Assessment of Fruit Traits and Antioxidant Capacity in Wild and Cultivated Genotypes of Ziziphus sp.
Abstract
:1. Introduction
2. Results
2.1. The Morphological Traits of Fruits and Leaves
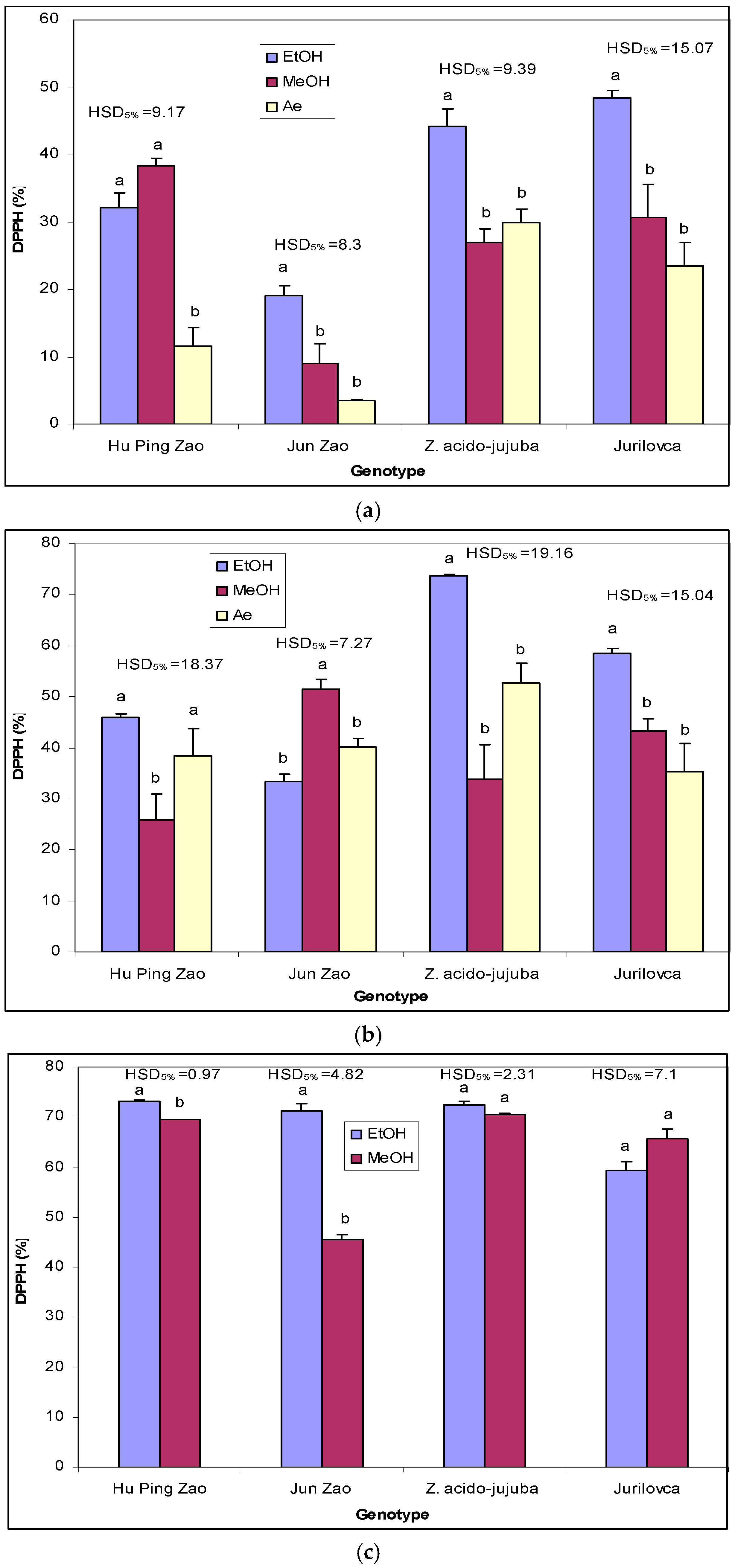
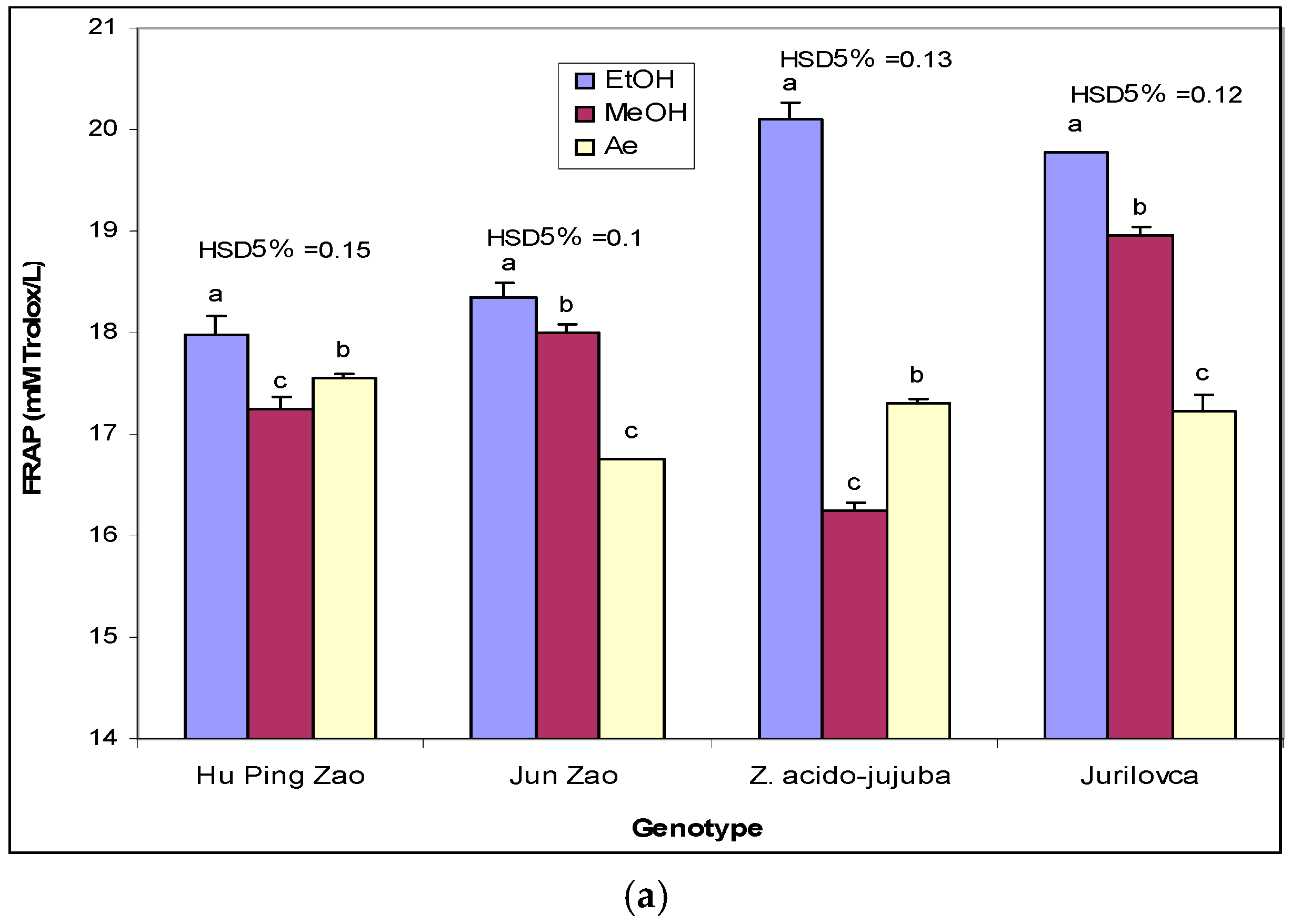
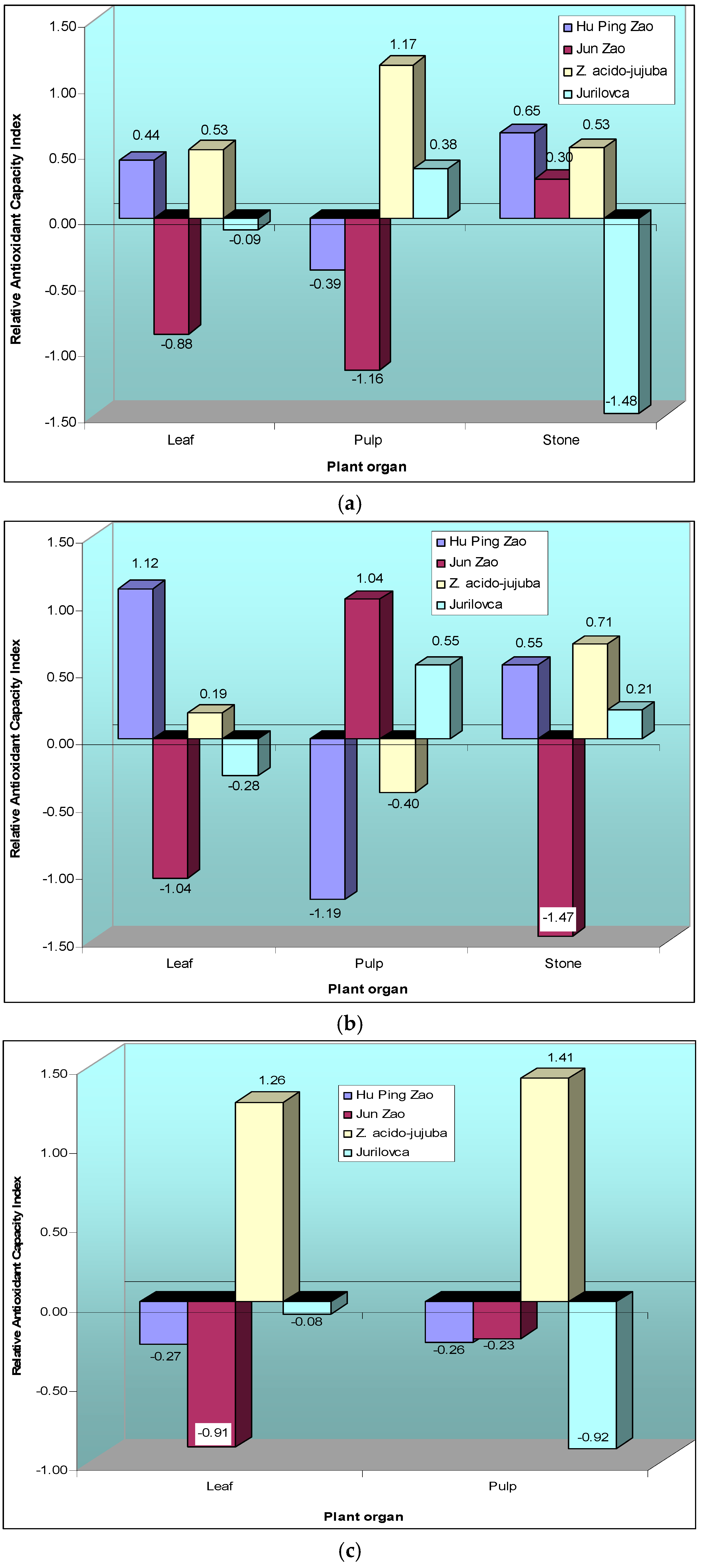
2.2. Antioxidant Potential
3. Materials and Methods
3.1. Biological Material
3.2. Sample Preparation
3.3. Antioxidant Capacity Determined by DPPH Assay
3.4. Antioxidant Capacity Determined by Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Statistical Analysis
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blondel, J. The ‘Design’ of Mediterranean Landscapes: A Millennial Story of Humans and Ecological Systems during the Historic Period. Hum. Ecol. 2006, 34, 713–729. [Google Scholar] [CrossRef]
- Ebert, A.W. Potential of underutilized traditional vegetables and legumes crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability 2014, 6, 319–335. [Google Scholar] [CrossRef]
- Scarano, A.; Semeraro, T.; Chieppa, M.; Santino, A. Neglected and Underutilized Plant Species (NUS) from the Apulia Region Worthy of Being Rescued and Re-Included in Daily Diet. Horticulturae 2021, 7, 177. [Google Scholar] [CrossRef]
- Sumalan, R.-L.; Ciulca, S.-I.; Sumalan, R.-M.; Popescu, S. Vegetable Landraces: The “Gene Banks” for Traditional Farmers and Future Breeding Programs. In Landraces-Traditional Variety and Natural Breed; InTech Open: London, UK, 2021. [Google Scholar]
- Baldermann, S.; Blagojević, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Schreiner, M. Are Neglected Plants the Food for the Future? Critical Rev. Plant Sci. 2016, 35, 106–119. [Google Scholar] [CrossRef]
- Semeraro, T.; Scarano, A.; Buccolieri, R.; Santino, A.; Aarrevaara, E. Planning of urban green spaces: An ecological perspective on human benefits. Land 2021, 10, 105. [Google Scholar] [CrossRef]
- Thompson, J.; Hodgkin, T.; Atta-Krah, K.; Jarvis, D.; Hoogendoorn, C.; Padulosi, S. Biodiversity in agroecosys-tems Farming with nature: The science and practice ofecoagriculture. In The Science and Practice of Ecoagriculture; Scherr, S.J., McNeely, J.A., Eds.; Island Press: Washington, DC, USA, 2007; pp. 46–60. [Google Scholar]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-Alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean Coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Karapatzak, E.; Dichala, O.; Papanastasi, K.; Manthos, I.; Ganopoulos, I.; Karydas, A.; Badeka, A.V.; Kosma, I.S.; Kyrkas, D.; Yfanti, P.; et al. A Multifaceted Evaluation Approach for Greek Native Neglected and Underutilized Forest Fruit Trees and Shrubs as Natural Sources of Antioxidants: Consolidating the Framework for Their Sustainable Agronomic Exploitation. Plants 2023, 12, 1642. [Google Scholar] [CrossRef] [PubMed]
- Malladi, A.; Johnson, L.K. Expression profiling of cell cycle genes reveals key facilitators of cell production during carpel development, fruit set, and fruit growth in apple (Malus x domestica Borkh.). J. Exp. Bot. 2011, 62, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, Z.; Li, S.; Lian, Q.; Fu, P.; He, Y.; Qiao, J.; Xu, K.; Liu, L.; Wu, M.; et al. Genomic analyses of diverse wild and cultivated accessions provide insights into the evolutionary history of jujube. Plant Biotechnol. J. 2021, 19, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, J.; Yin, X.; Lian, C.; Li, X. Genetic diversity and population structure of sour jujube, Ziziphus acidojujuba. Tree Genet. Genomes 2015, 11, 809. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Y. Fruit Tree Records of China; China Forestry Publishing House: Beijing, China, 2003; Chinese jujube volume. [Google Scholar]
- Meghwal, P.R.; Khan, M.A.; Tewari, J.C. Ber: Growing ber (Ziziphus mauritiana Lam) for Sustainable Income and Employment in Arid and Semi-Arid Regions; ICAR-Central Arid Zone Research Institute: Jodhpur, India, 2007; p. 26. [Google Scholar]
- Chundawat, B.S. Arid Fruit Culture; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 1990. [Google Scholar]
- Abdoul-Azize, S. Potential Benefits of Jujube (Zizyphus lotus L.) Bioactive Compounds for Nutrition and Health. J. Nutr. Metabol. 2016, 2016, 2867470. [Google Scholar] [CrossRef] [PubMed]
- Feyssa, D.H.; Njoka, J.T.; Asfaw, Z.; Nyangito, M.M. Wild edible fruits of importance for human nutrition in semi-arid parts of East Shewa Zone, Ethiopia: Associated indigenous knowledge and implications to food security. Pak. J. Nutr. 2011, 10, 40–50. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Shishir, M.R.I.; Bao, T.; Lu, Y.; Chen, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Bao, T.; Hao, X.; Shishir, M.R.I.; Karim, N.; Chen, W. Cold plasma: An emerging pretreatment technology for the drying of jujube slices. Food Chem. 2021, 337, 127783. [Google Scholar] [CrossRef]
- Meena, V.S.; Gora, J.S.; Singh, A.; Ram, C.; Meena, N.K.; Pratibha; Rouphael, Y.; Basile, B.; Kumar, P. Underutilized Fruit Crops of Indian Arid and Semi-Arid Regions: Importance, Conservation and Utilization Strategies. Horticulturae 2022, 8, 171. [Google Scholar] [CrossRef]
- Wojdylo, A.; Barrachina, A.A.C.; Legua, O.; Hernandez, F. Phenolic composition, ascorbic acid content, and antioxidant activity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chemistry 2016, 201, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.A.; Rauf, M.A.; Bashir, H.; Ahmad, A. The evaluation of biodiversity in some indigenous Indian jujube (Ziziphus mauritiana) germplasm through physico-chemical analysis. Acta Sci. Pol. Hortorum Cultus 2018, 17, 39–52. [Google Scholar] [CrossRef]
- Huang, J.; Chen, X.; He, A.; Ma, Z.; Gong, T.; Xu, K.; Chen, R. Integrative Morphological.; Physiological.; Proteomics Analyses of Jujube Fruit Development Provide Insights Into Fruit Quality Domestication From Wild Jujube to Cultivated Jujube. Front. Plant Sci. 2021, 12, 773825. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Heyduck, R. Ornamental Jujube Cultivar Evaluation in the Southwestern United States. HortTechnol. 2018, 28, 557–561. [Google Scholar] [CrossRef]
- Ivanišová, E.; Grygorieva, O.; Abrahamová, V.; Schubertova, Z.; Terentjeva, M.; Brindza, J. Characterization of morphological parameters and biological activity of jujube fruit (Ziziphus jujuba Mill.). J. Berry Res. 2017, 7, 249–260. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Wang, Z.; Song, L.; Cao, B. Fruit Morphology Measurements of Jujube Cultivar ‘Lingwu Changzao’ (Ziziphus jujuba Mill. cv. Lingwu Changzao) during Fruit Development. Horticulturae 2021, 7, 26. [Google Scholar] [CrossRef]
- Ciocârlan, V. Flora ilustrată a României (Illustrated Flora of Romania). Pterydophyta et Spermatophyta; Editura Ceres: Bucharest, Romania, 2000. [Google Scholar]
- Stanica, F. Preliminary results regarding the behavior of some Chinese Date varieties (Ziziphus jujuba Mill.) in Bucharest area. Sci. Pap. Ser. B 2000, XLIII, 203–206. [Google Scholar]
- Stanica, F. Characterization of Two Romanian Local Biotypes of Ziziphus jujuba. Acta Hort. 2009, 840, 259–262. [Google Scholar] [CrossRef]
- Ciulca, S. Metodologii de Experimentare în Agricultura si Biologie (Experimental Methodologies in Agriculture and Biology); Editura Agroprint: Timisoara, Romania, 2006; pp. 257–260. [Google Scholar]
- Sun, T.; Tanumihardjo, S.A. An integrated approach to evaluate food antioxidant capacity. J Food Sci. 2007, 72, 159–165. [Google Scholar] [CrossRef]
- Hurtado, M.; Vilanova, S.; Plazas, M.; Gramazio, P.; Fonseka, H.H.; Fonseka, R.; Prohens, J. Diversity and relationships of eggplants from three geographically distant secondary centers of diversity. PLoS ONE 2012, 7, e41748. [Google Scholar] [CrossRef] [PubMed]
- Ouborg, N.J. Integrating population genetics and conservation biology in the era of genomics. Biol. Lett. 2010, 6, 3–6. [Google Scholar] [CrossRef]
- Khadivi, A. Morphological characterization and interspecific variation among five species of Ziziphus genus to select superiors in Iran. BMC Plant Biol. 2023, 23, 550. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, E.; Erfani-Moghadam, J.; Fazeli, A.; Khadivi, A. Morphological variability within and among three species of Ziziphus genus using multivariate analysis. Sci. Hortic. 2017, 222, 180–186. [Google Scholar] [CrossRef]
- Grygorieva, O.; Abrahamova, V.; Karnatovska, M.; Bleha, R.; Brindza, J. Morpho logical characteristics of fruits, drupes and seeds in genotypes of Ziziphus jujuba Mill. Potravinarstvo Sci. J. Food Ind. 2014, 32, 27–42. [Google Scholar]
- Obeed, R.; Harhash, M.; Abdel-Mawgood, A. Fruit properties and genetic diversity of five ber (Ziziphus mauritiana Lamk) cultivars. Pak. J. Biol. Sci. 2008, 11, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, M.; Almutairi, K.F.; Al-Saif, A.M.; Ahmed, K.A. Fruit properties during the harvest period of eleven Indian jujube (Ziziphus mauritiana Lamk.) cultivars. Saudi J. Biol. Sci. 2021, 28, 3424–3432. [Google Scholar] [CrossRef]
- Guijun, Y.; Ferguson, A.R. Jujube Sub-Tropical Fruit Club of Qld Inc., 2010. Available online: http://stfc.org.au/jujube (accessed on 21 October 2024).
- Nowak, B.; Schulzová, B. Tropické Plody. (Tropical Fruits); Ikar: Bratislava, Slovakia, 2002; p. 239. [Google Scholar]
- Polívka, F. Užitkové a Pamétihodné Rostliny Cudzích Zemí. (Utility Plants and Memorable Foreign Countries); Volvox Globator: Praha, Chezch Republic, 2010; pp. 9–10. [Google Scholar]
- Cao, K.; Chang, Y.; Sun, R.; Shen, F.; Wu, T.; Wang, Y.; Zhang, X.; Han, Z. Candidate gene prediction via quantitative trait locus analysis of fruit shape index traits in apple. Euphytica 2015, 206, 381–391. [Google Scholar] [CrossRef]
- Yahia, Y.; Benabderrahim, M.A.; Tlili, N.; Bagues, M.; Nagaz, K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two ziziphus mill. Species. PLoS ONE 2020, 15, e0232599. [Google Scholar] [CrossRef]
- Riaz, M.U.; Raza, M.A.; Saeed, A.; Ahmed, M.; Hussain, T. Variations in Morphological Characters and Antioxidant Potential of Different Plant Parts of Four Ziziphus Mill. Species from the Cholistan. Plants 2021, 10, 2734. [Google Scholar] [CrossRef] [PubMed]
- Khadivi, A.; Mirheidari, F.; Moradi, Y.; Paryan, S. Identification of superior jujube (Ziziphus jujuba Mill.) genotypes based on morphological and fruit characterizations. Food Sci. Nutr. 2021, 9, 3165–3176. [Google Scholar] [CrossRef] [PubMed]
- Khadivi, A.; Beigi, F. Morphological and chemical characterizations of jujube (Ziziphus jujuba Mill.) to select superior accessions. Food Sci. Nutr. 2022, 10, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Mirheidari, F.; Khadivi, A.; Saeidifar, A.; Moradi, Y. Selection of superior genotypes of Indian jujube (Ziziphus mauritiana Lamk.) as revealed by fruit-related traits. Food Sci. Nutr. 2022, 10, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Jaskani, M.J.; Abbas Naqvi, S.; Awan, F.S. Exploitation of diversity in domesticated and wild ber (Ziziphus mauritiana lam.) germplasm for conservation and breeding in Pakistan. Sc. Hortic. 2019, 249, 228–239. [Google Scholar] [CrossRef]
- Fuller, D.Q. Long and attenuated: Comparative trends in the domestication of tree fruits. Veg. Hist. Archaeobot. 2018, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, X.; Zhao, K.; Liu, J.; Chen, G.; Zhang, Y.; Ma, J.; Sun, N. Cultivation and morphology of jujube (Ziziphus Jujuba Mill.) in the Qi River Basin of Northern China during the Neolithic Period. Sci Rep. 2024, 14, 2305. [Google Scholar] [CrossRef]
- Janick, J. The origins of fruits, fruit growing, and fruit breeding. Plant Breed. Rev. 2010, 25, 255–321. [Google Scholar]
- Fuller, D.Q.; Stevens, C.J. Between domestication and civilization: The role of agriculture and arboriculture in the emergence of the first urban societies. Veg. Hist. Archaeobot. 2019, 28, 263–282. [Google Scholar] [CrossRef]
- Koley, T.K.; Kaur, C.; Nagal, S.; Walia, S.; Jaggi, S.; Sarika, S.J. Antioxidant activity and phenolic content in genotypes of Indian jujube (Zizyphus mauritiana Lamk. ) Arab. J. Chem. 2016, 9, S1044–S1052. [Google Scholar] [CrossRef]
- Razi, M.F.; Anwar, R.; Basra, S.M.A.; Khan, M.M.; Khan, I.A. Morphological characterization of leaves and fruit of jujube (Ziziphus mauritiana Lamk.) germplasm in Faisalabad, Pakistan. Pak. J. Agric. Sci. 2013, 50, 211–216. [Google Scholar]
- Goswami, P.; Banerjee, R.; Mukherjee, A. Potential antigenotoxicity assessment of Ziziphus jujuba fruit. Heliyon. 2019, 5, e01768. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, P.; Yan, Y.; Huang, Y.; Bai, B.; Hou, X.; Zhang, L. Supercritical CO2 fluid extraction of flavonoid compounds from Xinjiang jujube (Ziziphus jujuba Mill.) leaves and associated biological activities and flavonoid compositions. Ind. Crops Prod. 2019, 139, 111508. [Google Scholar] [CrossRef]
- Elaloui, M.; Ennajah, A.; Ghazghazi, H.; BenYoussef, I.; Ben Othman, N.; Hajlaoui, M.R.; Khouja, A.; Laamouri, A. Quantification of total phenols, flavonoids and tannins from Ziziphus jujuba (mill.) and Ziziphus lotus (l.) (Desf). Leaf extracts and their effects on antioxidant and antibacterial activities. Int. J. Second. Met. 2017, 4, 18–26. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Yu, J.G.; Wang, M.; Ma, Y.J.; Li, C.L. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J. Food Sci. 2012, 77, 1218–1225. [Google Scholar] [CrossRef]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Tlili, N.; Mejri, H.; Yahia, Y.; Saadaoui, E.; Rejeb, S.; Khaldi, A.; Nasri, N. Phytochemicals and antioxidant activities of Rhus tripartitum (Ucria) fruits depending on locality and different stages of maturity. Food Chem. 2014, 160, 98–103. [Google Scholar] [CrossRef]
- Al-Saeedi, A.H.; Al- Ghafri, M.T.H.; Hossain, M.A. Comparative evaluation of total phenols, flavonoids content and antioxidant potential of leaf and fruit extracts of Omani Ziziphus jujuba L. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 78–83. [Google Scholar] [CrossRef]
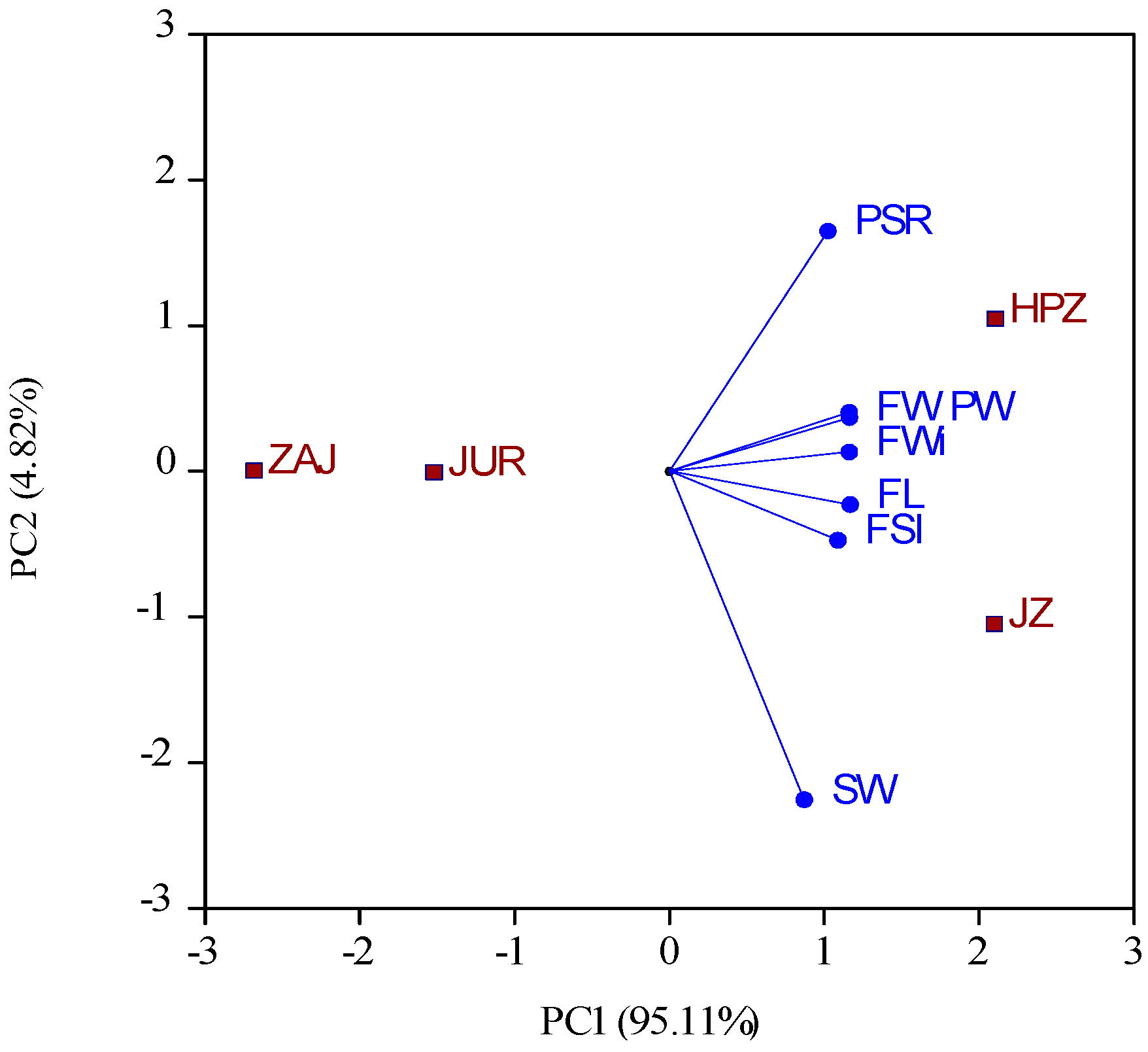




| Genotype | Fruit Length (mm) | Fruit Width (mm) | Fruit Shape Index | Fruit Fresh Weight (g) | Fruit Dry Weight (g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hu Ping Zao (Z. jujuba) | 37.4 ± 0.8 a | 26.70 ± 1.16 a | 1.42 ± 0.07 a | 11.65 ± 0.32 a | 3.76 ± 0.07 a | |||||
| Jun Zao (Z. jujuba) | 39.3 ± 0.5 a | 26.10 ± 0.66 a | 1.51 ± 0.04 a | 10.32 ± 0.31 b | 3.56 ± 0.11 a | |||||
| Z. acido-jujuba | 16.3 ± 0.4 c | 15.20 ± 0.53 b | 0.90 ± 0.02 c | 1.73 ± 0.03 d | 0.74 ± 0.01 c | |||||
| Jurilovca (Z. jujuba) | 20.2 ± 0.8 b | 16.30 ± 0.65 b | 1.24 ± 0.03 b | 3.43 ± 0.06 c | 1.40 ± 0.02 b | |||||
| F | 396.19 ** | 60.99 ** | 42.83 ** | 464.98 ** | 532.03 ** | |||||
| HSD5% | 2.4 | 3.1 | 0.16 | 0.87 | 0.25 | |||||
| Genotype | CV (%) | Range | CV (%) | Range | CV (%) | Range | CV (%) | Range | CV (%) | Range |
| Hu Ping Zao | 6.45 | 33–40 | 13.80 | 21–33 | 14.97 | 1.15–1.90 | 8.76 | 10.14–13.2 | 5.56 | 3.32–4.08 |
| Jun Zao | 4.33 | 36–42 | 7.97 | 22–28 | 7.48 | 1.41–1.77 | 9.73 | 8.66–11.24 | 9.92 | 2.96–4.06 |
| Z. acido-jujuba | 9.30 | 11–15 | 11.10 | 11–17 | 5.87 | 0.81–1.01 | 4.65 | 1.58–1.83 | 2.49 | 0.71–0.76 |
| Jurilovca | 12.08 | 16–24 | 12.62 | 13–21 | 7.28 | 1.12–1.31 | 5.50 | 3.05–3.61 | 5.33 | 1.3–1.52 |
| Genotype | Pulp Fresh Weight (g) | Pulp Dry Weight (g) | Stone Fresh Weight (g) | Stone Dry Weight (g) | Pulp: Stone Ratio (FW) | Pulp: Stone Ratio (DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu Ping Zao | 10.91 ± 0.28 a | 3.50 ± 0.07 a | 0.52 ± 0.02 b | 0.26 ± 0.01 b | 21.01 ± 0.31 a | 13.47 ± 0.57 a | ||||||
| Jun Zao | 9.51 ± 0.25 b | 3.11 ± 0.1 b | 0.81 ± 0.01 a | 0.44 ± 0.01 a | 11.76 ± 0.32 b | 7.02 ± 0.15 b | ||||||
| Z. acido-jujuba | 1.33 ± 0.01 d | 0.45 ± 0.01 d | 0.42 ± 0.01 c | 0.28 ± 0.01 b | 3.22 ± 0.05 d | 1.60 ± 0.02 d | ||||||
| Jurilovca | 2.98 ± 0.05 c | 1.13 ± 0.02 c | 0.45 ± 0.01 c | 0.27 ± 0.01 b | 6.63 ± 0.13 c | 4.21 ± 0.11 c | ||||||
| F | 453.93 ** | 559.16 ** | 219.08 ** | 117.4 ** | 110.19 ** | 292.81 ** | ||||||
| HSD5% | 0.85 | 0.24 | 0.05 | 0.03 | 0.88 | 1.14 | ||||||
| Genotype | CV (%) | Range | CV (%) | Range | CV (%) | Range | CV (%) | Range | CV (%) | Range | CV (%) | Range |
| Hu Ping Zao | 9.03 | 9.53–12.54 | 6.17 | 3.06–3.82 | 10.45 | 0.44–0.62 | 11.06 | 0.23–0.32 | 4.61 | 19.69–22.42 | 13.29 | 10.25–15.61 |
| Jun Zao | 10.38 | 7.89–11.18 | 10.53 | 2.54–3.59 | 2.72 | 0.77–0.84 | 7.05 | 0.39–0.48 | 8.58 | 10.25–13.63 | 6.65 | 6.0–7.61 |
| Z. acido-jujuba | 3.48 | 1.26–1.39 | 3.53 | 0.42–0.47 | 5.71 | 0.37–0.45 | 3.14 | 0.26–0.3 | 5.08 | 2.98–3.43 | 4.21 | 1.51–1.71 |
| urilovca | 5.46 | 2.66–3.14 | 5.26 | 1.03–1.21 | 9.43 | 0.39–0.53 | 9.01 | 0.23–0.31 | 6.34 | 5.92–7.37 | 8.52 | 3.81–5.04 |
| Extraction | Genotype | Plant Organ | HSD5% | ||
|---|---|---|---|---|---|
| Solvent | Leaf | Pulp | Stone | ||
| EtOH | Hu Ping Zao | 32.19 ± 2.07 B z | 45.83 ± 0.79 C y | 73.22 ± 0.34 A x | 5.61 |
| Jun Zao | 19.03 ± 1.46 C z | 33.41 ± 1.34 D y | 71.23 ± 1.43 A x | 6.13 | |
| Z. acido-jujuba | 44.30 ± 2.54 A y | 73.75 ± 0.27 A x | 72.37 ± 0.77 A x | 6.69 | |
| Jurilovca | 48.49 ± 0.99 A y | 58.40 ± 1.13 B x | 59.45 ± 1.64 B x | 5.56 | |
| HSD5% | 8.43 | 4.39 | 5.27 | ||
| MeOH | Hu Ping Zao | 38.26 ± 1.18 A y | 25.95 ± 4.87 D z | 69.57 ± 0.09 A x | 12.56 |
| Jun Zao | 8.97 ± 2.96 B y | 51.55 ± 1.76 A x | 45.65 ± 0.99 B x | 8,99 | |
| Z. acido-jujuba | 27.03 ± 1.95 A y | 33.90 ± 6.64 C y | 70.46 ± 0.3 A x | 17.34 | |
| Jurilovca | 30.67 ± 4.85 A z | 43.35 ± 2.38 B y | 65.59 ± 1.96 A x | 14.4 | |
| HSD5% | 13.86 | 6.49 | 5.04 | ||
| Ae | Hu Ping Zao | 11.6 ± 2.78 B y | 38.43 ± 5.43 A x | 16.93 | |
| Jun Zao | 3.51 ± 0.22 B y | 40.05 ± 1.87 A x | 5.23 | ||
| Z. acido-jujuba | 29.96 ± 1.94 A y | 52.7 ± 3.8 A x | 11.84 | ||
| Jurilovca | 23.55 ± 3.42 A x | 35.27 ± 5.48 A x | 17.94 | ||
| HSD5% | 10.91 | 19.91 | |||
| Extraction | Genotype | Plant Organ | HSD5% | ||
|---|---|---|---|---|---|
| Solvent | Leaf | Pulp | Stone | ||
| EtOH | Hu Ping Zao | 13.07 ± 0.84 A x | 3.13 ± 0.05 C y | 2.67 ± 0.01 A y | 2.09 |
| Jun Zao | 6.17 ± 0.47 C x | 2.19 ± 0.09 D y | 2.59 ± 0.05 A y | 1.2 | |
| Z. acido-jujuba | 9.96 ± 0.57 B x | 4.85 ± 0.02 A y | 2.65 ± 0.03 A z | 1.43 | |
| Jurilovca | 3.48 ± 0.07 D y | 4.06 ± 0.08 B x | 2.23 ± 0.06 B z | 0.31 | |
| HSD5% | 2.52 | 0.3 | 0.2 | ||
| MeOH | Hu Ping Zao | 12.17 ± 0.37 A x | 1.65 ± 0.2 B y | 2.52 ± 0.01 A y | 1.05 |
| Jun Zao | 3.24 ± 0.49 C x | 3.37 ± 0.11 A x | 1.68 ± 0.04 C y | 1.27 | |
| Z. acido-jujuba | 7.85 ± 0.56 B x | 2.35 ± 0.37 AB y | 2.62 ± 0.01 A y | 1.68 | |
| Jurilovca | 2.21 ± 0.35 C x | 3.18 ± 0.17 A x | 2.38 ± 0.07 B x | 0.99 | |
| HSD5% | 2.93 | 1.06 | 0.13 | ||
| Ae | Hu Ping Zao | 4.03 ± 0.39 B x | 2.84 ± 0.29 B y | 1.16 | |
| Jun Zao | 1.55 ± 0.09 C y | 2.74 ± 0.13 B x | 0.44 | ||
| Z. acido-jujuba | 10.16 ± 0.66 A x | 3.75 ± 0.27 A y | 1.97 | ||
| Jurilovca | 1.49 ± 0.22 C x | 2.27 ± 0.35 B x | 1.14 | ||
| HSD5% | 1.81 | 0.89 | |||
| Extraction | Genotype | Plant Organ | HSD5% | ||
|---|---|---|---|---|---|
| Solvent | Leaf | Pulp | Stone | ||
| EtOH | Hu Ping Zao | 17.98 ± 0.18 C x | 16.81 ± 0.46 B y | 16.64 ± 0.03 B y | 0.43 |
| Jun Zao | 18.35 ± 0.14 B x | 16.45 ± 0.12 B z | 16.98 ± 0.12 A y | 0.15 | |
| Z. acido-jujuba | 20.10 ± 0.17 A x | 17.69 ± 0.08 A y | 17.02 ± 0.03 A z | 0.13 | |
| Jurilovca | 19.78 ± 0.01 A x | 17.59 ± 0.03 A y | 17.00 ± 0.08 A z | 0.09 | |
| HSD5% | 0.33 | 0.72 | 0.17 | ||
| MeOH | Hu Ping Zao | 17.24 ± 0.12 C x | 17.03 ± 0.15 A y | 16.93 ± 0.17 B y | 0.16 |
| Jun Zao | 18.01 ± 0.06 B x | 17.02 ± 0.06 A z | 17.32 ± 0.02 A y | 0.06 | |
| Z. acido-jujuba | 16.25 ± 0.08 D y | 15.83 ± 0.43 B z | 16.75 ± 0.01 C x | 0.28 | |
| Jurilovca | 18.95 ± 0.09 A x | 16.70 ± 0.01 A y | 16.59 ± 0.06 C z | 0.07 | |
| HSD5% | 0.22 | 0.51 | 0.2 | ||
| Ae | Hu Ping Zao | 17.56 ± 0.03 A x | 16.08 ± 0.02 A y | 0.06 | |
| Jun Zao | 16.76 ± 0.01 C x | 16.24 ± 0.05 A y | 0.08 | ||
| Z. acido-jujuba | 17.31 ± 0.05 B x | 15.26 ± 0.19 B y | 0.46 | ||
| Jurilovca | 17.22 ± 0.16 B x | 16.43 ± 0.08 A y | 0.14 | ||
| HSD5% | 0.19 | 0.66 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șumălan, R.L.; Copolovici, D.M.; Crișan, M.; Stănică, F.; Șumălan, R.M.; Lupitu, A.; Vicas, S.I.; Mot, S.; Copolovici, L.; Ciulca, S. Assessment of Fruit Traits and Antioxidant Capacity in Wild and Cultivated Genotypes of Ziziphus sp. Plants 2025, 14, 134. https://doi.org/10.3390/plants14010134
Șumălan RL, Copolovici DM, Crișan M, Stănică F, Șumălan RM, Lupitu A, Vicas SI, Mot S, Copolovici L, Ciulca S. Assessment of Fruit Traits and Antioxidant Capacity in Wild and Cultivated Genotypes of Ziziphus sp. Plants. 2025; 14(1):134. https://doi.org/10.3390/plants14010134
Chicago/Turabian StyleȘumălan, Radu Liviu, Dana Maria Copolovici, Manuela Crișan, Florin Stănică, Renata Maria Șumălan, Andreea Lupitu, Simona Ioana Vicas, Silvia Mot, Lucian Copolovici, and Sorin Ciulca. 2025. "Assessment of Fruit Traits and Antioxidant Capacity in Wild and Cultivated Genotypes of Ziziphus sp." Plants 14, no. 1: 134. https://doi.org/10.3390/plants14010134
APA StyleȘumălan, R. L., Copolovici, D. M., Crișan, M., Stănică, F., Șumălan, R. M., Lupitu, A., Vicas, S. I., Mot, S., Copolovici, L., & Ciulca, S. (2025). Assessment of Fruit Traits and Antioxidant Capacity in Wild and Cultivated Genotypes of Ziziphus sp. Plants, 14(1), 134. https://doi.org/10.3390/plants14010134












