Melatonin-Mediated Regulation of Antioxidant Defense Enhances the Resistance of Tea Plants (Camellia sinensis L.) to Lead-Induced Stress
Abstract
1. Introduction
2. Results
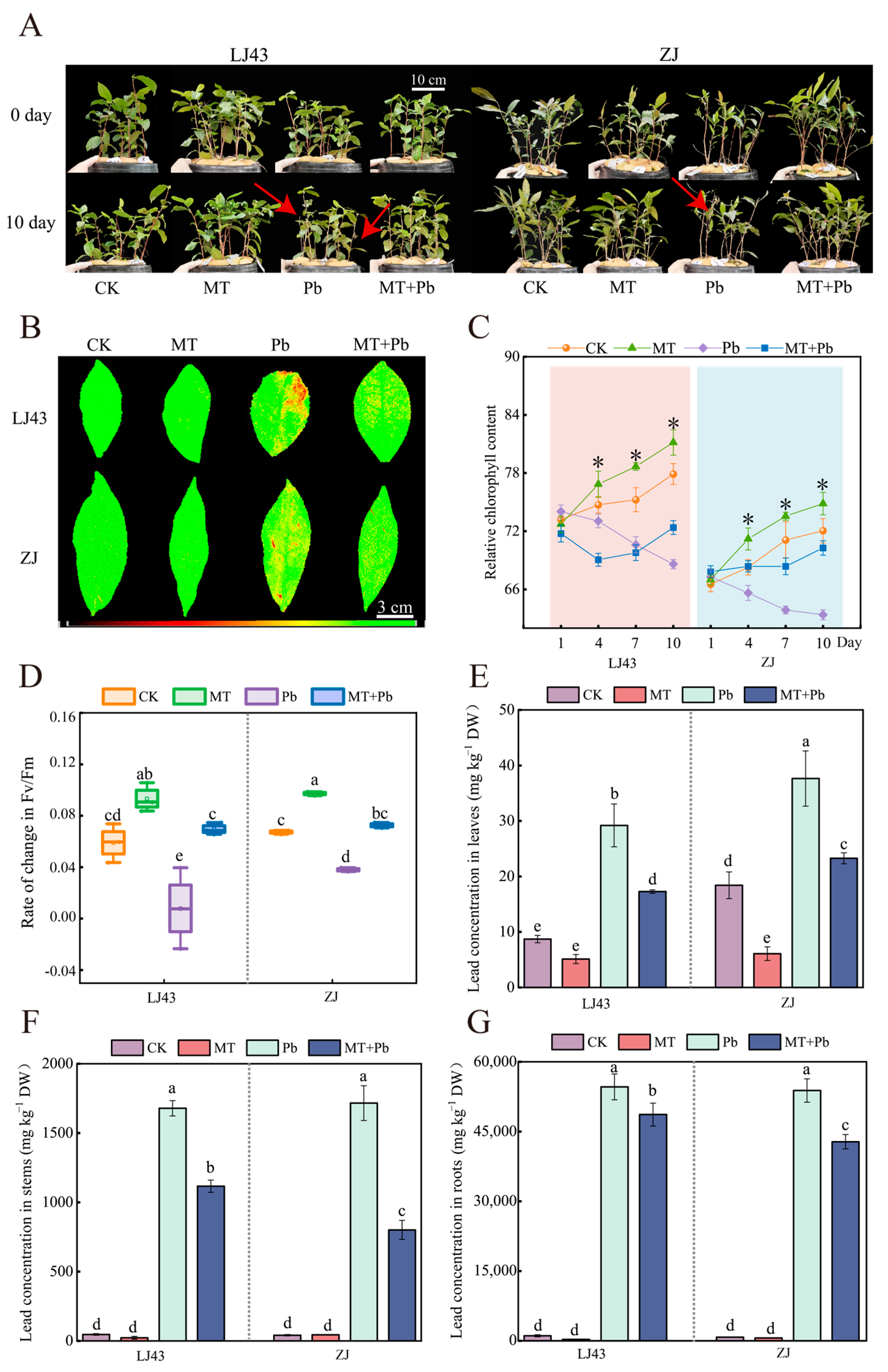
2.1. Exogenous Melatonin Alleviates Pb Stress in Tea Plants by Reducing Pb Accumulation and Enhancing Photosynthetic Performance
2.2. Exogenous Melatonin Enhances Antioxidant Defense to Mitigate Pb-Induced Oxidative Damage in Tea Plant Cells
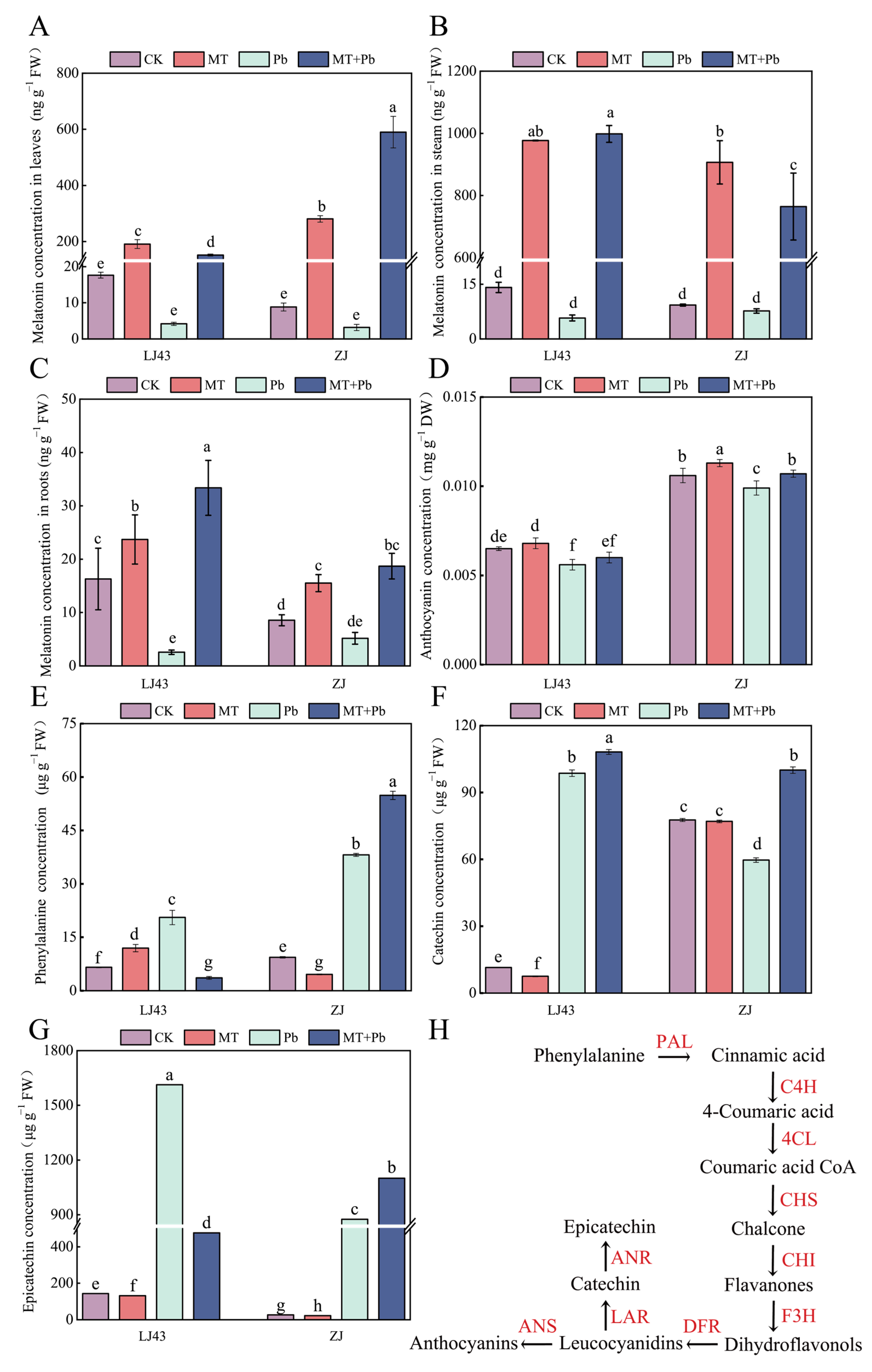
2.3. Exogenous Melatonin Regulates Osmotic Regulators and Secondary Metabolites, Enhancing the Tolerance of Tea Plants to Pb Stress
2.4. Exogenous Melatonin Enhances the Anthocyanin and Catechin Synthesis and Reduces the Pb Transportation
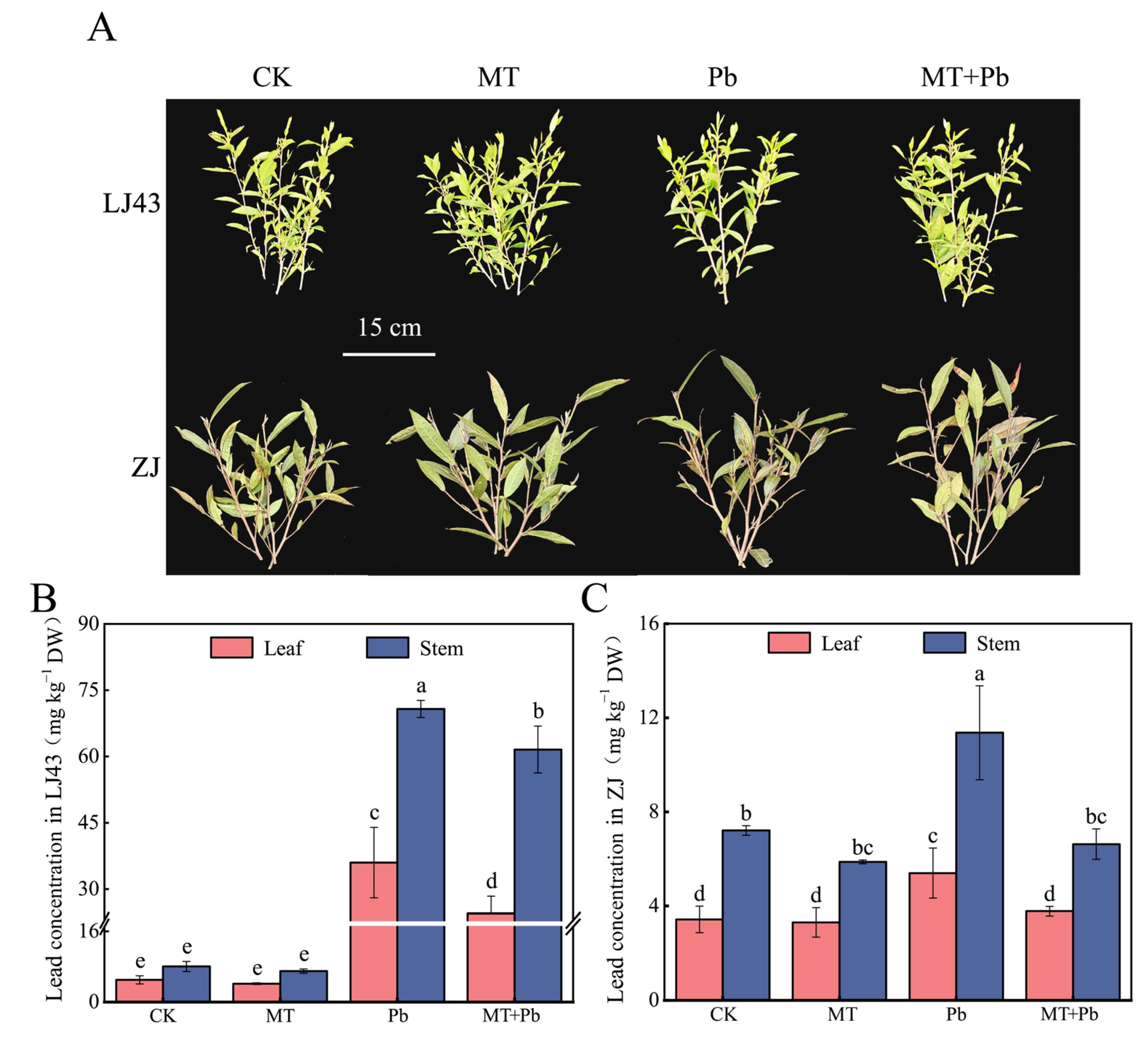
2.5. Melatonin Reduced Pb Accumulation in Tea Leaves in the Field
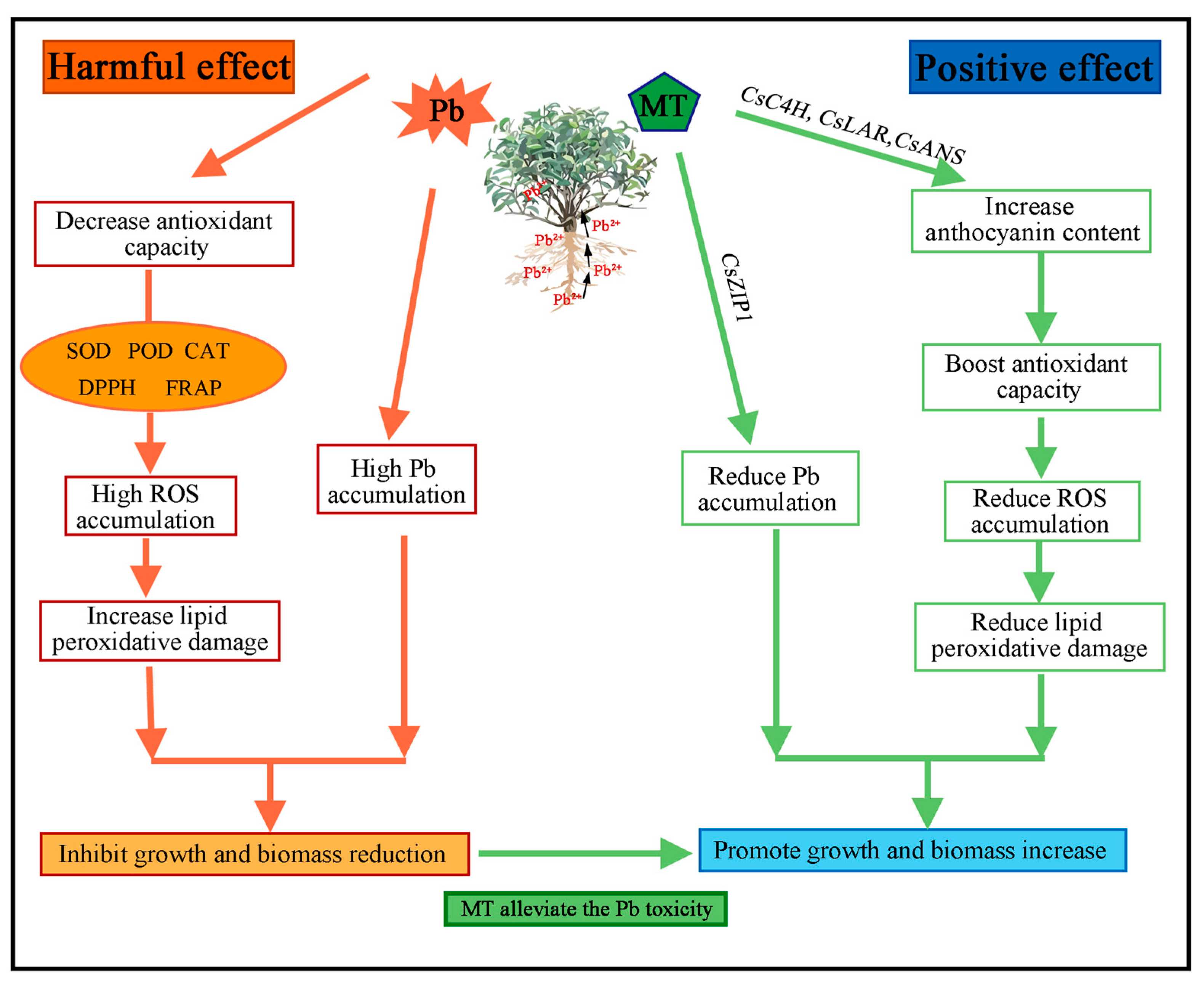
3. Discussion
4. Materials and Methods
4.1. Plant Species and Experimental Design
4.1.1. Hydroponic Experiment
- CK: Foliar spraying with pure water, no Pb treatment applied to the roots;
- MT: Foliar spraying with a 100 μmol L−1 melatonin solution, no Pb treatment applied to the roots;
- Pb: Foliar spraying with pure water, and root treatment with a nutrient solution containing a Pb concentration of 0.29 mmol L−1;
- MT+Pb: Foliar spraying with a 100 μmol L−1 melatonin solution, and root treatment with a nutrient solution containing a Pb concentration of 0.29 mmol L−1.
4.1.2. Field Experiment
- CK: Foliar spraying with pure water, no Pb treatment applied to the roots.
- MT: Foliar spraying with 100 μmol L−1 melatonin solution, no Pb treatment applied to the roots.
- Pb: Foliar spraying with pure water, Pb solution (1.93 mmol L−1) applied to the roots.
- MT+Pb: Foliar spraying with 100 μmol L−1 melatonin solution, Pb solution (1.93 mmol L−1) applied to the roots.
4.2. Measurement Methods
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pb | Lead |
| LJ43 | Longjing 43 |
| ZJ | Zijuan |
| MT | Melatonin |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| GSSG | Glutathiose |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| O2− | Superoxide anion |
| ROS | Reactive oxygen species |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| PCs | Phytochelatins |
References
- Kumar, A.; Kumar, A.; MMS, C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Al Azzawi, T.N.I.; Imran, M.; Hussain, A.; Mun, B.-G.; Pande, A.; Yun, B.-W. Effects of lead (Pb)-induced oxidative stress on morphological and physio-biochemical properties of rice. Biocell 2021, 45, 1413–1423. [Google Scholar] [CrossRef]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—A mini review. Environ. Sci. Pollut. Res. 2015, 22, 18318–18332. [Google Scholar] [CrossRef]
- Feleafel, M.; Mirdad, Z. Hazard and effects of pollution by lead on vegetable crops. J. Agric. Environ. Ethics 2013, 26, 547–567. [Google Scholar] [CrossRef]
- Lamhamdi, M.; El Galiou, O.; Bakrim, A.; Novoa-Munoz, J.C.; Arias-Estevez, M.; Aarab, A.; Lafont, R. Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J. Biol. Sci. 2013, 20, 29–36. [Google Scholar] [CrossRef]
- Muradoğlu, F.; Encu, T.; Gündoğdu, M.; Canal, S.B. Influence of lead stress on growth, antioxidative enzyme activities and ion change in root and leaf of strawberry. Fresenius Environ. Bull. 2016, 25, 623–632. [Google Scholar]
- Cao, L.T.T.; Bourquin, L.D. Relationship of arsenic and lead in soil with fruit and leaves of apple trees at selected orchards in Michigan. J. Food Prot. 2020, 83, 935–942. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K. Abiotic stress responses in tea [Camellia sinensis L. (O) Kuntze]: An overview. Rev. Agric. Sci. 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Thakur, M.; Singh, K.; Jasrotia, R.; Kumar, R.; Radziemska, M. Global Perspectives on Lead Contamination and Health Risks in Surface Water, Rice Grains, and Soils. Land Degrad. Dev. 2025, 36, 2159–2169. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Li, Y.C.; Peng, Y.; Wen, X.; Ni, X. Distribution, accumulation, and potential risks of heavy metals in soil and tea leaves from geologically different plantations. Ecotoxicol. Environ. Saf. 2020, 195, 110475. [Google Scholar] [CrossRef]
- El Khattabi, O.; Lamwati, Y.; Henkrar, F.; Collin, B.; Levard, C.; Colin, F.; Smouni, A.; Fahr, M. Lead-induced changes in plant cell ultrastructure: An overview. Biometals 2025, 38, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Z.; Zhang, Y.; Wei, Y.; Jiang, Z. Effects of lead stress on the growth, physiology, and cellular structure of privet seedlings. PLoS ONE 2018, 13, e0191139. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Mariz-Ponte, N.; Santos, C. Lead induces oxidative stress in Pisum sativum plants and changes the levels of phytohormones with antioxidant role. Plant Physiol. Biochem. 2019, 137, 121–129. [Google Scholar] [CrossRef]
- Ashraf, U.; Tang, X. Yield and quality responses, plant metabolism and metal distribution pattern in aromatic rice under lead (Pb) toxicity. Chemosphere 2017, 176, 141–155. [Google Scholar] [CrossRef]
- Schwalfenberg, G.; Genuis, S.J.; Rodushkin, I. The benefits and risks of consuming brewed tea: Beware of toxic element contamination. J. Toxicol. 2013, 2013, 370460. [Google Scholar] [CrossRef]
- Karak, T.; Bhagat, R. Trace elements in tea leaves, made tea and tea infusion: A review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Shariatifar, N.; Nazmara, S.; Jafari, M.; Arabameri, M.; Karami, L. Determination of element concentration of brewed tea consumed in Iran using ICP-OES: A risk assessment study. Biol. Trace Elem. Res. 2025, 203, 1221–1235. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Carrillo, J.T.; Borthakur, D. Methods for metal chelation in plant homeostasis. Plant Physiol. Biochem. 2021, 163, 95–107. [Google Scholar] [CrossRef]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Wong, H.L.; Sakamoto, T.; Kawasaki, T.; Umemura, K.; Shimamoto, K. Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 2004, 135, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Zheng, S.J.; Matsumoto, H.; Hiradate, S. Detoxifying aluminium with buckwheat. Nature 1997, 390, 569–570. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, H.; Lin, W. Effects of exogenous melatonin on carbon and nitrogen metabolism of rice seedlings under salt stress. Chin. J. Ecol. 2023, 42, 1635–1643. [Google Scholar]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Cui, G.B.; Zhao, X.X.; Liu, S.D.; Sun, F.L.; Zhang, C.; Xi, Y.J. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, G.; Miao, M.; Li, Y.; Li, H. Effects of exogenous melatonin on growth and physiological characteristics of Chinese cabbage seedlings under aluminum stress. In Proceedings of the 2017 6th International Conference on Energy and Environmental Protection (ICEEP 2017), Zhuhai, China, 29–30 June 2017; pp. 1119–1123. [Google Scholar]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant 2020, 168, 256–277. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Al-Amri, S.M. Exogenous melatonin-mediated modulation of arsenic tolerance with improved accretion of secondary metabolite production, activating antioxidant capacity and improved chloroplast ultrastructure in rosemary herb. Ecotoxicol. Environ. Saf. 2019, 180, 333–347. [Google Scholar] [CrossRef]
- Xie, C.; Xiong, X.; Huang, Z.; Sun, L.; Ma, J.; Cai, S.; Yu, F.; Zhong, W.; Chen, S.; Li, X. Exogenous melatonin improves lead tolerance of bermudagrass through modulation of the antioxidant defense system. Int. J. Phytoremediat. 2018, 20, 1408–1417. [Google Scholar] [CrossRef]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef]
- Wang, K.; He, J.; Zhao, N.; Zhao, Y.; Qi, F.; Fan, F.; Wang, Y. Effects of melatonin on growth and antioxidant capacity of naked oat (Avena nuda L) seedlings under lead stress. PeerJ 2022, 10, e13978. [Google Scholar] [CrossRef]
- Ullah, F.; Saqib, S.; Zaman, W.; Khan, W.; Zhao, L.; Khan, A.; Khan, W.; Xiong, Y.-C. Mitigating drought and heavy metal stress in maize using melatonin and sodium nitroprusside. Plant Soil 2024, 11, 1–23. [Google Scholar] [CrossRef]
- Ai, J.; Song, J.; Yan, Z.; Wang, Z.; Chen, W.; Wu, Y.; Wang, Y.; Pan, L.; Xu, Y.; Liu, P. Effects of Exogenous Melatonin on Physiological Response and DNA Damage of Ardisia mamillata and A. crenata Under Lead Stress. Chin. Bull. Bot. 2022, 57, 171–181. [Google Scholar]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Sun, H.-l.; Wang, X.-y.; Shang, Y.; Wang, X.-q.; Du, G.-d.; LÜ, D.-g. Preharvest application of melatonin induces anthocyanin accumulation and related gene upregulation in red pear (Pyrus ussuriensis). J. Integr. Agric. 2021, 20, 2126–2137. [Google Scholar] [CrossRef]
- Li, X.; Li, M.-H.; Deng, W.-W.; Ahammed, G.J.; Wei, J.-P.; Yan, P.; Zhang, L.-P.; Fu, J.-Y.; Han, W.-Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; Wang, W.; Ma, R.; Ding, C.; Wei, K.; Wang, L.; Ge, S.; Shi, Y.; Li, X. Transcriptomic and metabolomic insights into temperature-dependent changes in catechin and anthocyanin accumulation in tea plants with different leaf colors. Plant Stress 2024, 14, 100705. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Chakraborty, S.; Raychaudhuri, S.S. Melatonin improves the lead tolerance in Plantago ovata by modulating ROS homeostasis, phytohormone status and expression of stress-responsive genes. Plant Cell Rep. 2025, 44, 39. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef]
- Hossain, A.; Ahmad, Z.; Adeel, M.; Rahman, M.A.; Alam, M.J.; Ahmed, S.; Aftab, T. Emerging roles of osmoprotectants in heavy metal stress tolerance in plants. In Heavy Metal Toxicity in Plants; CRC Press: Boca Raton, FL, USA, 2021; pp. 95–110. [Google Scholar]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Upadhyay, R.; Saini, R.; Shukla, P.; Tiwari, K. Role of secondary metabolites in plant defense mechanisms: A molecular and biotechnological insights. Phytochem. Rev. 2025, 24, 953–983. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef] [PubMed]
- Dotaniya, M.; Dotaniya, C.; Solanki, P.; Meena, V.; Doutaniya, R. Lead contamination and its dynamics in soil–plant system. In Lead in Plants and the Environment; Gupta, D.K., Chatterjee, S., Walther, C., Eds.; Springer: Cham, Switzerland, 2020; pp. 83–98. [Google Scholar]
- Flegal, A.R.; Smith, D.R. Measurements of environmental lead contamination and human exposure. Rev. Environ. Contam. Toxicol. Contin. Residue Rev. 1995, 143, 1–45. [Google Scholar]
- Han, W.-Y.; Zhao, F.-J.; Shi, Y.-Z.; Ma, L.-F.; Ruan, J.-Y. Scale and causes of lead contamination in Chinese tea. Environ. Pollut. 2006, 139, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 239–273. [Google Scholar]
- Hassan, M.U.; Mahmood, A.; Awan, M.I.; Maqbool, R.; Aamer, M.; Alhaithloul, H.A.; Huang, G.; Skalicky, M.; Brestic, M.; Pandey, S. Melatonin-induced protection against plant abiotic stress: Mechanisms and prospects. Front. Plant Sci. 2022, 13, 902694. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species generation, hazards, and defense mechanisms in plants under environmental (abiotic and biotic) stress conditions. In Handbook of Plant and Crop Physiology, 4th ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 617–658. [Google Scholar]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Li, T.; Li, X.-Y.; Luo, L.-X.; Li, Z.; Liu, C.-L.; Ge, S.-F.; Zhu, Z.-L.; Li, Y.-Y.; Jiang, H.; et al. Enhancement of Apple Stress Resistance via Proline Elevation by Sugar Substitutes. Int. J. Mol. Sci. 2024, 25, 9548. [Google Scholar] [CrossRef]
- Bandurska, H. Drought stress responses: Coping strategy and resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Yang, H.; Fang, R.; Luo, L.; Yang, W.; Huang, Q.; Yang, C.; Hui, W.; Gong, W.; Wang, J. Potential roles of melatonin in mitigating the heavy metals toxicity in horticultural plants. Sci. Hortic. 2023, 321, 112269. [Google Scholar] [CrossRef]
- Altaf, M.A.; Hao, Y.; Shu, H.; Mumtaz, M.A.; Cheng, S.; Alyemeni, M.N.; Ahmad, P.; Wang, Z. Melatonin enhanced the heavy metal-stress tolerance of pepper by mitigating the oxidative damage and reducing the heavy metal accumulation. J. Hazard. Mater. 2023, 454, 131468. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, X.-N.; Zhang, L.; Yan, P.; Zhang, L.-P.; Fu, J.-Y.; Han, W.-Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 2021, 403, 123922. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.G.; Youxin, Y. Anthocyanin-mediated arsenic tolerance in plants. Environ. Pollut. 2021, 292, 118475. [Google Scholar]
- Sun, S.; Liu, A.; Li, Z.; Guo, T.; Chen, S.; Ahammed, G.J. Anthocyanin synthesis is critical for melatonin-induced chromium stress tolerance in tomato. J. Hazard. Mater. 2023, 453, 131456. [Google Scholar] [CrossRef]
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T. Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 11445. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. (Amst. Neth.) 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.; McGrath, S. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Song, W.-Y. Arsenic uptake and translocation in plants. Plant Cell Physiol. 2016, 57, 4–13. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.á.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A.; Yadav, B.; Chhaya; Narayan, O.P. Metal transporters in organelles and their roles in heavy metal transportation and sequestration mechanisms in plants. Physiol. Plant 2021, 173, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yamaji, N.; Ma, J.F. Metal transport systems in plants. Annu. Rev. Plant Biol. 2024, 75, 1–25. [Google Scholar] [CrossRef]
- Fenglin, D.; Naoki, Y.; Jixing, X.; Feng, M.J. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013, 163, 1353–1362. [Google Scholar]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef]
- Menguer, P.K.; Farthing, E.; Peaston, K.A.; Ricachenevsky, F.K.; Fett, J.P.; Williams, L.E. Functional analysis of the rice vacuolar zinc transporter OsMTP1. J. Exp. Bot. 2013, 64, 2871–2883. [Google Scholar] [CrossRef]
- eGaitan-Solis, E.; eTaylor, N.; eSiritunga, D.; eStevens, W.; Schachtman, D.P. Overexpression of the transporters AtZIP1 and AtMTP1 in cassava changes zinc accumulation and partitioning. Front. Plant Sci. 2015, 6, 492. [Google Scholar]
- Khan, A.; Jie, Z.; Xiangjun, K.; Ullah, N.; Short, A.W.; Diao, Y.; Zhou, R.; Xiong, Y.-C. Pre treatment of melatonin rescues cotton seedlings from cadmium toxicity by regulating key physio-biochemical and molecular pathways. J. Hazard. Mater. 2023, 445, 130530. [Google Scholar] [CrossRef]
- Limson, J.; Nyokong, T.; Daya, S. The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: An adsorptive voltammetric study. J. Pineal Res. 1998, 24, 15–21. [Google Scholar] [CrossRef]
- Altaf, M.A.; Sharma, N.; Srivastava, D.; Mandal, S.; Adavi, S.; Jena, R.; Bairwa, R.K.; Gopalakrishnan, A.V.; Kumar, A.; Dey, A. Deciphering the melatonin-mediated response and signalling in the regulation of heavy metal stress in plants. Planta 2023, 257, 115. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M. Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.; Helal, M.; Chaudri, A.; Lawlor, K.; McGrath, S. Soil Solid-Phase Controls Lead Activity in Soil Solution. J. Environ. Qual. 2002, 31, 162–167. [Google Scholar]
- Shchukin, V.; Zhigilei, E.; Erina, A.; Shvetsova, Y.N.; Kuz’mina, N.; Luttseva, A. Validation of an ICP-MS method for the determination of mercury, lead, cadmium, and arsenic in medicinal plants and related drug preparations. Pharm. Chem. J. 2020, 54, 968–976. [Google Scholar] [CrossRef]
- Pape, C.; Lüning, K. Quantification of melatonin in phototrophic organisms. J. Pineal Res. 2006, 41, 157–165. [Google Scholar] [CrossRef]
- Viegas, T.R.; Mata, A.L.; Duarte, M.M.; Lima, K.M. Determination of quality attributes in wax jambu fruit using NIRS and PLS. Food Chem. 2016, 190, 1–4. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria× anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Lotkowska, M.E.; Tohge, T.; Fernie, A.R.; Xue, G.-P.; Balazadeh, S.; Mueller-Roeber, B. The Arabidopsis transcription factor MYB112 promotes anthocyanin formation during salinity and under high light stress. Plant Physiol. 2015, 169, 1862–1880. [Google Scholar] [CrossRef]
- Ciganda, V.; Gitelson, A.; Schepers, J. Non-destructive determination of maize leaf and canopy chlorophyll content. J. Plant Physiol. 2009, 166, 157–167. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Gao, F. Plant Physiology Experiment Guidance; China Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Ukeda, H.; Kawana, D.; Maeda, S.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on the reduction of highly water-soluble tetrazolium salts by xanthine-xanthine oxidase. Biosci. Biotechnol. Biochem. 1999, 63, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.H.; Borg, L.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Reuveni, R.; Shimoni, M.; Karchi, Z.; Kuc, J. Peroxidase activity as a biochemical marker for resistance of muskmelon. Phytopathology 1992, 82, 749–753. [Google Scholar] [CrossRef]



| pH | Total Nitrogen (%) | Organic Matter (%) | Available Phosphorus (mg kg−1) | Available Potassium (mg kg−1) | Exchangeable Magnesium (mg kg−1) |
|---|---|---|---|---|---|
| 5.4 | 0.1 | 0.1 | 4.3 | 211.8 | 915.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, J.; Liang, X.; Zhan, S.; Bai, Y.; Ruan, L. Melatonin-Mediated Regulation of Antioxidant Defense Enhances the Resistance of Tea Plants (Camellia sinensis L.) to Lead-Induced Stress. Plants 2025, 14, 1417. https://doi.org/10.3390/plants14101417
Li J, Yang J, Liang X, Zhan S, Bai Y, Ruan L. Melatonin-Mediated Regulation of Antioxidant Defense Enhances the Resistance of Tea Plants (Camellia sinensis L.) to Lead-Induced Stress. Plants. 2025; 14(10):1417. https://doi.org/10.3390/plants14101417
Chicago/Turabian StyleLi, Jianwu, Jiao Yang, Xin Liang, Shuping Zhan, Yixuan Bai, and Li Ruan. 2025. "Melatonin-Mediated Regulation of Antioxidant Defense Enhances the Resistance of Tea Plants (Camellia sinensis L.) to Lead-Induced Stress" Plants 14, no. 10: 1417. https://doi.org/10.3390/plants14101417
APA StyleLi, J., Yang, J., Liang, X., Zhan, S., Bai, Y., & Ruan, L. (2025). Melatonin-Mediated Regulation of Antioxidant Defense Enhances the Resistance of Tea Plants (Camellia sinensis L.) to Lead-Induced Stress. Plants, 14(10), 1417. https://doi.org/10.3390/plants14101417






