Rooting, Growth, and Root Morphology of the Cuttings of Ficus carica L. (cv. “Dottato”): Cutting Types and Length and Growth Medium Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Cutting Collection and Preparation
2.2. Cutting, Rooting, and Evaluation of the Rooting Percentage
2.3. Transplanting and Growing Rooted Cuttings in Pots
2.4. Plant Biomass, Partitioning, and Above-Ground Morphology
2.5. Root Morphology Analysis
2.6. Statistics
3. Result and Discussion
3.1. Cutting Rooting Ability
3.1.1. Biometric and Above-Ground Morphological Traits
3.1.2. Root Morphological Traits
3.2. Multivariate Analysis
3.3. Correlations Between Cutting Growth and Morphology in the Soil/Peat/Sand Mixture and in Perlite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trad, M.; Le Bourvellec, C.; Gaaliche, B.; Renard, C.M.; Mars, M. Nutritional Compounds in Figs from the Southern Mediterranean Region. Int. J. Food Prop. 2013, 17, 491–499. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT. FAO Database. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 10 November 2024).
- Mars, M. Fig (Ficus carica L.) genetic resources and breeding. Acta Hortic. 2003, 605, 19–27. [Google Scholar] [CrossRef]
- Rasool, I.F.u.; Aziz, A.; Khalid, W.; Koraqi, H.; Siddiqui, S.A.; AL-Farga, A.; Lai, W.F.; Ali, A. Industrial Application and Health Prospective of Fig (Ficus carica) By-Products. Molecules 2023, 28, 960. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, Z.; Tarazkar, M.H.; Polat, A. World Fig Market. In The Fig: Botany, Production and Uses; Sarkhosh, A., Yavari, A.M., Ferguson, L., Eds.; CABI: Wallingford, UK, 2022; pp. 453–470. [Google Scholar]
- Boliani, A.C.; Ferreira, A.F.A.; Monteiro, L.N.H.; da Silva, M.S.C.; Rombola, A.D. Advances in propagation of Ficus carica L. Rev. Bras. Frutic. 2019, 41, e026. [Google Scholar] [CrossRef]
- Aljane, F.; Nahdi, S. Propagation of Some Local Fig (Ficus carica L.) Cultivars. By Aljane F. Propagation et conservation des cultivars du figuier (Ficus carica L.) en Tunisie. J. Algérien Zones Arid. 2006, 5, 29–37. [Google Scholar]
- Antunes, L.E.C.; Chalfun, N.N.J.; Pasqual, M.; Dutra, L.F.; Cavalcante-Alves, J.M. Factors affecting on rooting of figs (Ficus carica L.) cuttings. Acta Hortic. 2003, 605, 141–146. [Google Scholar] [CrossRef]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Mafrica, R.; Marchese, A.; Bruno, M.; Costa, F.; Fretto, S.; Marra, F.P.; Pangallo, S.; Quartararo, A.; Caruso, T. Morphological and molecular variability within the fig cultivar ‘Dottato’ in the Italian protected designation origin area ‘Fichi di Cosenza’. Acta Hortic. 2017, 1173, 29–33. [Google Scholar] [CrossRef]
- Costa, F.; Di Vaio, C.; Ferrara, G.; Fretto, S.; Mafrica, R.; Marchese, A.; Quartararo, A.; Marra, F.P.; Mennone, C.; Caruso, T. Genetic diversity of fig (Ficus carica L.) genotypes grown in Southern Italy reveled by the use of SSR markers. Acta Hortic. 2017, 1173, 75–79. [Google Scholar] [CrossRef]
- Mafrica, R.; De Bruno, A.; Piscopo, A.; Poiana, M.; Bruno, M.; Caruso, T. Cultivar and accessions of fig (Ficus carica L.) for breba production selected within the autochthonous germplasm of Calabria (South Italy). Acta Hortic. 2021, 1310, 29–34. [Google Scholar] [CrossRef]
- Mafrica, R.; De Bruno, A.; Piscopo, A.; Poiana, M. Performance evaluation of 40 fig accessions cultivated in Calabria: Study of qualitative parameters of breba production. J. Saudi Soc. Agric. Sci. 2023, 22, 98–106. [Google Scholar] [CrossRef]
- Nicola, S. Understanding root system to improve seedling quality. HortTechnology 1998, 8, 544–549. [Google Scholar] [CrossRef]
- Chalfun, N.N.J.; Cavalcete, J.M.; Noberto, M.P.M.; Pasaual, M.; Putra, L.F. Rooting of fig (Ficus carica L.) cutting; cutlintime and IBA. Acta Hortic. 2003, 605, 137–140. [Google Scholar] [CrossRef]
- Siddiqui, M.I.; Hussain, S.A. Effect of indole butyric acid and types of cuttings on root initiation of ficus Hawaii. Sarhad J. Agric. 2007, 23, 919–925. [Google Scholar]
- Rajeswara-Reddy, K.V.; Pulla-Reddy, C.; Goud, P.V. Effect of Auxins on the rooting of fig (Ficus carica L.) hardwood and semi hardwood cuttings. Indian J. Agric. Res. 2008, 42, 75–78. [Google Scholar]
- Zarei, N.; Aboutalebi, A.; Roshanzadeh, H. Evaluation the rooting behavior of Caprifig cultivars cuttings in Fars province. Sci. Agric. 2013, 3, 26–29. [Google Scholar]
- Aljane, F.; Nahdi, S. Propagation of Some Local Fig (Ficus carica L.) Cultivars by Hardwood Cuttings under the Field Conditions in Tunisia. Int. Sch. Res. Not. 2014, 2014, 809450. [Google Scholar] [CrossRef][Green Version]
- Sorgonà, A.; Cacco, G. Linking the physiological parameters of nitrate uptake with root morphology and topology in wheat (Triticum durum Desf.) and in citrus rootstock (Citrus volkameriana Ten e Pasq). Can. J. Bot. 2002, 80, 494–503. [Google Scholar] [CrossRef]
- Tato, L.; Lattanzio, V.; Ercole, E.; Dell’Orto, M.; Sorgonà, A.; Linsalata, V.; Salvioli di Fossalunga, A.; Novero, M.; Astolfi, S.; Abenavoli, M.R.; et al. Plasticity, exudation and microbiome-association of the root system of Pellitory-of-the-wall plants grown in environments impaired in iron availability. Plant Physiol. Biochem. 2021, 168, 27–42. [Google Scholar] [CrossRef]
- Vescio, R.; Abenavoli, M.R.; Sorgonà, A. Single and Combined Abiotic Stress in Maize Root Morphology. Plants 2021, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Tellah, S.; Badiani, M.; Trifilò, P.; Lo Gullo, M.A.; Ounane, G.; Ounane, S.M. Morpho-physiological traits contributing to water stress tolerance in a peanut (Arachis hypogaea L.) landraces collection from the Algerian Maghreb. Agrochimica 2014, 58, 126–147. [Google Scholar]
- Tucker, S.S.; Craine, J.M.; Nippert, J.B. Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere 2011, 2, 1–19. [Google Scholar] [CrossRef]
- Rewald, B.; Ephrath, J.E.; Rachmilevitch, S. A root is a root is a root? Water uptake rates of Citrus root orders. Plant Cell Environ. 2011, 34, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Caruso, T.; Mafrica, R.; Bruno, M.; Fratia, D.; Vescio, R.; Sorgonà, A. Effects of arbuscular mycorrhizal fungi formulations on the root morphological traits of rooted cuttings of two fig (Ficus carica L.) cultivars. Acta Hortic. 2021, 1310, 61–68. [Google Scholar] [CrossRef]
- Caruso, T.; Mafrica, R.; Bruno, M.; Vescio, R.; Sorgonà, A. Root architectural traits of rooted cuttings of two fig cultivars: Treatments with arbuscular mycorrhizal fungi formulation. Sci. Hortic. 2021, 283, 110083. [Google Scholar] [CrossRef]
- Kerbiriou, P.J.; Stomph, T.J.; Lammerts van Bueren, E.T.; Struik, P.C. Influence of transplant size on the above- and below-ground performance of four contrasting field-grown lettuce cultivars. Front. Plant Sci. 2013, 4, 379. [Google Scholar] [CrossRef]
- Tian, N.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Influence of Container Type and Growth Medium on Seedling Growth and Root Morphology of Cyclocarya paliurus during Nursery Culture. Forests 2017, 8, 387. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Feinstein, L.M.; Kershner, M.W.; Blackwood, C.B. The distribution of below-ground traits is explained by intrinsic species differences and intraspecific plasticity in response to root neighbours. J. Ecol. 2013, 101, 933–942. [Google Scholar] [CrossRef]
- Ryser, P.; Lambers, H. Root and leaf attributes accounting for the performance of fast and slow-growing grasses at different nutrient supply. Plant Soil 1995, 170, 51–265. [Google Scholar] [CrossRef]
- Afifi, A.; Clark, V.A.; May, S. Computer-Aided Multivariate Analysis, 4th ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Peche, P.M.; Moura, P.H.A.; Curi, P.N.; Melo, E.T.; Figueiredo, A.L.; Zambon, C.R.; Coutinho, G.; Pio, R. Rooting of apical cuttings of fig trees with a lopping system. Acta Hortic. 2017, 1173, 217–220. [Google Scholar] [CrossRef]
- Khapare, L.S.; Dahale, M.H.; Bhusari, R.B. Effect of plant growth regulators on rooting in cuttings of fig (Ficus carica L.) cv. Dinkar. Asian Sci. 2012, 7, 25–27. [Google Scholar]
- Singh, G.; Rattanpal, H.S. Effect of Indole Butyric Acid on Quantitative Measurement Responses of Nursery Plants of Fig (Ficus carica L.) Cv. Brown Turkey. Chem. Sci. Rev. Lett. 2017, 6, 88–93. [Google Scholar]
- Nava, G.A.; Júnior, A.W.; Mezalira, E.J.; Cassol, D.A.; Alegretti, A.L. Rooting of hardwood cuttings of Roxo de Valinhos fig (Ficus carica L.) with different propagation strategies. Rev. Ceres 2014, 61, 989–996. [Google Scholar] [CrossRef]
- Sousa, C.M.; Busquet, R.N.; Vasconcellos, M.A.; Miranda, R.M. Effects of auxin and misting on the rooting of herbaceous and hardwood cuttings from the fig tree. Rev. Ciência Agronômica 2013, 44, 334–338. [Google Scholar] [CrossRef]
- Bisi, R.B.; Locatelli, G.; Barbosa, C.; Pio, R.; Balbi, R.V. Rooting of stem segments from fig tree cultivars. Acta Sci. Agron. 2016, 38, 379–385. [Google Scholar] [CrossRef][Green Version]
- Arsov, T.; Saraginovski, N. Effect of auxin phytohormones on the rooting of some local populations of fig (Ficus carica L.) hardwood cuttings from North Macedonia. Acta Hortic. 2021, 1310, 49–54. [Google Scholar] [CrossRef]
- Mirsoleimani, A.; Zinati, Z.; Abbasi, S. New insights into the identification of biochemical traits linked to rooting percentage in fig (Ficus carica L.) cuttings. J. Berry Res. 2024, 14, 227–245. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.R.; Patel, M.J.; Singh, S. Effect of different levels of IBA and NAA on rooting of hardwood and semi hardwood cutting in fig. Int. J. Agric. Sci. Res. (IJASR) 2017, 7, 519–524. [Google Scholar]
- Inglis, J.E. Effects of some growth substances on the promotion and rooting of interfascicular shoots in Pinus caribaea. Plant Propagator 1984, 30, 4–6. [Google Scholar]
- Kane, M. Systems of rooting lignified and semi-lignified cuttings of Bombacopsis quinata in the nursery. In Informe de Investigación; Monterrey Forestal Ltda: Cartagena, Colombia, 1989; Volume 5. [Google Scholar]
- Hawrami, I.J.B.; Noori, I.M. Rooting of blackberry (Rubus fruticosus L.) hardwood cuttings as influenced by cutting time and cutting length. Kufa J. Agric. Sci. 2024, 16, 33–48. [Google Scholar] [CrossRef]
- Schuler, J.L.; McCarthy, W. Development of eastern cottonwood cuttings as modified by cutting length and surface area available for rooting. New For. 2015, 46, 547–559. [Google Scholar] [CrossRef]
- Oliveira, H.D.; Baliza, P.D.; Rezende, T.T.; De Carvalho, P.S.; Guimarães, J.R. Influence of cutting length and environment on the growth of coffee seedlings obtained by rooting. Coffee Sci. 2010, 5, 183–189. [Google Scholar]
- Mbabu, P.; Spethmann, W. Effect of Length of Cuttings, Substrate pH and Mineral Nutrition on Rooting of Pyrus communis Cultivars. Eur. J. Hort. Sci. 2005, 70, 189–194. [Google Scholar]
- Vâtcă, S.D.; Gâdea, Ș.; Vidican, R.; Șandor, M.; Stoian, V.; Vâtcă, A.; Horvath, A.; Stoian, V.A. Primary Growth Effect of Salix viminalis L. CV. Inger and Tordis in Controlled Conditions by Exploring Optimum Cutting Lengths and Rhizogenesis Treatments. Sustainability 2022, 14, 9272. [Google Scholar] [CrossRef]
- Shamsuddin, M.S.; Shahari, R.; Amri, C.N.A.C.; Tajudin, N.S.; Mispan, M.R.; Salleh, M.S. Early Development of Fig (Ficus carica L.) Root and Shoot Using Different Propagation Medium and Cutting Types. Trop. Life Sci. Res. 2021, 32, 83–90. [Google Scholar] [CrossRef] [PubMed]
- de la Riva, E.G.; Olmo, M.; Poorter, H.; Ubera, J.L.; Villar, R. Leaf Mass per Area (LMA) and Its Relationship with Leaf Structure and Anatomy in 34 Mediterranean Woody Species along a Water Availability Gradient. PLoS ONE 2016, 11, e0148788. [Google Scholar] [CrossRef] [PubMed]
- Sivaji, T.; Madhavi, K.; Sudha, V.V. Effect of type of cuttings and IBA concentrations on the propagation of fig (Ficus carica) cv. Poona fig under open conditions. Trends Biosci. 2014, 7, 1087–1089. [Google Scholar]
- Patel, H.R.; Patel, M.J. Role of Auxins on Rooting of Different Types of Cuttings in Fig. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1317–1322. [Google Scholar] [CrossRef][Green Version]
- Tinker, P.B.; Nye, P.H. Solute Movement in the Rhizosphere; Oxford University Press: New York, NY, USA; Oxford, UK, 2000. [Google Scholar]
- Wasson, A.; Richards, R.; Chatrath, R.; Misra, S.; Prasad, S.S.; Rebetzke, G.; Kirkegaard, J.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, X.; Feng, Y.; Jilani, G. Differential response of root morphology to potassium deficient stress among rice genotypes varying in potassium efficiency. J. Zhejiang Univ. Sci. 2008, 9, 427–434. [Google Scholar] [CrossRef]
- Hawkins, B.J.; Robbins, S.; Porter, R.B. Nitrogen uptake over entire root systems of tree seedlings. Tree Physiol. 2014, 34, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Lazof, D.B.; Rufty, T.W.; Redinbaugh, M.G. Localization of nitrate absorption and translocation within morphological regions of the corn root. Plant Physiol. 1992, 100, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Ohland, T.; Pio, R.; Chagas, E.A.; Barbosa, W.; Dalastra, I.M.; Kotz, T.E. Enraizamento de estacas apicais lenhosas de figueira ‘Roxo de Valinhos’ com aplicação de AIB e cianamida hidrogenada. Rev. Bras. Frutic. 2009, 31, 273–279. [Google Scholar] [CrossRef]
- Di Mambro, R.; Sabatini, S.; Dello Ioio, R. Patterning the Axes: A Lesson from the Root. Plants 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T., Jr.; Geneve, R.L. Plant Propagation: Principles and Practices, 7th ed.; Prentice Hall: Hoboken, NJ, USA, 2002; p. 880. [Google Scholar]
- Fitter, A.H. Characteristics and functions of root systems. In Plants Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkaki, K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1991; pp. 3–25. [Google Scholar]
- Christie, E.K.; Moorby, J. Physiological responses of semiarid grasses. I. The influence of phosphorus supply on growth and phosphorus absorption. Aust. J. Agric. Res. 1975, 26, 423–436. [Google Scholar] [CrossRef]
- Goss, M.J. Effect of mechanical impedance on growth of seedlings. J. Exp. Bot. 1977, 28, 96–111. [Google Scholar] [CrossRef]
- Miguel, M.A.; Postma, J.A.; Lynch, J.P. Phene Synergism between Root Hair Length and Basal Root Growth Angle for Phosphorus Acquisition. Plant Physiol. 2015, 167, 1430–1439. [Google Scholar] [CrossRef]
- Ryser, P. Intra and interspecific variation in root length, root turnover and the underlying parameters. In Inherent Variation in Plant Growth: Physiological Mechanisms and Ecological Consequences; Lambers, H., Poorter, H., VanVuuren, M.M.I., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 441–465. [Google Scholar]
- Romano, A.; Sorgonà, A.; Lupini, A.; Araniti, F.; Stevanato, P.; Cacco, G.; Abenavoli, M.R. Morpho-physiological responses of sugar beet (Beta vulgaris L.) genotypes to drought stress. Acta Phys. Plant 2013, 35, 853–865. [Google Scholar] [CrossRef]
- Peman, J.; Voltas, J.; Gil-Pelegrin, E. Morphological and functional variability in the root system of Quercus ilex L. subject to confinement: Consequences for afforestation. Ann. For. Sci. 2006, 63, 425–430. [Google Scholar] [CrossRef]
- Wells, C.E.; Eissenstat, D.M. Marked differences in survivorship among apple roots of different diameters. Ecology 2001, 82, 882–892. [Google Scholar] [CrossRef]
- Rytter, R.M. The effect of limited availability of N or water on C allocation to fine roots and annual fine root turnover in Alnus incana and Salix viminalis. Tree Physiol. 2013, 33, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rao, I.M.; Horst, W.J. Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil 2013, 372, 3–25. [Google Scholar] [CrossRef]
- Rao, I.M.; Miles, J.W.; Beebe, S.E.; Horst, W.J. Root adaptations to soils with low fertility and aluminium toxicity. Ann. Bot. 2016, 118, 593–605. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernández-Fernández, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.G.; Ribalta, F.M.; Pazos-Navarro, M.; Leonforte, A.; Croser, J.S. Discrimination of boron tolerance in Pisum sativum L. genotypes using a rapid, high-throughput hydroponic screen and precociously germinated seed grown under far-red enriched light. Plant Methods 2017, 13, 70. [Google Scholar] [CrossRef]
- Singh, D.H.; Dikshit, H.K.; Singh, R.A. A new phenotyping technique for screening for drought tolerance in lentil (Lens culinaris Medik.). Plant Breed. 2013, 132, 185–190. [Google Scholar] [CrossRef]


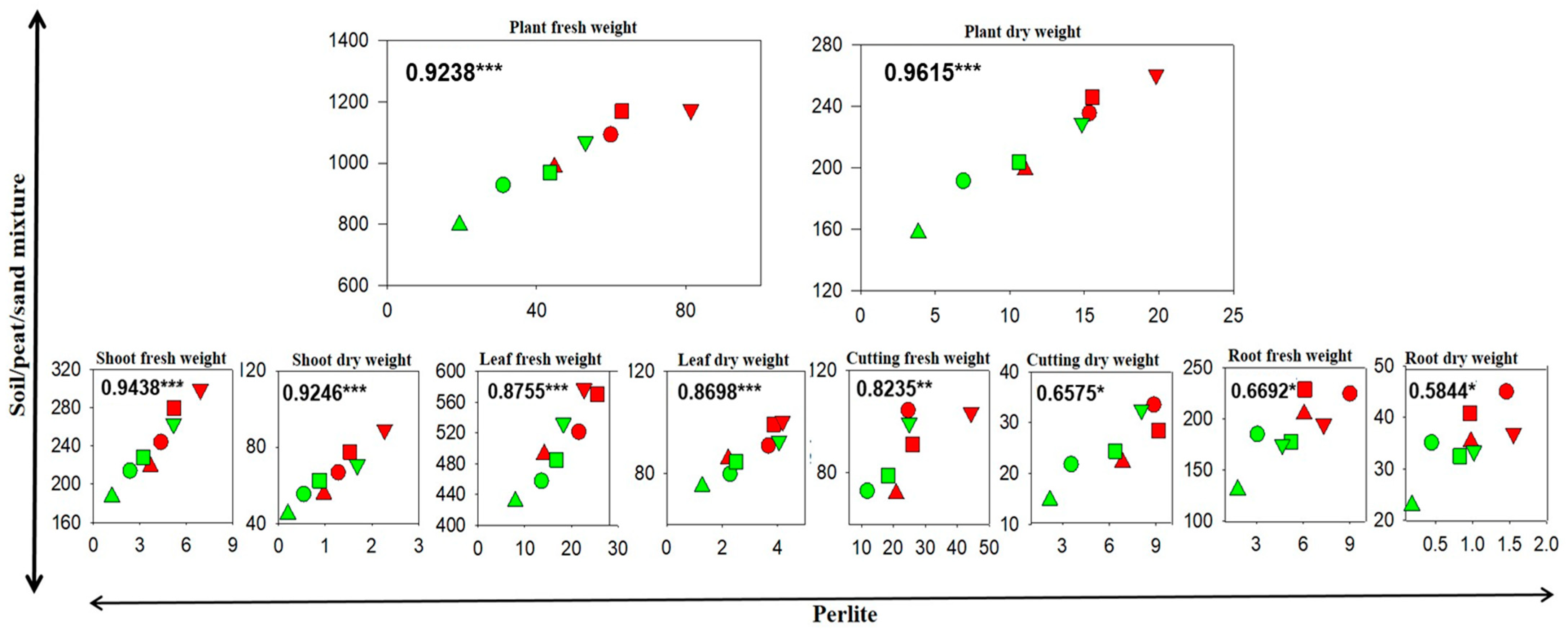
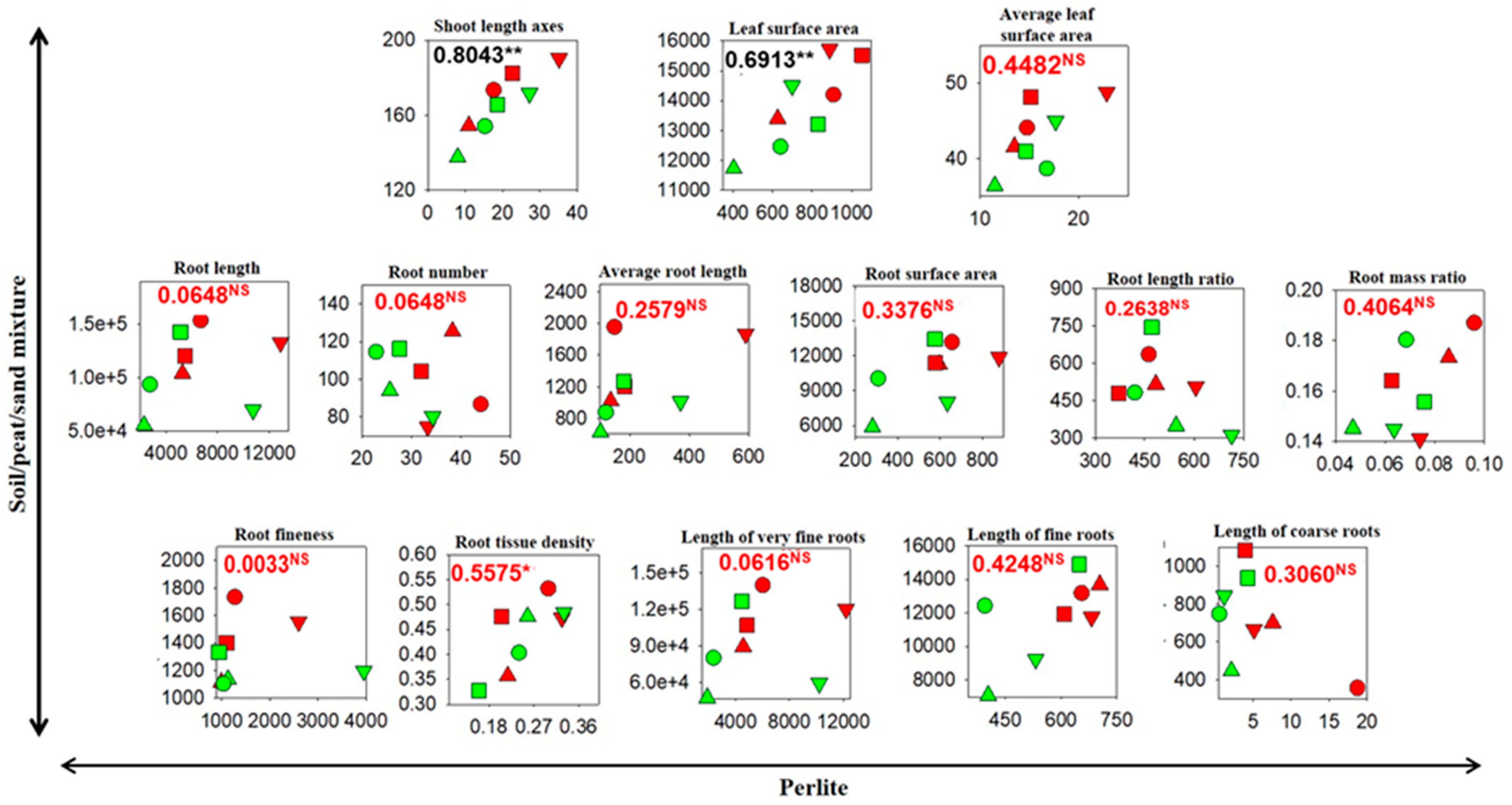
| Parameter | Statistics # | Cutting Length (CL) | Cutting Type (CT) | CL Average | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| Rooting rate (%) | CT 19.26 *** CL 11.36 ** CT × CL 0.56 NS | A | 59.0 ± 1.8 | 76.4 ± 3.9 | 81.3 ± 5.0 | 86.8 ± 3.1 | 75.9 X |
| B | 50.0 ± 3.3 | 60.4 ± 4.7 | 73.6 ± 3.1 | 80.6 ± 4.2 | 66.2 Y | ||
| CT average | 54.5 C | 68.4 B | 77.4 AB | 83.7 A | |||
| Parameters | Statistics # | Cutting Length (CL) | Cutting Type (CT) | CL Average | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| Plant fresh biomass (g) | CT 18.55 *** CL 55.73 *** CT × CL 0.41 NS | A | 45.3 a ± 12.3 | 60.4 a ± 11.7 | 63.3 a ± 11.3 | 81.1 a ± 17.3 | 62.2 X |
| B | 19.2 b ± 4.0 | 31.3 b ± 8.2 | 44.2 b ± 13.1 | 53.3 b ± 14.2 | 36.8 Y | ||
| CT average | 32.3 C | 45.97 BC | 53.8 AB | 67.2 A | |||
| Shoot axes fresh biomass (g) | CT 12.63 *** CL 22.73 *** CT × CL 0.14 NS | A | 3.7 a ± 1.2 | 4.4 a ± 1.8 | 5.2 a ± 0.9 | 6.9 a ± 2.1 | 5.1 X |
| B | 1.2 b ± 0.3 | 2.4 b ± 1.6 | 3.2 b ± 1.6 | 1.7 b ± 0.9 | 3.0 Y | ||
| CT average | 2.45 C | 3.41 BC | 4.21 B | 6.04 A | |||
| Leaf fresh biomass (g) | CT 9.52 *** CL 21.00 *** CT × CL 0.43 NS | A | 14.2 a ± 7.0 | 21.7 a ± 4.5 | 26.0 a ± 6.0 | 23.0 a ± 5.0 | 21.2 X |
| B | 8.0 b ± 2.6 | 13.7 b ± 4.8 | 17.0 b ± 6.0 | 18.0 b ± 4.0 | 14.2 Y | ||
| CT average | 11.1 B | 17.7 AB | 21.5 A | 20.5 A | |||
| Cutting fresh biomass (g) | CT 21.57 *** CL 48.88 *** CT × CL 1.65 NS | A | 21.0 a ± 6.0 | 25.0 a ± 6.0 | 26.0 a ± 7.0 | 44.0 a ± 11.0 | 29.0 X |
| B | 8.0 b ± 2.0 | 12.0 b ± 13.0 | 18.0 b ± 5.0 | 25.0 b ± 7.0 | 15.9 Y | ||
| CT average | 14.6 B | 18.4 AB | 22.2 AB | 34.7 A | |||
| Root fresh biomass (g) | CT 1.60 NS CL 18.90 *** CT × CL 1.87 NS | A | 6.1 a ± 2.6 | 9.0 a ± 4.1 | 6.1 a ± 1.2 | 7.3 a ± 3.6 | 7.1 |
| B | 1.8 b ± 0.5 | 3.1 b ± 1.2 | 5.2 b ± 2.6 | 4.7 b ± 3.2 | 3.7 | ||
| CT average | 3.9 A | 6.1 A | 5.7 A | 6.0 A | |||
| Plant dry biomass (g) | CT 22.50 *** CL 54.07 *** CT × CL 1.00 NS | A | 11.0 ± 3.2 | 15.4 ± 3.3 | 15.5 ± 3.3 | 19.8 ± 3.9 | 15.4 X |
| B | 3.9 ± 0.7 | 6.9 ± 1.4 | 10.6 ± 3.3 | 14.8 ± 3.5 | 9.1 Y | ||
| CT average | 7.4 B | 11.1 B | 13.1 AB | 17.3 A | |||
| Shoot axes dry biomass (g) | CT 12.41 *** CL 16.64 ** CT × CL 0.06 NS | A | 1.0 ± 0.4 | 1.3 ± 0.5 | 1.5 ± 0.2 | 2.3 ± 1.1 | 1.5 X |
| B | 0.2 ± 0.1 | 0.6 ± 0.3 | 0.9 ± 0.5 | 1.7 ± 0.9 | 0.8 Y | ||
| CT average | 0.6 C | 0.9 B | 1.2 B | 1.2 A | |||
| Leaf dry biomass (g) | CT 12.04 *** CL 11.52 ** CT × CL 1.08 NS | A | 2.2 ± 0.4 | 3.7 ± 0.4 | 3.9 ± 0.5 | 4.2 ± 0.5 | 3.5 X |
| B | 1.3 ± 0.2 | 2.3 ± 0.3 | 2.5 ± 0.3 | 4.1 ± 0.4 | 2.5 Y | ||
| CT average | 1.8 B | 3.0 AB | 3.2 AB | 4.1 A | |||
| Cutting dry biomass (g) | CT 18.15 *** CL 58.12 *** CT × CL 1.05 NS | A | 6.9 ± 0.9 | 8.9 ± 1.1 | 9.2 ± 1.0 | 11.8 ± 0.9 | 9.2 X |
| B | 2.2 ± 0.2 | 3.6 ± 0.3 | 6.4 ± 0.8 | 8.1 ± 0.6 | 5.1 Y | ||
| CT average | 4.52 B | 6.25 AB | 7.79 AB | 9.94 A | |||
| Root dry biomass (g) | CT 1.89 NS CL 8.64 ** CT × CL 0.81 NS | A | 1.0 ± 0.2 | 1.5 ± 0.5 | 1.0 ± 0.1 | 1.6 ± 0.5 | 1.2 X |
| B | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.2 | 1.0 ± 0.4 | 0.6 Y | ||
| CT average | 0.6 A | 1.0 A | 0.9 A | 1.3 A | |||
| Parameters | Statistics # | Cutting Length (CL) | Cutting Type (CT) | CL Average | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| Plant fresh biomass (g) | CT 13.63 *** CL 38.09 *** CT × CL 0.64 NS | A | 987 ± 26 | 1092 ± 40 | 1170 ± 48 | 1174 ± 31 | 1106 X |
| B | 798 ± 26 | 927 ± 32 | 969 ± 52 | 1070 ± 37 | 941 Y | ||
| CT average | 893 C | 1009 B | 1069 AB | 1122 A | |||
| Shoot axes fresh biomass (g) | CT 53.43 *** CL 66.07 *** CT × CL 0.14 NS | A | 219 ± 5 | 243 ± 2 | 280 ± 7 | 299 ± 7 | 260 X |
| B | 187 ± 6 | 213 ± 6 | 228 ± 8 | 263 ± 9 | 223 Y | ||
| CT average | 203 D | 228 C | 254 B | 281 A | |||
| Leaf fresh biomass (g) | CT 19.05 *** CL 45.47 *** CT × CL 0.76 NS | A | 492 ± 8 | 521 ± 7 | 570 ± 16 | 577 ± 12 | 540 X |
| B | 431 ± 12 | 457 ± 12 | 485 ± 16 | 532 ± 18 | 476 Y | ||
| CT average | 461 B | 489 B | 528 A | 555 A | |||
| Cutting fresh biomass (g) | CT 8.19 ** CL 8.71 ** CT × CL 1.01 NS | A | 72 ± 9 | 104 ± 8 | 91 ± 7 | 103 ± 12 | 93 X |
| B | 50 ± 5 | 73 ± 5 | 79 ± 8 | 99 ± 10 | 75 Y | ||
| CT average | 61 B | 88 A | 85 A | 101 A | |||
| Root fresh biomass (g) | CT 1.38 NS CL 9.76 ** CT × CL 0.59 NS | A | 204 ± 25 | 224 ± 33 | 229 ± 23 | 194 ± 5 | 213 X |
| B | 130 ± 10 | 184 ± 20 | 177 ± 23 | 175 ± 15 | 167 Y | ||
| CT average | 167 | 204 | 203 | 185 | |||
| Plant dry biomass (g) | CT 16.86 *** CL 33.78 *** CT × CL 0.17 NS | A | 199 ± 8 | 235 ± 11 | 246 ± 10 | 261 ± 11 | 235 X |
| B | 158 ± 5 | 191 ± 9 | 204 ± 13 | 229 ± 9 | 195 Y | ||
| CT average | 178 C | 213 B | 225 AB | 245 A | |||
| Shoot axes dry biomass (g) | CT 96.12 *** CL 115.11 *** CT × CL 2.06 NS | A | 55 ± 0.5 | 66 ± 1.5 | 77 ± 1.9 | 89 ± 2.1 | 72 X |
| B | 45 ± 1.2 | 55 ± 2.0 | 62 ± 2.6 | 71 ± 1.9 | 58 Y | ||
| CT average | 50 D | 61 C | 70 B | 80 A | |||
| Leaf dry biomass (g) | CT 19.05 *** CL 45.47 *** CT × CL 0.76 NS | A | 86 ± 1.4 | 91 ± 1.3 | 99 ± 2.9 | 101 ± 2.1 | 94 X |
| B | 75 ± 2.1 | 80 ± 2.1 | 84 ± 2.8 | 93 ± 3.2 | 83 Y | ||
| CT average | 80 B | 85 B | 92 A | 97 A | |||
| Cutting dry biomass (g) | CT 7.84 *** CL 8.06 ** CT × CL 0.98 NS | A | 22 ± 3 | 33 ± 3 | 28 ± 2 | 34 ± 5 | 30 X |
| B | 15 ± 2 | 22 ± 2 | 24 ± 3 | 33 ± 4 | 23 Y | ||
| CT average | 19 B | 28 A | 26 AB | 33 A | |||
| Root dry biomass (g) | CT 1.78 NS CL 6.22 * CT × CL 0.31 NS | A | 35 ± 6 | 45 ± 7 | 41 ± 4 | 37 ± 3 | 39 X |
| B | 23 ± 1 | 35 ± 5 | 32 ± 5 | 33 ± 4 | 31 Y | ||
| CT average | 29 | 40 | 37 | 35 | |||
| Parameters | Statistics # | Cutting Length (CL) | Cutting Type (CT) | CL Average | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| Total length of the whole root system (cm) | CT 6.29 *** CL 2.59 NS CT × CL 0.2750 NS | A | 5273 ± 3048 | 6734 ± 3808 | 5458 ± 2523 | 12,883 ± 11,166 | 7587 |
| B | 2302 ± 2001 | 2803 ± 1735 | 5125 ± 3105 | 10,746 ± 5663 | 5244 | ||
| CT average | 3787 B | 4769 B | 5291 B | 11,815 A | |||
| Total surface area of the whole root system (cm2) | CT 2.17 NS CL 5.72 * CT × CL 0.67 NS | A | 596 ± 358 | 659 ± 213 | 579 ± 248 | 878 ± 541 | 678 x |
| B | 281 ± 296 | 311 ± 190 | 575 ± 305 | 634 ± 353 | 450 y | ||
| CT average | 439 | 485 | 577 | 756 | |||
| Average diameter of the whole root system (cm) | CT 1.60 NS CL 0.83 NS CT × CL 0.23 NS | A | 0.60 ± 0.59 | 0.43 ± 0.60 | 0.53 ± 0.36 | 0.73 ± 0.37 | 0.58 |
| B | 0.38 ± 0.24 | 0.37 ± 0.18 | 0.44 ± 0.18 | 0.73 ± 0.64 | 0.48 | ||
| CT average | 0.50 | 0.40 | 0.49 | 0.73 | |||
| Adventitous root number (n) | CT 0.23 NS CL 5.74 * CT × CL 1.57 NS | A | 38.33 ± 15.37 | 44.17 ± 15.37 | 32.00 ± 8.07 | 33.33 ± 20.06 | 36.9 x |
| B | 25.67 ± 7.94 | 23.00 ± 11.52 | 27.67 ± 6.89 | 34.33 ± 16.13 | 27.6 y | ||
| CT average | 32.00 | 33.58 | 29.83 | 33.83 | |||
| Average length of the adventitous root system (cm) | CT 4.00 ** CL 0.71 NS CT × CL 0.34 NS | A | 136 ± 66 | 151 ± 56 | 182 ± 97 | 590 ± 766 | 265 |
| B | 102 ± 96 | 122 ± 73 | 180 ± 85 | 371 ± 254 | 194 | ||
| CT average | 119 B | 137 B | 181 AB | 480 A | |||
| Parameters | Statistics # | Cutting Length (CL) | Cutting Type (CT) | CL Average | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| Total length of the whole root system (cm) | CT 2.17 NS CL 5.59 * CT × CL 1.64 NS | A | 103,766 ± 35,088 | 152,747 ± 67,185 | 119,838 ± 36,323 | 132,528 ± 63,805 | 127,220 x |
| B | 55,295 ± 12,306 | 92,783 ± 27,501 | 142,410 ± 76,696 | 69,542 ± 43,411 | 90,007 y | ||
| CT average | 79,530 | 122,765 | 131,124 | 101,035 | |||
| Total surface area of the whole root system (cm2) | CT 1.58 NS CL 3.56 NS CT × CL 1.40 NS | A | 11,261 ± 2995 | 13,131 ± 5624 | 11,380 ± 2968 | 11,859 ± 5358 | 11,908 |
| B | 5856 ± 850 | 10,005 ± 2675 | 13,442 ± 4636 | 8021 ± 6464 | 9330 | ||
| CT average | 8559 | 11,568 | 12,410 | 9940 | |||
| Average diameter of the whole root system (cm) | CT 0.84 NS CL 0.14 NS CT × CL 4.27 * | A | 0.56 a ± 0.32 | 0.53 a ± 0.37 | 0.34 b ± 0.11 | 0.32 a ± 0.19 | 0.44 |
| B | 0.27 b ± 0.10 | 0.29 b ± 0.07 | 0.69 a ± 0.20 | 0.39 a ± 0.27 | 0.41 | ||
| CT average | 0.41 | 0.41 | 0.51 | 0.36 | |||
| Adventitous root number (n) | CT 1.82 NS CL 0.09 NS CT × CL 1.22 NS | A | 126 ± 73 | 86 ± 39 | 104 ± 19 | 75 ± 23 | 101 |
| B | 94 ± 25 | 114 ± 34 | 116 ± 21 | 80 ± 20 | 98 | ||
| CT average | 109.80 | 100.30 | 110.20 | 77.50 | |||
| Average length of the adventitous root system (cm) | CT 2.17 NS CL 5.59 * CT × CL 1.64 NS | A | 1019 ± 662 | 1948 ± 816 | 1199 ± 457 | 1866 ± 841 | 1508 x |
| B | 624 ± 201 | 866 ± 292 | 1270 ± 680 | 1011 ± 776 | 942 y | ||
| CT average | 822 | 1407 | 1235 | 1438 | |||
| Parameters | Statistics # | Cutting Length (CL) | Cutting Type (CT) | CL Average | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| RLR (cm/g) | CT 1.43 * CL 0.40 NS CT × CL 0.15 NS | A | 484 ± 236 | 464 ± 264 | 378 ± 227 | 606 ± 443 | 48 |
| B | 545 ± 385 | 422 ± 279 | 467 ± 240 | 714 ± 338 | 538 | ||
| CT average | 515 AB | 443 AB | 422 B | 660 A | |||
| RMR (g/g) | CT 0.37 NS CL 1.75 NS CT × CL 0.89 NS | A | 0.09 ± 0.03 | 0.10 ± 0.08 | 0.05 ± 0.02 | 0.07 ± 0.05 | 0.08 |
| B | 0.05 ± 0.01 | 0.07 ± 0.04 | 0.09 ± 0.05 | 0.06 ± 0.04 | 0.06 | ||
| CT average | 0.07 | 0.08 | 0.07 | 0.07 | |||
| Root fineness (cm/cm3) | CT 22.62 *** CL 1.42 NS CT × CL 2.55 NS | A | 993 ± 171 | 1291 ± 881 | 1100 ± 169 | 2600 ± 1421 | 1496 |
| B | 1132 ± 352 | 1055 ± 154 | 944 ± 209 | 3953 ± 1438 | 1771 | ||
| CT average | 1063 B | 1173 B | 1022 B | 3276 A | |||
| Root tissue density (g/cm3) | CT 1.11 NS CL 0.07 NS CT × CL 0.16 NS | A | 0.22 ± 0.17 | 0.30 ± 0.26 | 0.17 ± 0.08 | 0.33 ± 0.19 | 0.26 |
| B | 0.26 ± 0.37 | 0.24 ± 0.16 | 0.22 ± 0.17 | 0.33 ± 0.14 | 0.25 | ||
| CT average | 0.24 | 0.27 | 0.183 | 0.33 | |||
| vFi (cm) | CT 6.69 *** CL 2.39 NS CT × CL 0.24 NS | A | 4560 ± 2625 | 6056 ± 3790 | 4840 ± 2325 | 12,190 ± 10,990 | 6912 |
| B | 1896 ± 1490 | 2410 ± 1550 | 4470 ± 2950 | 10,210 ± 5320 | 4744 | ||
| CT average | 3228 B | 4231 B | 4656 B | 11,198 A | |||
| Fi (cm) | CT 0.22 NS CL 2.77 NS CT × CL 0.58 NS | A | 704.20 ± 429 | 657.20 ± 176 | 608.60 ± 232 | 682 ± 364 | 663 |
| B | 403.90 ± 570 | 396.50 ± 227 | 650.05 ± 174 | 531 ± 401 | 495 | ||
| CT average | 554 | 526 | 629 | 606 | |||
| C (cm) | CT 0.95 NS CL 5.08 * CT × CL 1.77 NS | A | 7.60 ± 15.90 | 18.80 ± 21.90 | 3.90 ± 4.40 | 5.10 ± 6.20 | 8.86 X |
| B | 2.20 ± 4.50 | 0.65 ± 0.80 | 4.30 ± 6.80 | 1.20 ± 3.00 | 2.10 Y | ||
| CT average | 4.88 | 9.70 | 4.10 | 3.20 | |||
| Parameters | Statistics # | Cutting Length (CL) | Cutting Type (CT) | CL Average | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| RLR (cm/g) | CT 1.58 NS CL 0.65 NS CT × CL 1.97 NS | A | 516 ± 146 | 633 ± 228 | 480 ± 103 | 506 ± 239 | 534 |
| B | 350 ± 719 | 480 ± 117 | 743 ± 530 | 312 ± 199 | 470 | ||
| CT average | 433 | 556 | 611 | 410 | |||
| RMR (g/g) | CT 1.93 NS CL 0.67 NS CT × CL 0.31 NS | A | 0.17 ± 0.06 | 0.19 ± 0.05 | 0.16 ± 0.03 | 0.14 ± 0.02 | 0.17 |
| B | 0.15 ± 0.01 | 0.18 ± 0.05 | 0.16 ± 0.04 | 0.14 ± 0.03 | 0.16 | ||
| CT average | 0.16 | 0.18 | 0.16 | 0.14 | |||
| Root fineness (cm/cm3) | CT 1.16 NS CL 4.49 * CT × CL 1.46 NS | A | 1114 ± 438 | 1730 ± 3279 | 1401 ± 281 | 1553 ± 400 | 1450 x |
| B | 1135 ± 329 | 1098 ± 312 | 1332 ± 501 | 1196 ± 450 | 1190 y | ||
| CT average | 1125 | 1410 | 1366 | 1375 | |||
| Root tissue density (g/cm3) | CT 0.69 NS CL 0.82 NS CT × CL 2.08 NS | A | 0.36 ± 0.11 | 0.53 ± 0.13 | 0.48 ± 0.05 | 0.47 ± 0.15 | 0.46 |
| B | 0.48 ± 0.15 | 0.40 ± 0.06 | 0.33 ± 0.12 | 1.08 ± 1.35 | 0.42 | ||
| CT average | 0.42 | 0.47 | 0.40 | 0.47 | |||
| vFi (cm) | CT 2.13 NS CL 5.76 * CT × CL 1.64 NS | A | 89,330 ± 34,036 | 139,197 ± 63,160 | 106,783 ± 34,063 | 120,060 ± 61,127 | 113,842 x |
| B | 47,746 ± 12,258 | 79,605 ± 25,512 | 126,537 ± 73,671 | 59,424 ± 35,309 | 78,328 y | ||
| CT average | 68,538 A | 109,400 A | 116,660 A | 89,742 A | |||
| Fi (cm) | CT 1.16 NS CL 4.49 * CT × CL 1.46 NS | A | 13,685 ± 4576 | 13,136 ± 5566 | 11,910 ± 3462 | 11,739 ± 2988 | 12,618 x |
| B | 7079 ± 1475 | 12,384 ± 4586 | 14,862 ± 3142 | 9244 ± 9071 | 10,893 y | ||
| CT average | 10,382 | 12,760 | 13,386 | 10,492 | |||
| C (cm) | CT 1.80 NS CL 0.07 NS CT × CL 0.07 NS | A | 701 ± 580 | 353 ± 244 | 1084 ± 550 | 666 ± 175 | 701 |
| B | 446 ± 377 | 745 ± 272 | 939 ± 265 | 843 ± 1002 | 744 | ||
| CT average | 574 | 549 | 1012 | 755 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafrica, R.; Bruno, M.; Fiozzo, V.; Caridi, R.; Sorgonà, A. Rooting, Growth, and Root Morphology of the Cuttings of Ficus carica L. (cv. “Dottato”): Cutting Types and Length and Growth Medium Effects. Plants 2025, 14, 160. https://doi.org/10.3390/plants14020160
Mafrica R, Bruno M, Fiozzo V, Caridi R, Sorgonà A. Rooting, Growth, and Root Morphology of the Cuttings of Ficus carica L. (cv. “Dottato”): Cutting Types and Length and Growth Medium Effects. Plants. 2025; 14(2):160. https://doi.org/10.3390/plants14020160
Chicago/Turabian StyleMafrica, Rocco, Marcello Bruno, Vincenzo Fiozzo, Roberta Caridi, and Agostino Sorgonà. 2025. "Rooting, Growth, and Root Morphology of the Cuttings of Ficus carica L. (cv. “Dottato”): Cutting Types and Length and Growth Medium Effects" Plants 14, no. 2: 160. https://doi.org/10.3390/plants14020160
APA StyleMafrica, R., Bruno, M., Fiozzo, V., Caridi, R., & Sorgonà, A. (2025). Rooting, Growth, and Root Morphology of the Cuttings of Ficus carica L. (cv. “Dottato”): Cutting Types and Length and Growth Medium Effects. Plants, 14(2), 160. https://doi.org/10.3390/plants14020160









