Agro-Industrial Waste from Pistacia vera: Chemical Profile and Bioactive Properties
Abstract
1. Introduction
2. Results
2.1. Chemical Profile
2.1.1. Total Phenolic (TPC), Flavonoid Content (FC), and Antioxidant Activity
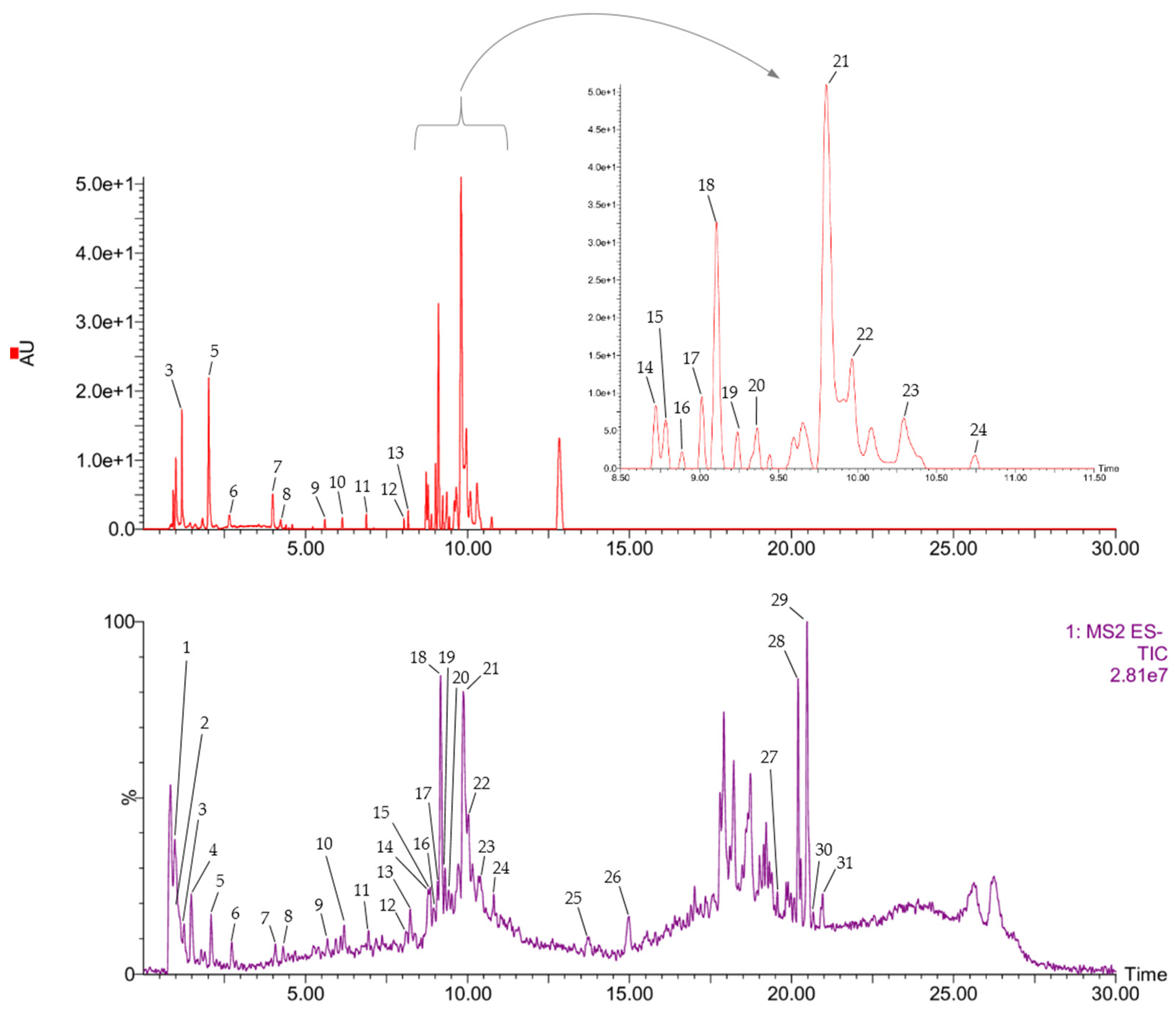
2.1.2. UPLC-DAD-ESI-MS/MS Analysis
2.2. Nematicidal and Anticholinesterases Activities
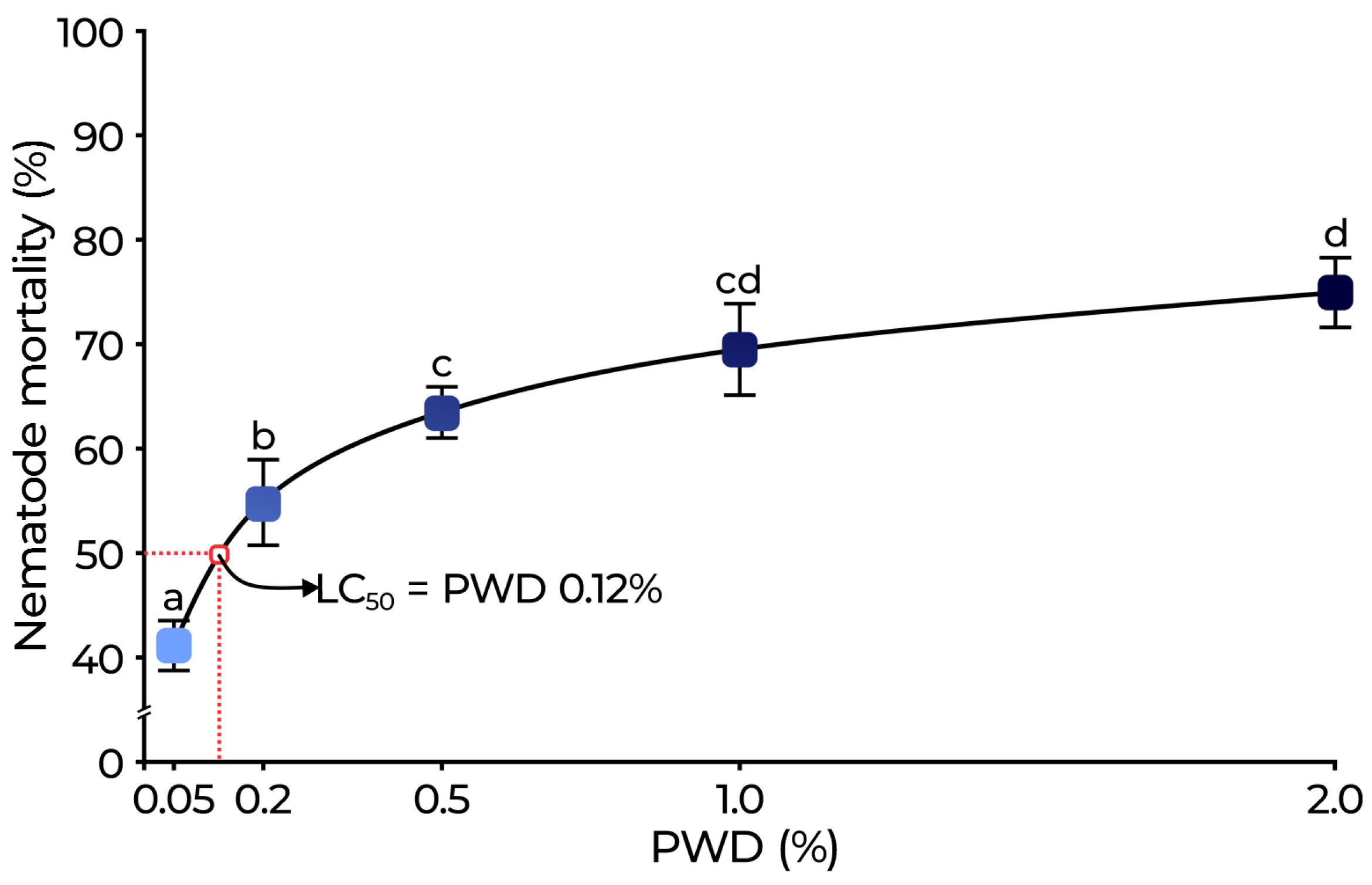
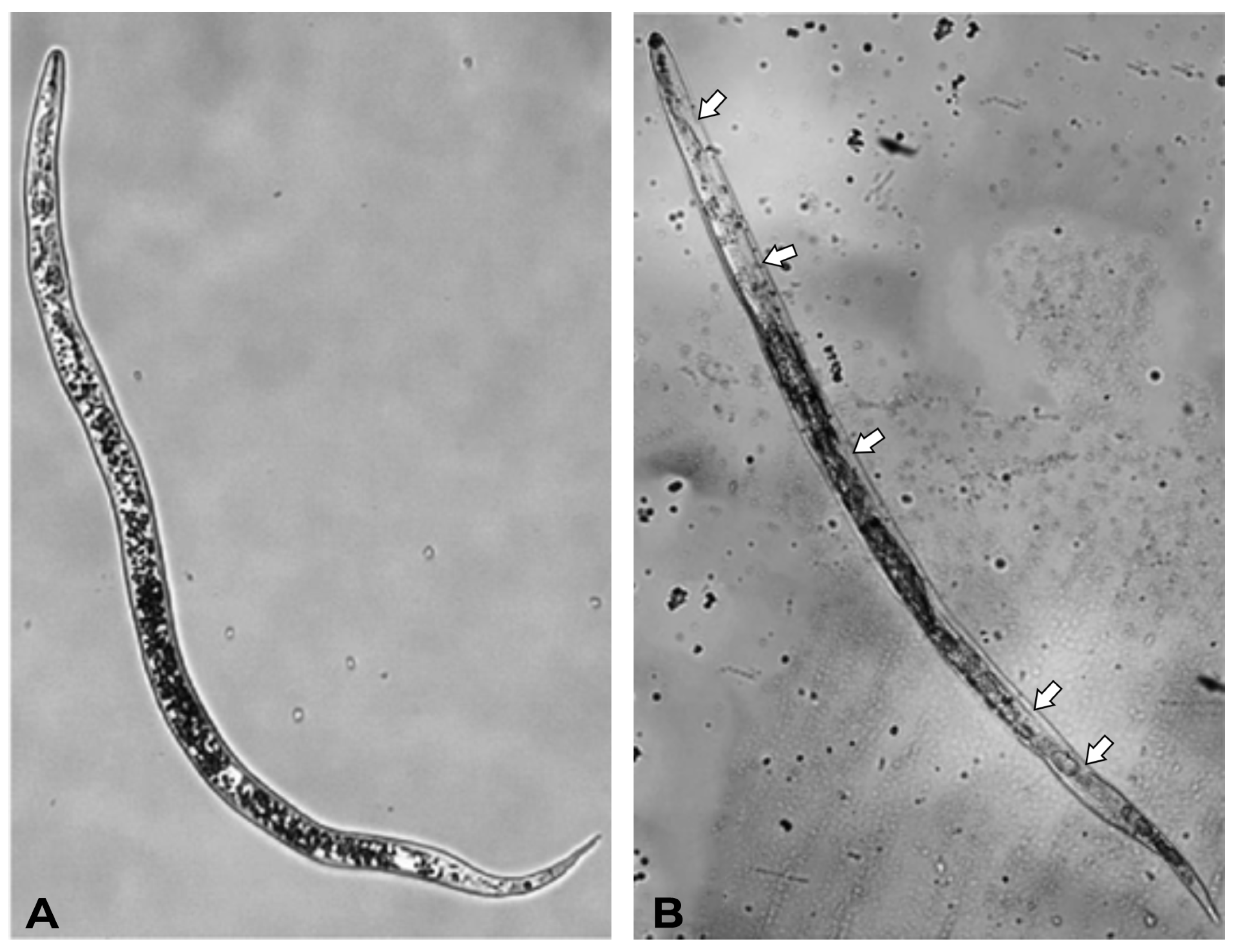
2.2.1. Nematicidal Activity
2.2.2. Anticholinesterases Activity
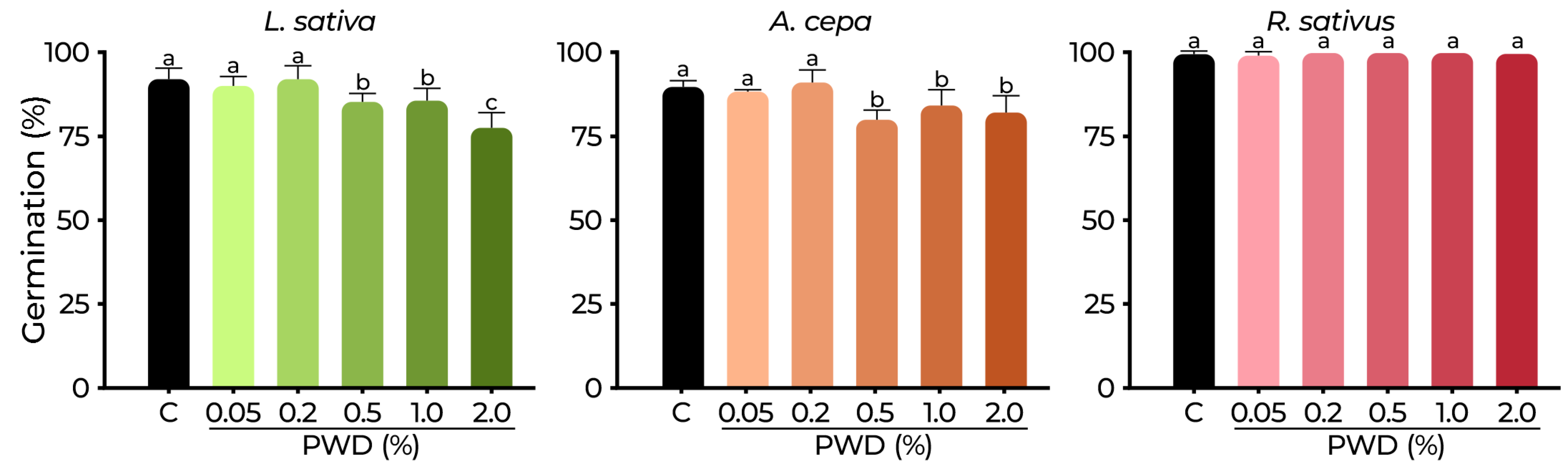
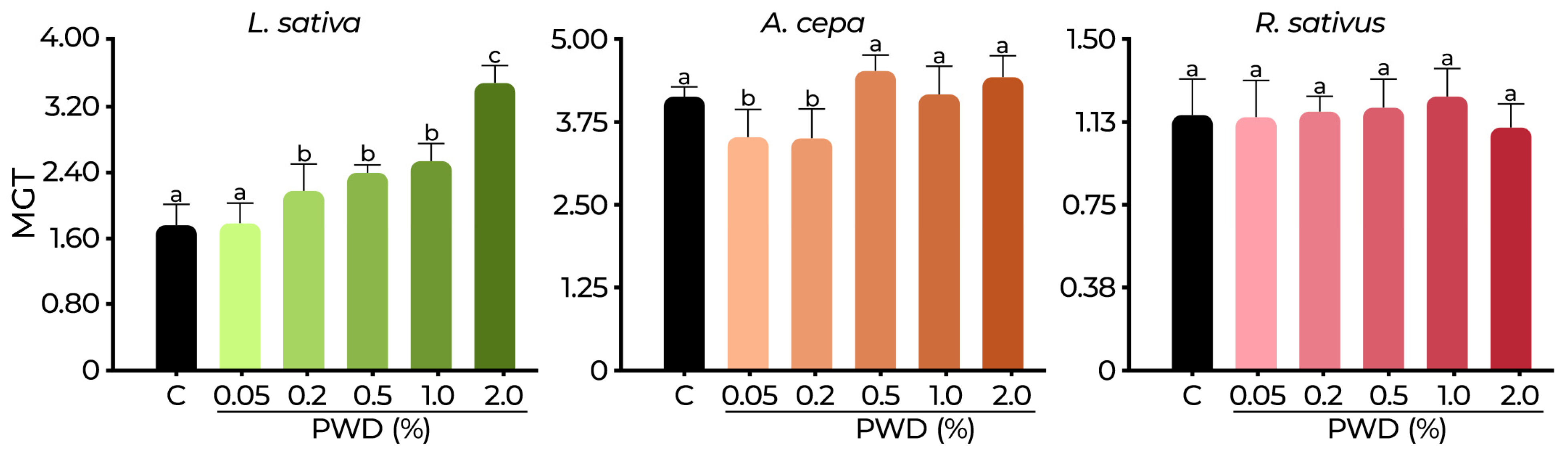
2.3. Phytotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Samples and Extraction Procedure
4.3. UPLC-DAD-ESI-MS/MS Analysis
4.4. Assessment of Total Phenolic Content (TPC) and Flavonoid Content (FC)
4.5. Antioxidant Activity (DPPH, ABTS, and FRAP Assays)
4.6. Nematicidal Activity
4.7. Cholinesterase Inhibitory Activities
4.8. Phytotoxic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. Chemical composition, antioxidant and anti-inflammatory properties of pistachio hull extracts. Food Chem. 2016, 210, 85–95. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Crops and Livestock Products; FAO: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 17 March 2025).
- Toghiani, J.; Fallah, N.; Nasernejad, B.; Mahboubi, A.; Taherzadeh, M.J.; Afsham, N. Production of protein-rich fungal biomass from pistachio dehulling waste using edible Neurospora intermedia. Sci. Rep. 2025, 15, 5873. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Maccarronello, A.E.; Cardullo, N.; Silva, A.M.; Di Francesco, A.; Costa, P.C.; Rodrigues, F.; Muccilli, V. From waste to bioactive compounds: A response surface methodology approach to extract antioxidants from Pistacia vera shells for postprandial hyperglycaemia management. Food Chem. 2024, 443, 138504. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Abbas, M.; Zia, S.; Maan, A.A.; Khan, M.K.I.; Hassoun, A.; Aadil, R.M. An appealing review of industrial and nutraceutical applications of pistachio waste. Crit. Rev. Food Sci. Nutr. 2024, 64, 3103–3121. [Google Scholar] [CrossRef]
- Din, M.S.U.; Mubeen, M.; Hussain, S.; Ahmad, A.; Hussain, N.; Ali, M.A.; Nasim, W. World nations priorities on climate change and food security. Build. Clim. Resil. Agric. 2022, 365, 365–384. [Google Scholar]
- Mokhtarpour, A.; Naserian, A.A.; Valizadeh, R.; Mesgaran, M.D.; Pourmollae, F. Extraction of phenolic compounds and tannins from pistachio by-products. Ann. Res. Rev. Biol. 2014, 4, 1330. [Google Scholar] [CrossRef]
- Zalazar-García, D.; Feresin, G.E.; Rodriguez, R. Optimal operational variables of phenolic compound extractions from pistachio industry waste (Pistacia vera var. Kerman) using the response surface method. Biomass Convers. Biorefin. 2022, 12, 3761–3770. [Google Scholar] [CrossRef]
- Goli, A.; Barzegar, M.; Sahari, M. Antioxidant Activity and Total Phenolic Compounds of Pistachio (Pistacia vera) Hull Extracts. Food Chem. 2005, 92, 521–525. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., Variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2022, 11, 18. [Google Scholar] [CrossRef]
- Akyuz, M.; Yabo, L. The Chemical Composition and Antidiabetic, Neuroprotective and Cytotoxic Activities of Soft Hulls (Mesocarp) of Pistachio (Pistacia vera) Fruits. J. Chem. Soc. Pak. 2023, 45, 551–567. [Google Scholar]
- Mokhtarpour, A.; Naserian, A.A.; Tahmasbi, A.M.; Valizadeh, R. Effect of feeding pistachio by-products silage supplemented with polyethylene glycol and urea on Holstein dairy cows performance in early lactation. Livest. Sci. 2012, 148, 208–213. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Upgrading Strategies for Managing Nematode Pests on Profitable Crops. Plants 2024, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Zhao, J.T.; Zhao, W.; Chi, Y.K.; Ali, Q.; Ali, F.; Khan, A.R.; Yu, Q.; Yu, J.W.; Wu, W.C.; et al. Biocontrol of plant parasitic nematodes by bacteria and fungi: A multi-omics approach for the exploration of novel nematicides in sustainable agriculture. Front. Microbiol. 2024, 15, 1433716. [Google Scholar] [CrossRef]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef]
- Soliman, G.M.; Mohamed, S.A.; Emam, M.T.; Hagagg, L.F.; Hassan, A.M.; El-Hady, E.S. Impact of Genetically Modified Bacteria and Natural Rocks on Root-Knot Nematode Bio-Control, Improving Productivity and Chemical Properties of Grapes. Egypt. J. Chem. 2024, 67, 457–474. [Google Scholar] [CrossRef]
- Holden-Dye, L.; Walker, R.J. Neurobiology of plant parasitic nematodes. Invert. Neurosci. 2011, 11, 9–19. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.; Kesba, H.H.; Mohamed, H.G.; Kamel, D.F.; Ahmed, F.S. Sublethal concentrations of conventional nematicides alter the physiological activities of Meloidogyne incognita and suppress parasitism. Sci. Rep. 2023, 13, 229. [Google Scholar] [CrossRef]
- Almutairi, F.M.; Khan, A.; Ajmal, M.R.; Khan, R.H.; Khan, M.F.; Lal, H.; Ullah, M.F.; Ahmad, F.; Ahamad, L.; Khan, A.; et al. Phytochemical Analysis and Binding Interaction of Cotton Seed Cake Derived Compounds with Target Protein of Meloidogyne incognita for Nematicidal Evaluation. Life 2022, 12, 2109. [Google Scholar] [CrossRef]
- Moeini, R.; Memariani, Z.; Asadi, F.; Bozorgi, M.; Gorji, N. Pistacia genus as a potential source of neuroprotective natural products. Planta Med. 2019, 85, 1326–1350. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.; Srivastava, A. Production of biopesticides from agricultural waste as an alternative to chemical pesticides. In Agro-Waste to Microbe Assisted Value Added Product: Challenges and Future Prospects: Recent Developments in Agro-Waste Valorization Research; Springer Nature: Cham, Switzerland, 2024; pp. 365–379. [Google Scholar]
- Priac, A.; Badot, P.M.; Crini, G. Treated wastewater phytotoxicity assessment using Lactuca sativa: Focus on germination and root elongation test parameters. C. R. Biol. 2017, 340, 188–194. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef]
- Batool, M.; El-Badri, A.M.; Wang, C.; Mohamed, I.A.; Wang, Z.; Khatab, A.; Zhou, G. The role of storage reserves and their mobilization during seed germination under drought stress conditions of rapeseed cultivars with high and low oil contents. Crop Environ. 2022, 1, 231–240. [Google Scholar] [CrossRef]
- Ersan, S.; Üstündağ, Ö.G.; Carle, R.; Schweiggert, R.M. Identification of phenolic compounds in red and green pistachio (Pistacia vera L.) hulls (exo- and mesocarp) by HPLC-DAD-ESI-(HR)-MS. J. Agric. Food Chem. 2016, 64, 5334–5344. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, M.; Escribano-Bailón, M.T.; Guenni, K.; Salhi Hannachi, A.; Dueñas, M. Qualitative and quantitative analyses of phenolic compounds by HPLC–DAD–ESI/MS in Tunisian Pistacia vera L. Leaves unveiled a rich source of phenolic compounds with a significant antioxidant potential. Food Meas. 2019, 13, 2448–2460. [Google Scholar] [CrossRef]
- Saludes-Zanfaño, M.I.; González-Hernández, A.I.; Vivar-Quintana, A.M.; Morales-Corts, M.R. Phytotoxicity of phenolic compounds of Pistacia vera leaves and its potential use as bioherbicide. Crop Prot. 2024, 184, 106812. [Google Scholar] [CrossRef]
- Andres, M.F.; Coloma, A.G. Agro-industrial by-products and waste as sources of biopesticides. In Recent Advances and Prospective in the Biocontrol of Plant Diseases; Dumas, B., Prigent-Combaret, C., Eds.; ITSE: London, UK, 2022. [Google Scholar]
- Kawashty, S.A.; Mosharrafa, S.A.M.; El-Gibali, M.; Saleh, N.A.M. The flavonoids of four Pistacia species in Egypt. Biochem. Syst. Ecol. 2000, 28, 915–917. [Google Scholar] [CrossRef]
- Elakremi, M.; Sillero, L.; Ayed, L.; Ben Mosbah, M.; Labidi, J.; Ben Salem, R.; Moussaoui, Y. Pistacia vera L. leaves as a renewable source of bioactive compounds via microwave-assisted extraction. Sustain. Chem. Pharm. 2022, 29, 100815. [Google Scholar] [CrossRef]
- Saludes-Zanfaño, M.I.; Vivar-Quintana, A.M.; Morales-Corts, M.R. Pistacia root and leaf extracts as potential bioherbicides. Plants 2022, 11, 916. [Google Scholar] [CrossRef]
- Chaves Lobón, N.; González Félix, M.; Alías Gallego, J.C. Comparison of the allelopathic potential of non-native and native species of Mediterranean ecosystems. Plants 2023, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Toghiani, J.; Fallah, N.; Nasernejad, B.; Mahboubi, A.; Taherzadeh, M.J.; Afsham, N. Sustainable pistachio dehulling waste management and its valorization approaches: A review. Curr. Pollut. Rep. 2023, 9, 60–72. [Google Scholar] [CrossRef]
- Bloise, E.; Di Bello, M.P.; Carbone, L.; Mazzetto, S.E.; Mele, G. Anacardic acid: A promising building block for the sustainable preparation of vesicular nanosystems. Waste Biomass Valor. 2021, 12, 4367–4374. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization and comparative evaluation of volatile, phenolic and antioxidant properties of pistachio (Pistacia vera L.) hull. Chem. J. Essent. Oil Res. 2017, 29, 262–270. [Google Scholar] [CrossRef]
- Buyukkurt, K.O.; Guclu, G.; Kelebek, H.; Sellib, S. Comparative elucidation of the phenolic profile and antioxidant and antimicrobial activities of unripe and ripe Pistacia lentiscus L. fruits and their oils as affected by ripening. J. Sci. Food Agric. 2025. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M. Biological screening of Viola betonicifolia Smith whole plant. Afr. J. Pharm. Pharmacol. 2011, 5, 2323–2329. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Reiner, D.A.; Dallemole-Giaretta, R.; dos Santos, I.; Oldoni, T.L.C.; Lopes, E.A.; Chiarani, A. Efeito nematicida de um subproduto da indústria vinícola em Meloidogyne javanica (Treub) Chitwood. Ciênc. Téc. Vitivinícola 2016, 31, 24–30. [Google Scholar] [CrossRef]
- Ardakani, A.S.; Hosseininejad, S.A. Identification of chemical components from essential oils and aqueous extracts of some medicinal plants and their nematicidal effects on Meloidogyne incognita. J. Basic Appl. Zool. 2022, 83, 14. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Jaremko, M.; Abdelsalam, N.R.; Abdelhamid, M.M.; El-Argawy, E.; Ghozlan, M.H. Biocontrol potential of endophytic fungi against phytopathogenic nematodes on potato (Solanum tuberosum L.). Sci. Rep. 2024, 14, 15547. [Google Scholar] [CrossRef]
- Barbosa, P.; Faria, J.M.S.; Cavaco, T.; Figueiredo, A.C.; Mota, M.; Vicente, C.S.L. Nematicidal Activity of Phytochemicals against the Root-Lesion Nematode Pratylenchus penetrans. Plants 2024, 13, 726. [Google Scholar] [CrossRef]
- Wuyts, N.; Swennen, R.; De Waele, D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology 2006, 8, 89–101. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Araujo-Filho, J.H.; Grangeiro, T.B.; Gondim, D.M.; Segalin, J.; Pinto, P.M.; Vasconcelos, I.M. Enhanced synthesis of antioxidant enzymes, defense proteins and leghemoglobin in rhizobium-free cowpea roots after challenging with Meloidogyne incognita. Proteomes 2014, 2, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Hajji-Hedfi, L.; Larayedh, A.; Hammas, N.C.; Regaieg, H.; Horrigue-Raouani, N. Biological activities and chemical composition of Pistacia lentiscus in controlling Fusarium wilt and root-knot nematode disease complex on tomato. Eur. J. Plant Pathol. 2019, 155, 281–291. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.; Kesba, H.H.; Al-Sayed, A.A. Activity and reproductive capability of Meloidogyne incognita and sunflower growth response as influenced by root exudates of some medicinal plants. Biocatal. Agric. Biotechnol. 2019, 22, 101418. [Google Scholar] [CrossRef]
- Khorsandi, K.; Kianmehr, Z.; Hosseinzadeh, R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Njume, F.N.; Razzauti, A.; Soler, M.; Perschin, V.; Fazeli, G.; Bourez, A.; Laurent, P. A lipid transfer protein ensures nematode cuticular impermeability. Iscience 2022, 25, 105357. [Google Scholar] [CrossRef]
- Njom, V.S.; Winks, T.; Diallo, O.; Lowe, A.; Behnke, J.; Dickman, M.J.; Buttle, D.J. The effects of plant cysteine proteinases on the nematode cuticle. Parasites Vectors 2021, 14, 302. [Google Scholar] [CrossRef]
- Djiwanti, S.R.; Kammenga, J.E.; Murk, A.J. Nematicidal activity of plant extracts against the root-knot nematode, Meloidogyne incognita. Open Nat. Prod. J. 2009, 2, 77–85. [Google Scholar]
- Fanelli, E.; Vovlas, A.; D’Addabbo, T.; De Luca, F. Molecular mechanism of Cinnamomum zeylanicum and Citrus aurantium essential oils against the root-knot nematode, Meloidogyne incognita. Sci. Rep. 2025, 15, 6077. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, H.; Bargali, P.; Kumar, R.; Prakash, O.; Kumar, S.; Garzoli, S.; Isidorov, V.A. Comparison of chemical composition, nematicidal activity and acetylcholinesterase inhibition with in silico mechanistic insights of Hedychium flavescens essential oils and extracts from aerial parts and rhizomes. Biochem. Syst. Ecol. 2025, 121, 104987. [Google Scholar] [CrossRef]
- Docampo, M.; Olubu, A.; Wang, X.; Pasinetti, G.; Dixon, R.A. Glucuronidated flavonoids in neurological protection: Structural analysis and approaches for chemical and biological synthesis. J. Agric. Food Chem. 2017, 65, 7607–7623. [Google Scholar] [CrossRef]
- Alsharif, B.; Biasiotti, M.; Bader, A.; Kukula-Koch, W.; Davey, G.P.; Boylan, F. Acetylcholinesterase and monoamine oxidase inhibitory activities of Pistacia falcata Becc. ex Martelli extract. Nat. Prod. Res. 2025, 1–6. [Google Scholar] [CrossRef]
- Römheld, V. Chapter 11—Diagnosis of Deficiency and Toxicity of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 299–312. ISBN 9780123849052. [Google Scholar] [CrossRef]
- Moreno-Robles, A.; Cala Peralta, A.; Soriano, G.; Zorrilla, J.G.; Masi, M.; Vilariño-Rodríguez, S.; Fernández-Aparicio, M. Identification of allelochemicals with differential modes of phytotoxicity against Cuscuta campestris. Agriculture 2022, 12, 1746. [Google Scholar] [CrossRef]
- Alyousef, A.; Ibrahim, G. Inhibitory effect of fruit hull and leaves of pistachio on weed growth in pots. Int. J. PharmTech Res. 2015, 8, 365–369. [Google Scholar]
- Taghvaee, N.; Sadeghi, H. Allelopathic effect of Pistacia khinjuk leaf extracts on Chenopodium album, Physalis alkekengi and Amaran retroflexus. Agric. Adv. 2014, 3, 33–37. [Google Scholar]
- Gimenez Guerrero, M.; Luna, L.C.; Sapag, K.; Feresin, G.E.; Deiana, C. Grape stalks as a renewable source for the production of value-added products: Application of hydrothermal carbonization, analysis of the process and products. Biomass Convers. Biorefin. 2025. [Google Scholar] [CrossRef]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Manrique, S.; Gómez, J.; Piñeiro, M.; Sampietro, B.A.; Peschiutta, M.L.; Tapia, A.; Lima, B. Zuccagnia punctata Cav., a potential environmentally friendly and sustainable bionematicide for the control of Argentinean horticultural crops. Plants 2023, 12, 4104. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat 2021; InfoStat Transfer Center, FCA, National University of Córdoba: Córdoba, Argentina, 2021; Available online: http://www.infostat.com.ar (accessed on 7 March 2025).
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Parera, V.; Parera, C.A.; Feresin, G.E. Germination and early seedling growth of high Andean native plants under heavy metal stress. Diversity 2023, 15, 824. [Google Scholar] [CrossRef]
- Brenchley, J.L.; Probert, R.J. Seed germination responses to some environmental factors in the seagrass Zostera capricorni from eastern Australia. Aquat. Bot. 1998, 62, 177–188. [Google Scholar] [CrossRef]
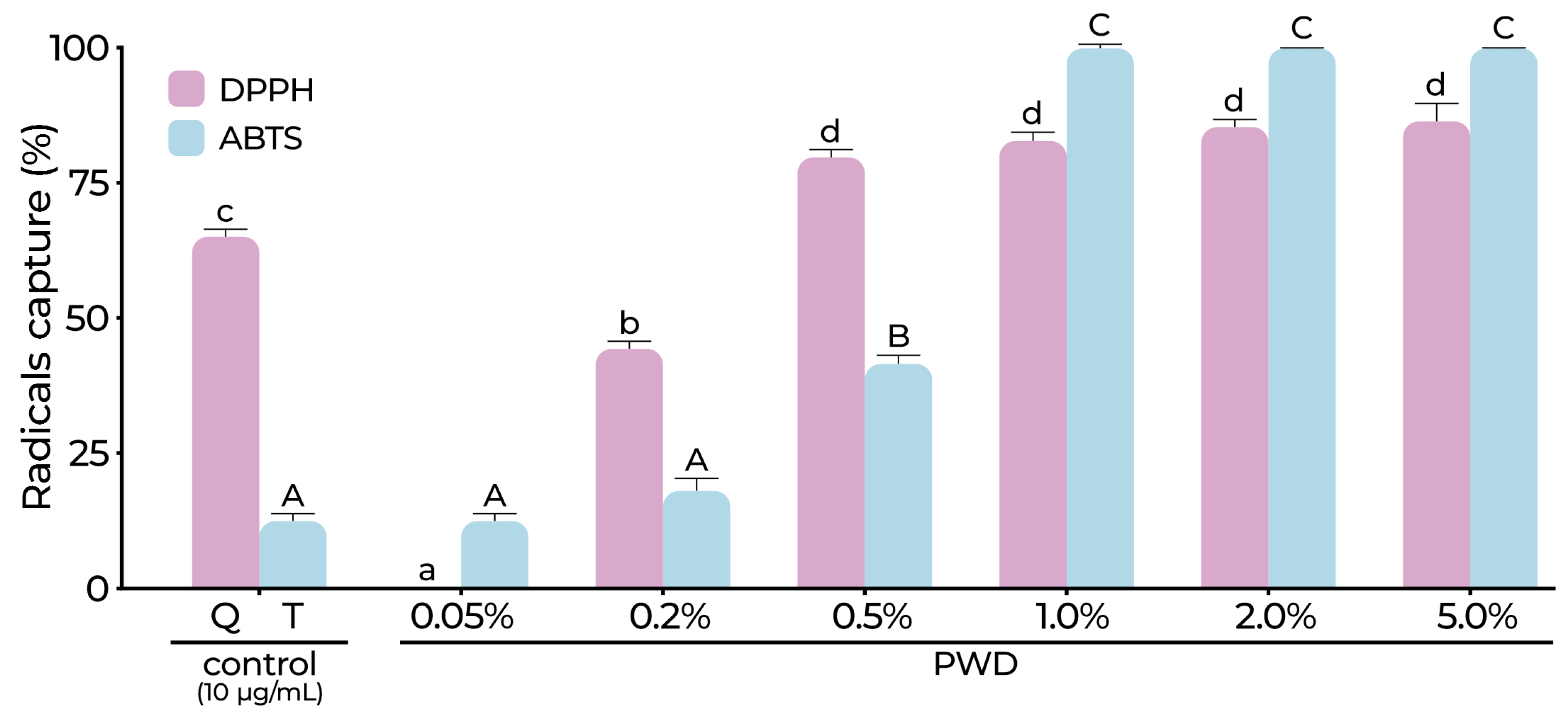


| TPC a | FC b | FRAP c |
|---|---|---|
| (mg GAE/g dPW) | (mg QE/g dPW) | (mM TE/g dPW) |
| 212.65 ± 21.93 | 0.022 ± 0.004 | 7.52 ± 0.19 |
| Peak | RT (min) | [M-H+]− | ESI-MS Fragmentation | UV-Vis (λ) | Tentative Identification | Ref. |
|---|---|---|---|---|---|---|
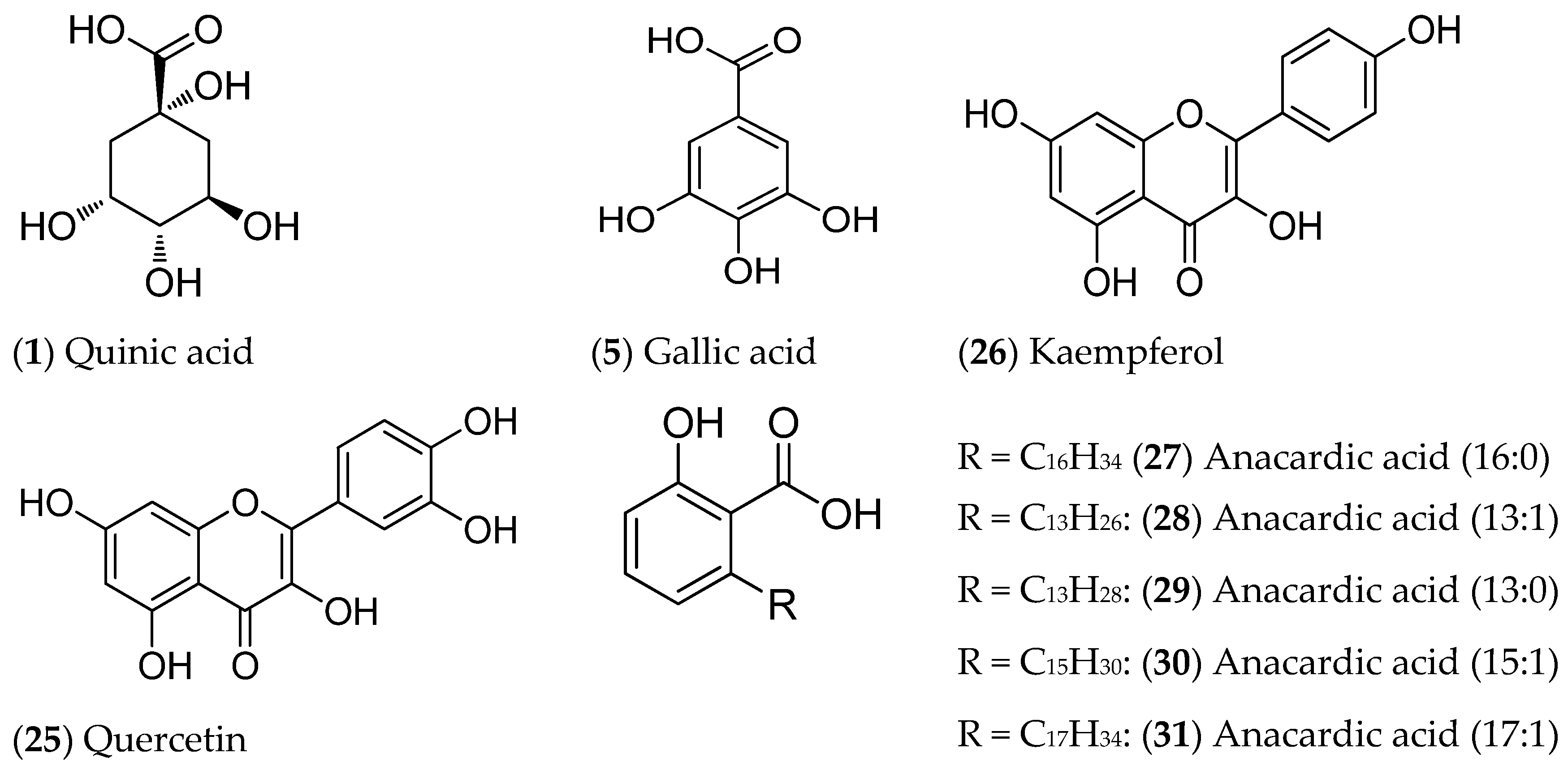
| 1 | 0.97 | 191(100) | 171(22), 127(12), 85(10) | n.d. | Quinic acid | [5,27] |
| 2 | 1.08 | 133(100) | 71(61), 115(50), 89(10) | n.d. | Malic acid | [5,27] |
| 3 | 1.26 | 171(100) | 109(60), 127(58) | 239 | Unidentified | [5,27] |
| 4 | 1.48 | 427(45) | 111(100), 191(90), 87(85) | n.d. | Quinic acid derivative | [28] |
| 5 | 2.09 | 169(100) | 125(98), 97(20), 107(6) | 271 | Gallic acid | [28] |
| 6 | 2.71 | 495(5) | 191(100), 343(25) | 274 | Digalloyl quinic acid | [27,28] |
| 7 | 4.07 | 153(40) | 109(100), 125(5) | 260 | Protocatechuic acid | [27] |
| 8 | 4.30 | 331(52) | 169(100), 125(15) | 260 | 1-O-galloyl-β-D-glucose | [27] |
| 9 | 5.71 | 495(35) | 343(100), 191(50) | 277 | Digalloyl quinic acid | [27,28] |
| 10 | 6.17 | 635(30) | 423(100), 465(12), 483(5) | 279 | Tri-O-galloyl-glucose isomer | [5] |
| 11 | 6.95 | 483(10) | 163(100), 325(30), 199(18) | 282 | Unidentified | |
| 12 | 8.12 | 787(100) | 465(15), 635(10), 617(5) | 276 | Tetragalloyl hexose | [5,27] |
| 13 | 8.24 | 787(75) | 631(100), 463(10) | 272, 359 | Unidentified | |
| 14 | 8.78 | 635(10) | 317(100), 493(52), 515(30) | 264, 300, 357 | Unidentified | |
| 15 | 8.85 | 479(100) | 316(28), 392(8) | 266, 292, 358 | Myricetin-3-O-hexoside | [28] |
| 16 | 8.94 | 625(18) | 479(100), 316(23) | 266, 290, 357 | Myricetin-3-O-hexoside | [28] |
| 17 | 9.08 | 939(70) | 469(100), 169(40), 625(29) | 280 | Pentagalloyl glucose isomer | [5,27] |
| 18 | 9.16 | 615(100) | 463(6), 313(4), 301(2) | 264, 290, 354 | Quercetin galloyl hexoside | [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] |
| 19 | 9.31 | 615(100) | 393(12), 463(8), 217(5) | 267, 356 | Unidentified | |
| 20 | 9.44 | 939(15) | 469(100), 393(16), 615(15) | 279 | Pentagalloyl glucose isomer | [5,27] |
| 21 | 9.87 | 477(60) | 301(100) | 257, 355 | Quercetin glucuronide | [28] |
| 22 | 10.04 | 609(20) | 463(100), 301(60), 545(12) | 266, 355 | Quercetin 3-O-rhamnoside-7-O-glucoside | [5,28] |
| 23 | 10.39 | 767(100) | 263(50), 463(12), 615(5) | 286, 398 | Myricetin digalloyl rhamnoside | [28] |
| 24 | 10.81 | 447(100) | 285(20), 284(18), 151(15) | 269, 355 | Kaempferol hexoside | [27,28] |
| 25 | 13.74 | 301(100) | 151(80), 217(25), 179(20) | n.d. | Quercetin | [5] |
| 26 | 14.98 | 285(100) | 199(5), 151(5), 175(5) | n.d. | Kaempferol | [5] |
| 27 | 19.56 | 361(100) | 299(25), 219(20), 317(20) | n.d. | (16:0) Anacardic acid | [5,27] |
| 28 | 20.20 | 317(70) | 273(100) | n.d. | (13:1) Anacardic acid | [5,27] |
| 29 | 20.47 | 319(55) | 275(100) | n.d. | (13:0) Anacardic acid | [5,27] |
| 30 | 20.52 | 345(80) | 301(100) | n.d. | (15:1) Anacardic acid | [5,27] |
| 31 | 20.95 | 373(80) | 329(100) | n.d. | (17:1) Anacardic acid | [5,27] |
| PWD (%) | % Inhibitory (Mean ± SD) | |
|---|---|---|
| AChE | BuChE | |
| 0.05 | nd | nd |
| 0.2 | nd | nd |
| 0.5 | nd | nd |
| 1.0 | 12.23 ± 1.27 | 6.78 ± 1.11 |
| 2.0 | 42.65 ± 2.15 | 58.90 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñeiro, M.; Parera, V.; Ortiz, J.E.; Llalla-Cordova, O.; Manrique, S.; Castro, B.; Ighani, M.; Luna, L.C.; Feresin, G.E. Agro-Industrial Waste from Pistacia vera: Chemical Profile and Bioactive Properties. Plants 2025, 14, 1420. https://doi.org/10.3390/plants14101420
Piñeiro M, Parera V, Ortiz JE, Llalla-Cordova O, Manrique S, Castro B, Ighani M, Luna LC, Feresin GE. Agro-Industrial Waste from Pistacia vera: Chemical Profile and Bioactive Properties. Plants. 2025; 14(10):1420. https://doi.org/10.3390/plants14101420
Chicago/Turabian StylePiñeiro, Mauricio, Victoria Parera, Javier E. Ortiz, Olimpia Llalla-Cordova, Sofia Manrique, Brisa Castro, Maximiliano Ighani, Lorena C. Luna, and Gabriela E. Feresin. 2025. "Agro-Industrial Waste from Pistacia vera: Chemical Profile and Bioactive Properties" Plants 14, no. 10: 1420. https://doi.org/10.3390/plants14101420
APA StylePiñeiro, M., Parera, V., Ortiz, J. E., Llalla-Cordova, O., Manrique, S., Castro, B., Ighani, M., Luna, L. C., & Feresin, G. E. (2025). Agro-Industrial Waste from Pistacia vera: Chemical Profile and Bioactive Properties. Plants, 14(10), 1420. https://doi.org/10.3390/plants14101420








