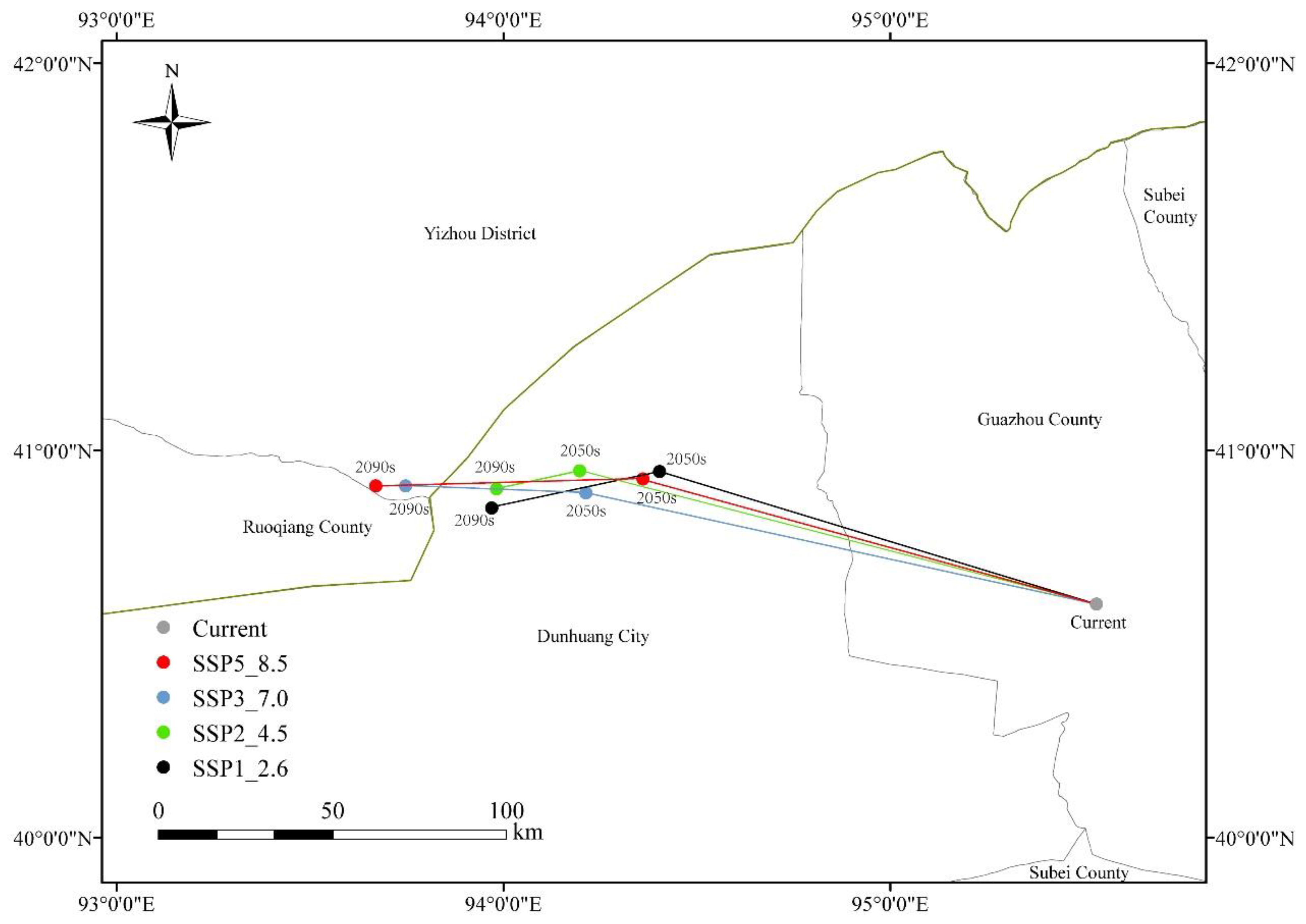
2.5. Centroid Migration of the Suitable Area
3. Results
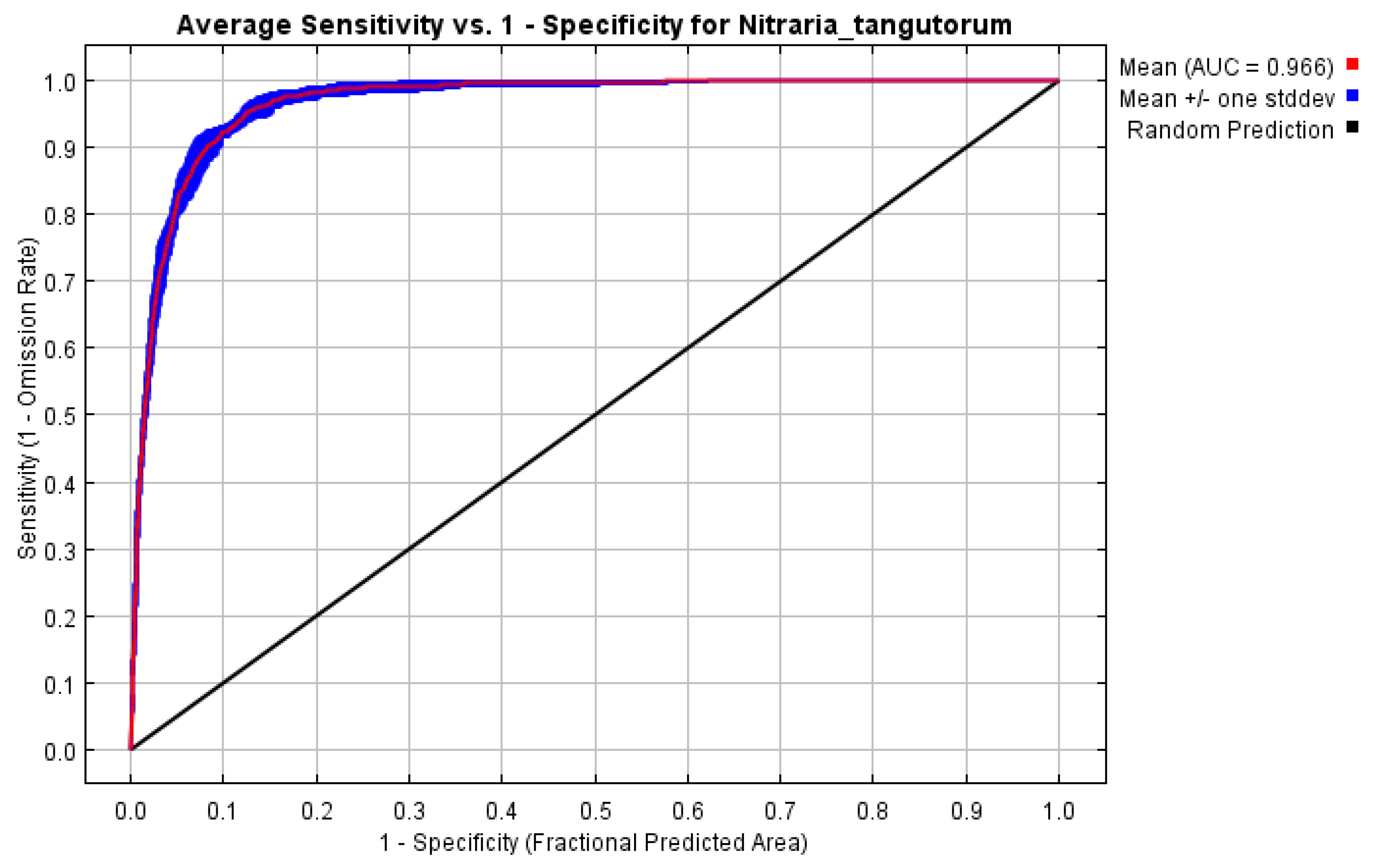
3.1. Model Optimization Results and Evaluation
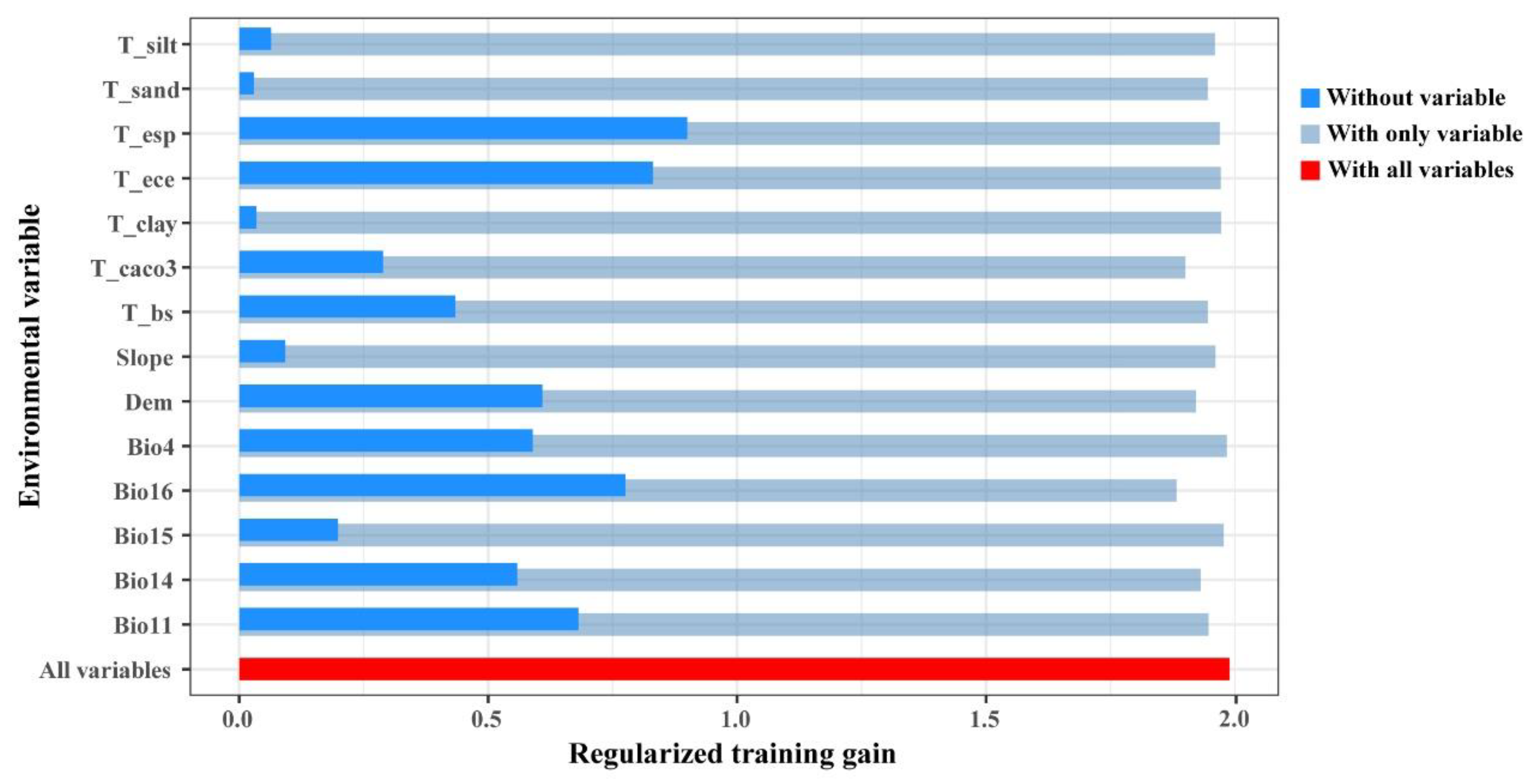
3.2. Key Environmental Variables Affecting the Distribution of N. tangutorum
3.3. The Current Suitable Habitat Distribution of N. tangutorum
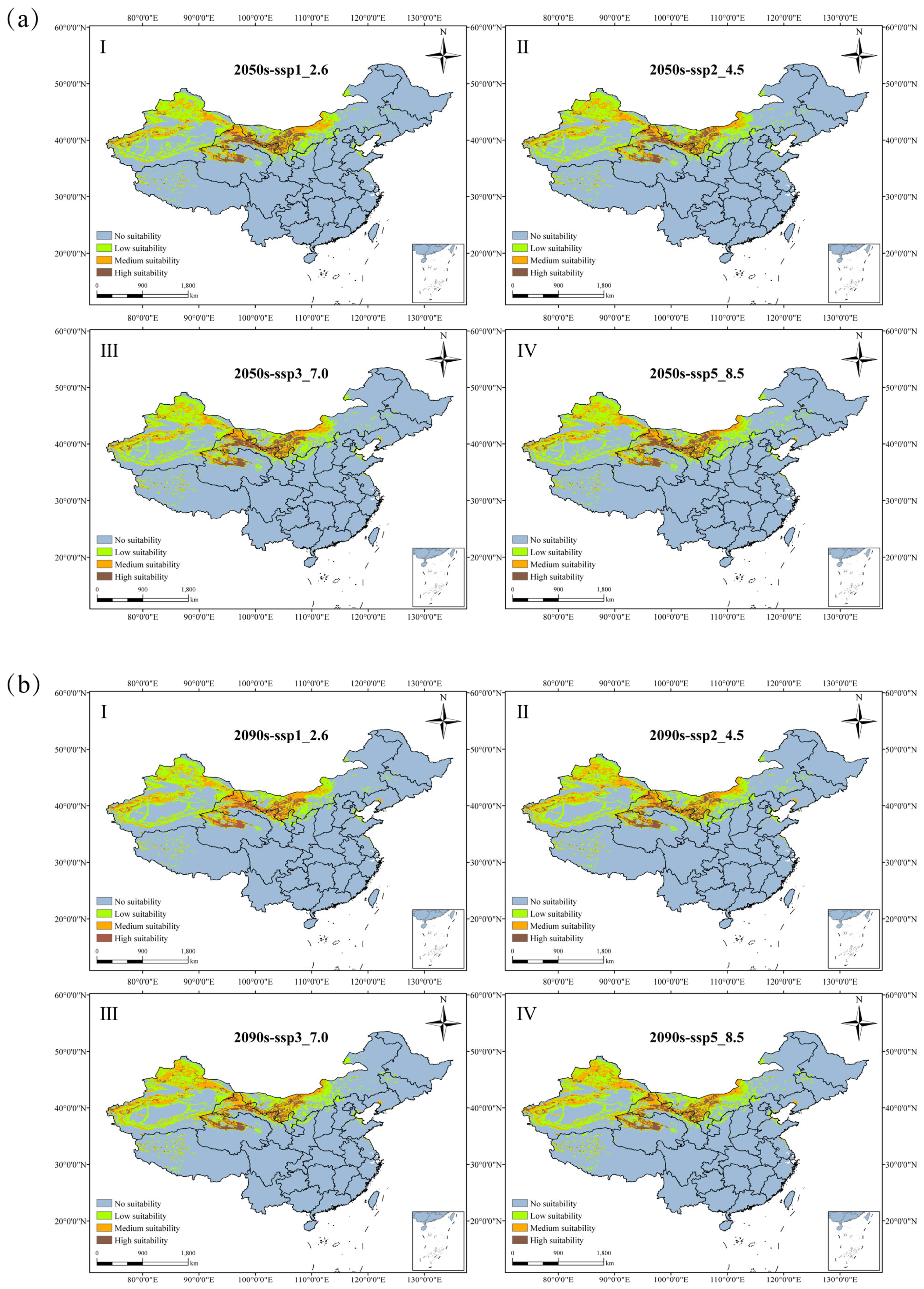
3.4. The Future Suitable Habitat Distribution of N. tangutorum
3.5. Centroid of Suitable Area of N. tangutorum Under Present and Different Future Climate Scenario
4. Discussion
4.1. Accuracy Analysis of the Optimized Model
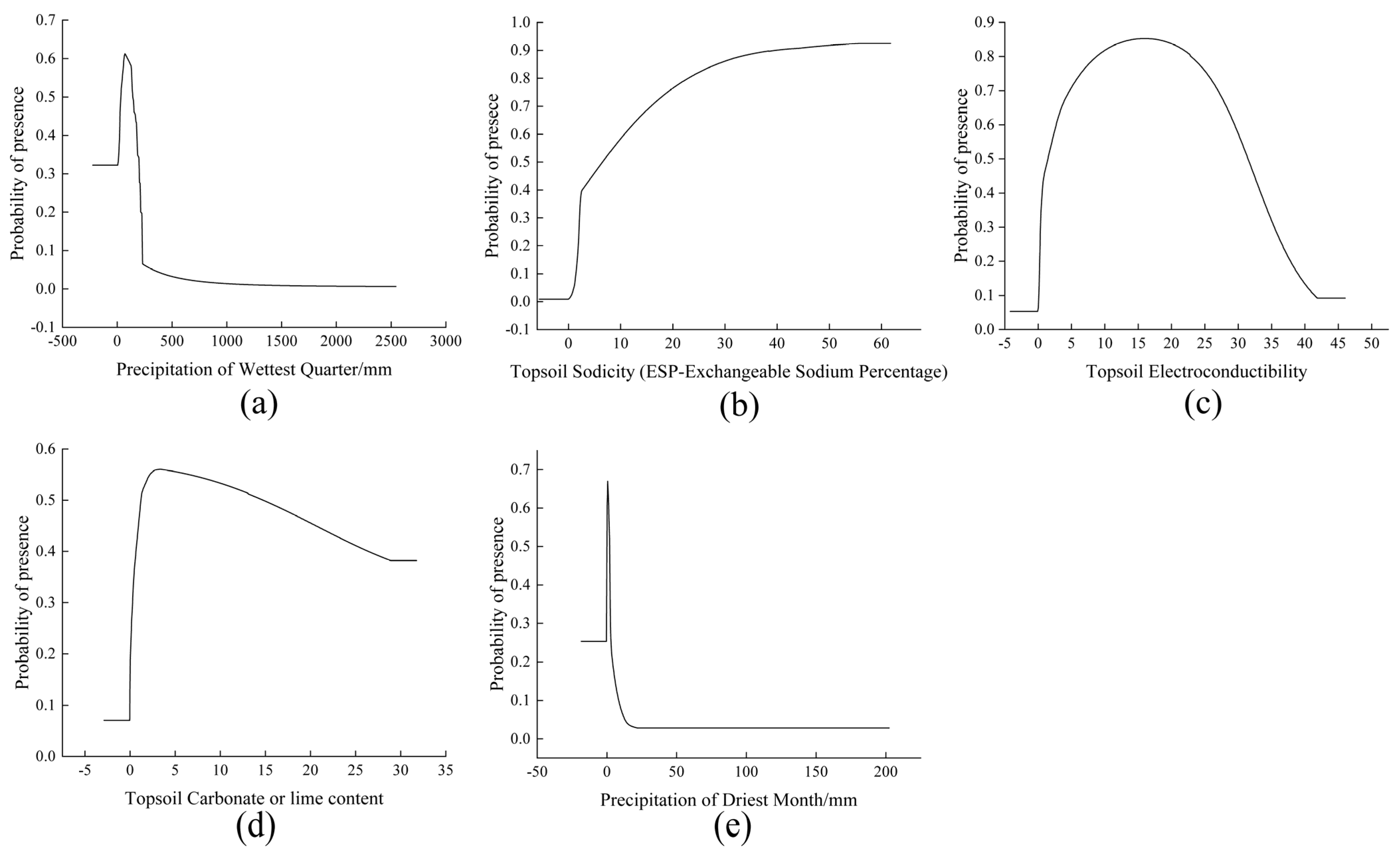
4.2. Effects of Environmental Variables on N. tangutorum Distribution
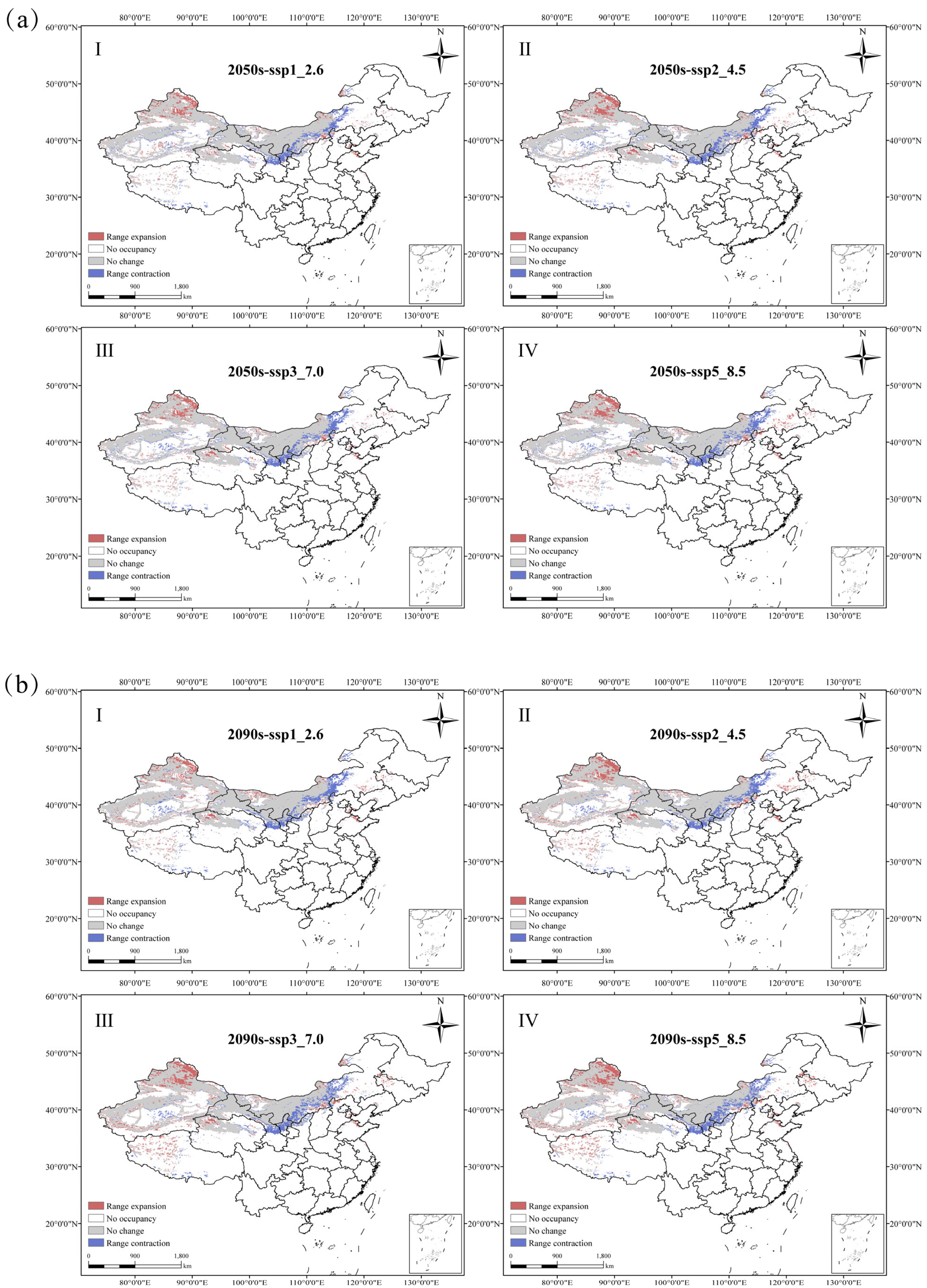
4.3. N. tangutorum Geographic Distribution and Spatial Pattern Change
4.4. Protection Strategies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lawler, J.; Shafer, S.L.; White, D. Projected climate-induced faunal change in the Western Hemisphere. Ecology 2009, 90, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, T.; Zang, G.; Zheng, Y. Prediction of suitable areas of Eremochloa ophiuroides in China under different climate scenarios based on MaxEnt model. J. Beijing For. Univ. 2024, 46, 91–102. (In Chinese) [Google Scholar] [CrossRef]
- Zhong, X.; Lei, C.; Liu, J.; Lin, C.; Qi, Y.; Li, H. FuXi-Extreme: Improving extreme rainfall and wind forecasts with diffusion model. Sci. China Earth Sci. 2024, 54, 3734–3747. (In Chinese) [Google Scholar] [CrossRef]
- Song, Z.; Du, Y.; Primack, R.; Miller-Rushing, A.; Ye, W.; Huang, Z. Surprising roles of climate in regulating flowering phenology in a subtropical ecosystem. Ecography 2021, 44, 1379–1390. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, X.; Mao, K.; Wang, M.; Wang, K.; Xi, Z.; Liu, J. Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. J. Biogeogr. 2018, 45, 1334–1344. [Google Scholar] [CrossRef]
- Crimmins, S.M.; Dobrowski, S.Z.; Greenberg, J.A.; Abatzoglou, J.T.; Mynsberge, A.R. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 2011, 331, 324–327. [Google Scholar] [CrossRef]
- Wiens, J. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 2016, 14, e2001104. [Google Scholar] [CrossRef]
- Hao, T.; Jane, E.; José, J.L.; Gurutzeta, G.A. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Wang, H. Potential geographical distribution of Populus euphratica in China under future climate change scenarios based on MaxEnt model. Acta Ecol. Sin. 2020, 40, 6552–6563. (In Chinese) [Google Scholar]
- Pili, A.; Tingley, R.; Sy, E.; Diesmos, M.; Diesmos, A. Niche shifts and environmental non-equilibrium undermine the usefulness of ecological niche models for invasion risk assessments. Sci. Rep. 2020, 10, 7972. [Google Scholar] [CrossRef]
- Bao, R.; Li, X.; Zheng, J. Feature tuning improves MAXENT predictions of the potential distribution of Pedicularis longiflora Rudolph and its variant. PeerJ 2022, 10, e13337. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Wang, L.; Zhao, J. Nitraria resources in China and their utilization. World For. Res. 2013, 26, 64–68. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.; Wu, B.; Zhu, Y.; Lu, Q.; Li, Y. Responses of Nitraria tangutorum branch morphology to increased rainfall in Minqin, Gansu Province. J. Desert Res. 2012, 32, 709–716. (In Chinese) [Google Scholar]
- Yang, Y.; Yang, F.; Li, X.; Shi, R.; Lu, J. Signal regulation of proline metabolism in callus of the halophyte Nitraria tangutorum Bobr. grown under salinity stress. Plant Cell Tissue Organ Cult. 2013, 112, 33–42. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.; Shi, R.; Fan, Q.; An, L. Salinity-induced physiological modification in the callus from halophyte Nitraria tangutorum Bobr. J. Plant Growth Regul. 2010, 29, 465–476. [Google Scholar] [CrossRef]
- Wang, S.; Kang, X. Current research situation and suggestion on Nitraria tangutorum Bobr. J. Plant Genet. Resour. 2005, 2, 231–235. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, S.; Li, J.; Wang, Y.; Zhang, Q. Hypoglycemic effect of Nitraria tangutorum fruit by inhibiting glycosidase and regulating IRS1/PI3K/AKT signalling pathway and its active ingredient identification by UPLC-MS. Food Funct. 2023, 14, 7869–7881. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Li, X.; Zhang, Y. Analysis and evaluation of forage quality of four Nitraria species. J. Gansu Agric. Univ. 2017, 52, 97–100. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Li, M.; Zhou, Y.; Li, Y. Determination and analysis of nutritional components in Cynomorium songaricum parasitic on two species of Nitraria. J. Gansu Agric. Univ. 2023, 58, 130–136. (In Chinese) [Google Scholar] [CrossRef]
- Gao, X.; Li, Q.; Zhang, L.; Wang, H. Prediction of the potential distribution of raspberry (Rubus idaeus) in China based on MaxEnt model. Sci. Rep. 2024, 14, 24438. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, S.; Chen, S. Predicting the potential habitat suitability of Saussurea species in China under future climate scenarios using the optimized Maximum Entropy (MaxEnt) model. J. Clean. Prod. 2024, 474, 143552. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Jie, W. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, H.; Zhang, W.; Wu, T. Introduction of BCC models and its participation in CMIP6. Clim. Change Res. 2019, 15, 533–539. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Hu, K.; Zeng, A.; Dong, D. Potential distribution of Asystasia gangetica ssp. Micrantha in China based on the MaxEnt ecological niche model. Southwest China J. Agric. Sci. 2024, 37, 860–868. (In Chinese) [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Wang, X.; Zhang, J. Distribution pattern and change prediction of Saposhnikovia divaricate suitable area in China under climate change. Ecol. Indic. 2022, 143, 109311. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Wang, L.; Li, H. Potential distribution of Apocynum based on MaxEnt optimization model. J. Beijing Norm. Univ. (Nat. Sci.) 2022, 59, 377–387. (In Chinese) [Google Scholar] [CrossRef]
- Kass, J.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.; Li, Y.; Liu, Y.; Wang, Y.; Wang, X. Spatial and temporal variations of the potential habitat of Asterothamnus centraliasiaticus on the Qinghai-Tibet Plateau under climate change. Chin. J. Ecol. 2024, 43, 1566–1575. (In Chinese) [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Geng, W.; Zhang, Y.; Liu, X.; Li, J. Prediction of the potential geographical distribution of Betula platyphylla Suk. in China under climate change scenarios. PLoS ONE 2022, 17, e0262540. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Xu, X.; Tong, L. MaxEnt modeling for predicting potential suitable distribution areas of Styrax odoratissimus. Ecol. Sci. 2020, 39, 119–124. (In Chinese) [Google Scholar] [CrossRef]
- Han, M.; Li, X.; Zhang, Y.; Wang, H. Simulation of Elymus sibiricus L. distribution in Tibetan Plateau based on MaxEnt model. Acta Agrestia Sin. 2021, 29, 374–382. (In Chinese) [Google Scholar]
- Wang, P.; Li, J.; Zhang, Q.; Zhao, L. Prediction of the potential distribution of Curcuma in China under current and future climate scenarios. Acta Prataculturae Sin. 2024, 33, 14–27. (In Chinese) [Google Scholar] [CrossRef]
- Elena, A.E. Plant hormesis and Shelford’s tolerance law curve. J. For. Res. 2021, 32, 1789–1802. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Zou, H. Optimizing MaxEnt model in the prediction of species distribution. Chin. J. Appl. Ecol. 2019, 30, 2116–2128. (In Chinese) [Google Scholar] [CrossRef]
- Perlman, P.; Chu, G.; Chang, Y.; Peng, M.; Wang, M. Prediction and analysis of two potential suitable areas of Reaumuria plants in Northwest China based on the maximum entropy (MaxEnt) model. J. Northwest For. Univ. 2020, 35, 18–25. (In Chinese) [Google Scholar] [CrossRef]
- Arman, J.; Nurbay, A.; Maidina, T.; Sayit, H. Predicting the potential geographic distribution and pattern of Tamarix taklamakanensis based on the maximum entropy model (Maxent). Jiangsu Agric. Sci. 2019, 47, 91–94. [Google Scholar] [CrossRef]
- Chang, H.; Liu, T.; Wang, D.; Ji, X. Haloxylon ammodendron’s potential distribution under climate change in arid areas of Northwest China. J. Desert Res. 2019, 39, 110–118. (In Chinese) [Google Scholar]
- Yang, Z.; Gao, Z. Impact of precipitation and underground water level in the edge of oases on growth and decline of Nitraria tangugtorum community. Chin. J. Appl. Ecol. 2000, 6, 923–926. (In Chinese) [Google Scholar] [CrossRef]
- Yao, Z.; Han, Q.; Lin, B. Prediction of distribution area of main noxious and miscellaneous weeds in Xinjiang based on Maxent model. Acta Ecol. Sin. 2023, 12, 5096–5109. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Mao, Y.; Liu, X. Flue gas desulfurization gypsum application for enhancing the desalination of reclaimed tidal lands. Ecol. Eng. 2015, 82, 566–570. [Google Scholar] [CrossRef]
- Fang, H.; Wang, Y.; Zhang, L.; Li, J. Analysis on the potential habitat suitability of Spartina alterniflora based on MaxEnt model. Acta Agrestia Sin. 2023, 31, 3514–3524. (In Chinese) [Google Scholar] [CrossRef]
- Dilmurat, Y.; Arzigul, T.; Mamat, Y. Comparative analysis of salt stress on germination of five Nitraria species. J. Agric. 2018, 8, 19–22. (In Chinese) [Google Scholar]
- Kim, Y.; Choo, B.; Cho, J. Effect of gypsum and rice straw compost application on improvements of soil quality during desalination of reclaimed coastal tideland soils: Ten years of long-term experiments. Catena 2017, 156, 131–138. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Wang, Y.; Liu, B. Biomass allocation and prediction in Nitraria nabkhas. Pratacult. Sci. 2021, 38, 1069–1077. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, W.; Luo, W.; Liu, B. Species diversity and vegetation distribution in nebkhas of Nitraria tangutorum in the desert steppes of China. Ecol. Res. 2015, 30, 735–744. [Google Scholar] [CrossRef]
- Duan, Y.; Zhu, G.; Du, Z.; Li, Y.; Lu, K.; Shi, J. Simulation and analysis of the suitable environment for Nitraria L. in the arid area of Northwest China. J. Arid Land Resour. Environ. 2021, 35, 124–129. (In Chinese) [Google Scholar] [CrossRef]
- Fan, Y.; Wang, L.; Zhang, Q.; Li, J. Prediction of potential suitable distribution area of Pinus bungeana in China under the background of climate change. Arid Zone Res. 2024, 41, 1719–1730. (In Chinese) [Google Scholar] [CrossRef]
- Song, Y.; Tang, X.; Jia, Y.; Liu, X.; Cui, Y. Fertilization effect of carbon dioxide on ecosystems in Gannan, China. Acta Ecol. Sin. 2021, 41, 7301–7311. (In Chinese) [Google Scholar] [CrossRef]
- Chen, S.; Dong, H.; Yue, Y.; Hao, Y.; Liu, X.; Cao, X.; Ma, J. Geographical distribution and dynamic change prediction of Hippophae rhamnoides subsp. sinensis under different climate scenarios. Arid Zone Res. 2024, 41, 1560–1571. (In Chinese) [Google Scholar] [CrossRef]
- Lai, W.; Shi, C.; Wen, G.; Lü, Z.; Ye, L.; Huang, Q.; Zhang, G. Potential impacts of climate change on the distribution of the relict plant Shaniodendron subaequale. Heliyon 2023, 9, e14402. [Google Scholar] [CrossRef]
- Morgan, J.W.; Venn, S.E. Alpine plant species have limited capacity for long-distance seed dispersal. Plant Ecol. 2017, 218, 813–819. [Google Scholar] [CrossRef]
- Zhang, M.; Temirbayeva, K.; Sanderson, S.; Chen, X. Young dispersal of xerophil Nitraria lineages in intercontinental disjunctions of the Old World. Sci Rep. 2015, 5, 13840. [Google Scholar] [CrossRef][Green Version]
- Diamond, S.E.; Frame, A.M.; Martin, R.A.; Buckley, L.B. Species’ traits predict phenological responses to climate change in butterflies. Ecology 2011, 92, 1005–1012. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 275243–275254. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Jing, W.; Wang, Q.; Yan, H.; Qiu, X.; Jian, H. Simulation of climate change effect on the global distribution of Rosa multiflora. Chin. J. Appl. Ecol. 2024, 35, 1897–1906. [Google Scholar] [CrossRef]
- He, X.; Burgess, K.; Gao, L.; Li, D. Distributional responses to climate change for alpine species of Cyananthus and Primula endemic to the Himalaya-Hengduan Mountains. Plant Divers. 2018, 41, 26–32. [Google Scholar] [CrossRef]
- Lv, T.; Liu, Y.; Zhou, Y.; Liu, T.; Zhang, X.; Su, X. Germplasm Collection and Preliminary Studies on Genealogical Differentiation of A Desert Species—Psammochloa villosa. Acta Agrestia Sin. 2018, 26, 733–740. [Google Scholar] [CrossRef]
- Hao, S.; Zhang, D.; Wen, Y. Potential Geographical Distribution of Lagerstroemia excelsa under Climate Change. Agriculture 2024, 14, 191. [Google Scholar] [CrossRef]

| Type | RM | FC | Delta.AICc | Mean.Diff.AUC | Mean.OR10 |
|---|---|---|---|---|---|
| default | 1 | LQHP | 967.58 | 0.052 | 0.21 |
| optimized | 3 | LQHPT | 0 | 0.04 | 0.20 |
| Variables | Description | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Bio16 | Precipitation of Wettest Quarter | 32.4 | 36 |
| T_esp | Topsoil Sodicity (ESP-Exchangeable Sodium Percentage) | 13.7 | 1.4 |
| T_ece | Topsoil Electroconductibility | 9.8 | 1.5 |
| T_CACO3 | Topsoil Carbonate or lime content | 9.2 | 10.2 |
| Bio14 | Precipitation of Driest Month | 6.3 | 5.3 |
| Dem | Elevation | 6 | 8.8 |
| Bio11 | Mean Temperature of Coldest Quarter | 5.2 | 9.5 |
| Bio4 | Temperature Seasonality | 5.2 | 1.5 |
| T_bs | Basic saturation | 3.6 | 6 |
| Bio15 | Precipitation Seasonality | 2.6 | 1.7 |
| T_sand | Topsoil sand content | 2 | 7.8 |
| T_silt | Topsoil silt content | 1.5 | 5.2 |
| Slope | Slope variability | 1.3 | 1.7 |
| T_clay | Topsoil clay content | 1 | 3.4 |
| Variable | Suboptimal Ranges | Optimal Ranges |
|---|---|---|
| Precipitation of Wettest Quarter (Bio16) | 0.96–200.20 mm | 34.17–139.32 mm |
| Topsoil Sodicity (ESP-Exchangeable Sodium Percentage) (T_esp) | 2.13–61.74% | 6.58–61.74% |
| Topsoil Electroconductibility (T_ece) | 0.39–35.40 dS·m−1 | 1.49–31.43 dS·m−1 |
| Topsoil Carbonate or lime content (T_CACO3) | 0.30–31.75% | 1.20–14.71% |
| Precipitation of Driest Month (Bio14) | 0.15–2.35 mm | 0.15–1.91 mm |
| Period | Climate Scenarios | Low Suitable Area | Medium Suitable Area | High Suitable Area | Total Suitable Area | Compare with the Current Increase |
|---|---|---|---|---|---|---|
| Current | 108.35 | 57.73 | 24.21 | 190.30 | 0 | |
| 2050s | ssp126 | 105.11 | 53.91 | 26.34 | 185.35 | −2.59% |
| ssp245 | 104.58 | 55.13 | 26.56 | 186.27 | −2.12% | |
| ssp370 | 103.88 | 53.87 | 27.07 | 184.82 | −2.88% | |
| ssp585 | 106.47 | 54.82 | 27.39 | 188.68 | −0.85% | |
| 2090s | ssp126 | 101.77 | 54.30 | 22.76 | 178.83 | −6.02% |
| ssp245 | 101.69 | 62.92 | 24.24 | 188.85 | −0.76% | |
| ssp370 | 103.05 | 64.10 | 25.38 | 192.54 | 1.18% | |
| ssp585 | 103.56 | 65.72 | 25.77 | 195.05 | 2.50% |
| Period | No Change Area | No Change Rate (%) | Range Contraction Area | Range Contraction Rate (%) | Range Expansion Area | Range Expansion Rate (%) | |
|---|---|---|---|---|---|---|---|
| 2050s | SSP126 | 153.84 | 80.85 | 23.68 | 12.44 | 19.10 | 10.03 |
| SSP245 | 151.13 | 79.42 | 26.39 | 13.87 | 20.68 | 10.87 | |
| SSP370 | 151.50 | 79.61 | 26.02 | 13.67 | 18.75 | 9.85 | |
| SSP585 | 151.58 | 79.66 | 25.94 | 13.63 | 22.16 | 11.64 | |
| 2090s | SSP126 | 151.12 | 79.41 | 26.40 | 13.87 | 19.21 | 10.09 |
| SSP245 | 152.08 | 79.92 | 25.44 | 13.37 | 23.56 | 12.38 | |
| SSP370 | 151.84 | 79.79 | 25.68 | 13.5 | 27.37 | 14.38 | |
| SSP585 | 151.23 | 79.47 | 26.30 | 13.82 | 28.31 | 14.88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jia, Z.; He, L.; Han, D.; Yang, Q.; Li, J.; Zhou, P. Impacts of Climate Change on the Spatial Distribution and Habitat Suitability of Nitraria tangutorum. Plants 2025, 14, 1446. https://doi.org/10.3390/plants14101446
Li L, Jia Z, He L, Han D, Yang Q, Li J, Zhou P. Impacts of Climate Change on the Spatial Distribution and Habitat Suitability of Nitraria tangutorum. Plants. 2025; 14(10):1446. https://doi.org/10.3390/plants14101446
Chicago/Turabian StyleLi, Lianxing, Zhiqing Jia, Lingxianzi He, Dong Han, Qiankun Yang, Jialuo Li, and Pingyi Zhou. 2025. "Impacts of Climate Change on the Spatial Distribution and Habitat Suitability of Nitraria tangutorum" Plants 14, no. 10: 1446. https://doi.org/10.3390/plants14101446
APA StyleLi, L., Jia, Z., He, L., Han, D., Yang, Q., Li, J., & Zhou, P. (2025). Impacts of Climate Change on the Spatial Distribution and Habitat Suitability of Nitraria tangutorum. Plants, 14(10), 1446. https://doi.org/10.3390/plants14101446






