Application of Common Culinary Herbs for the Development of Bioactive Materials
Abstract
1. Introduction
2. Results
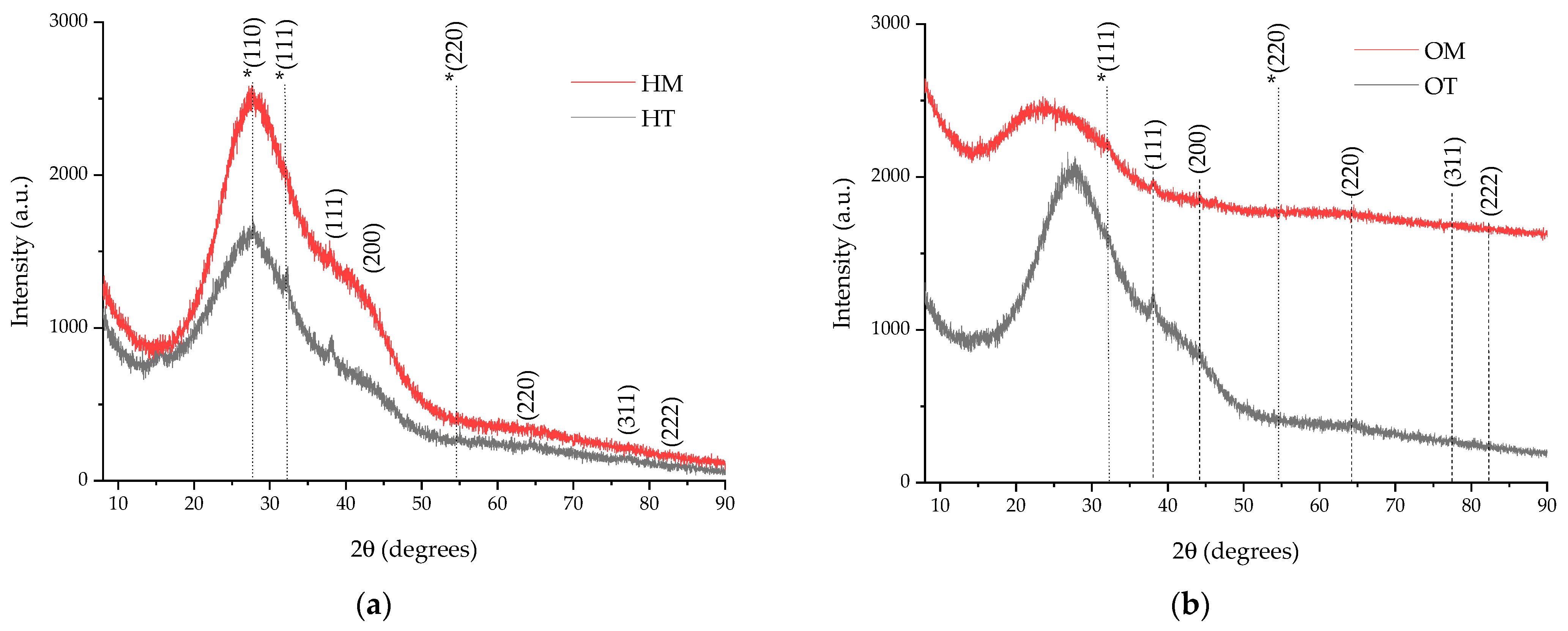
2.1. Extracts and NP Characterization
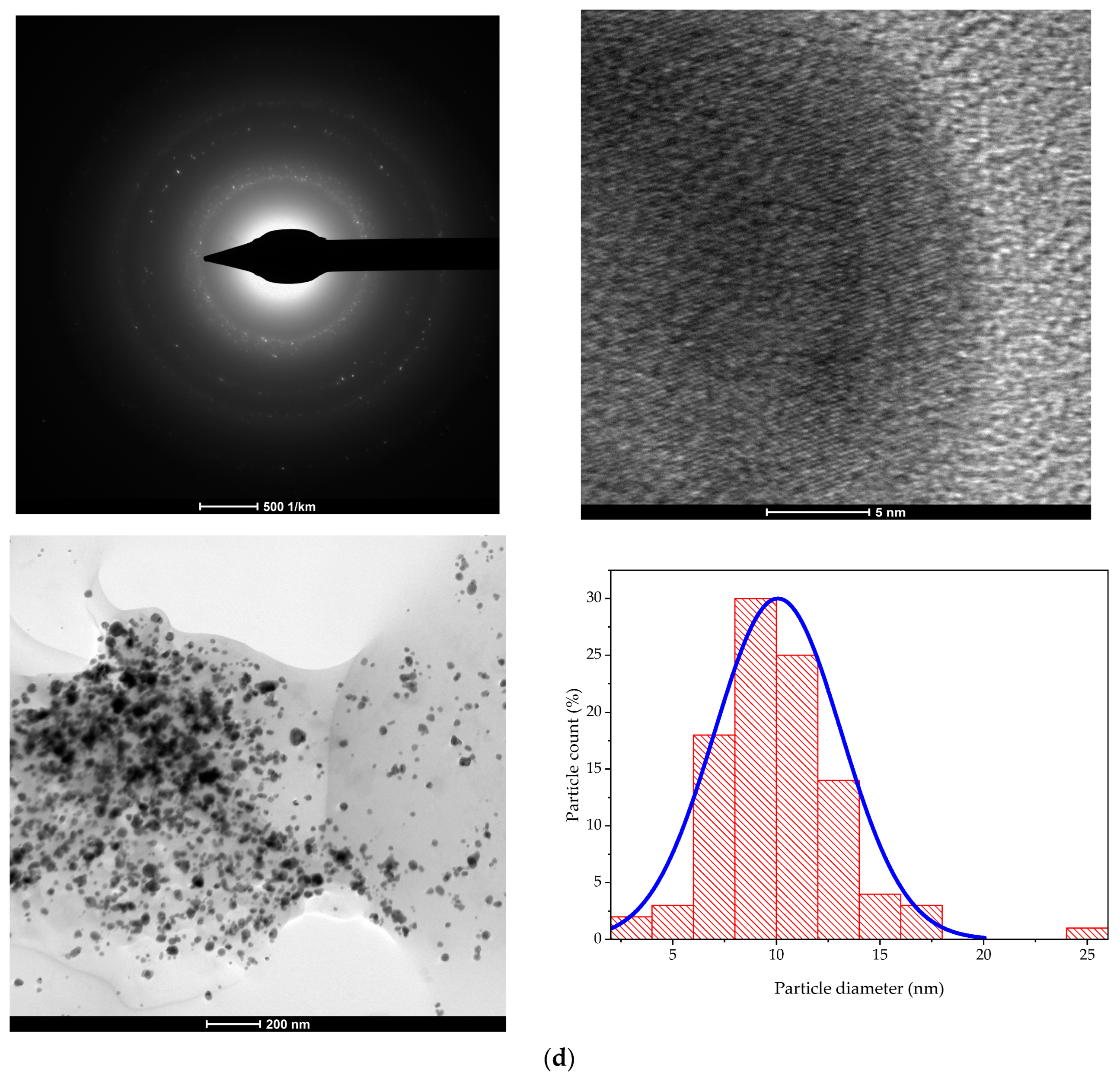
2.2. Characterization of Nanoparticles
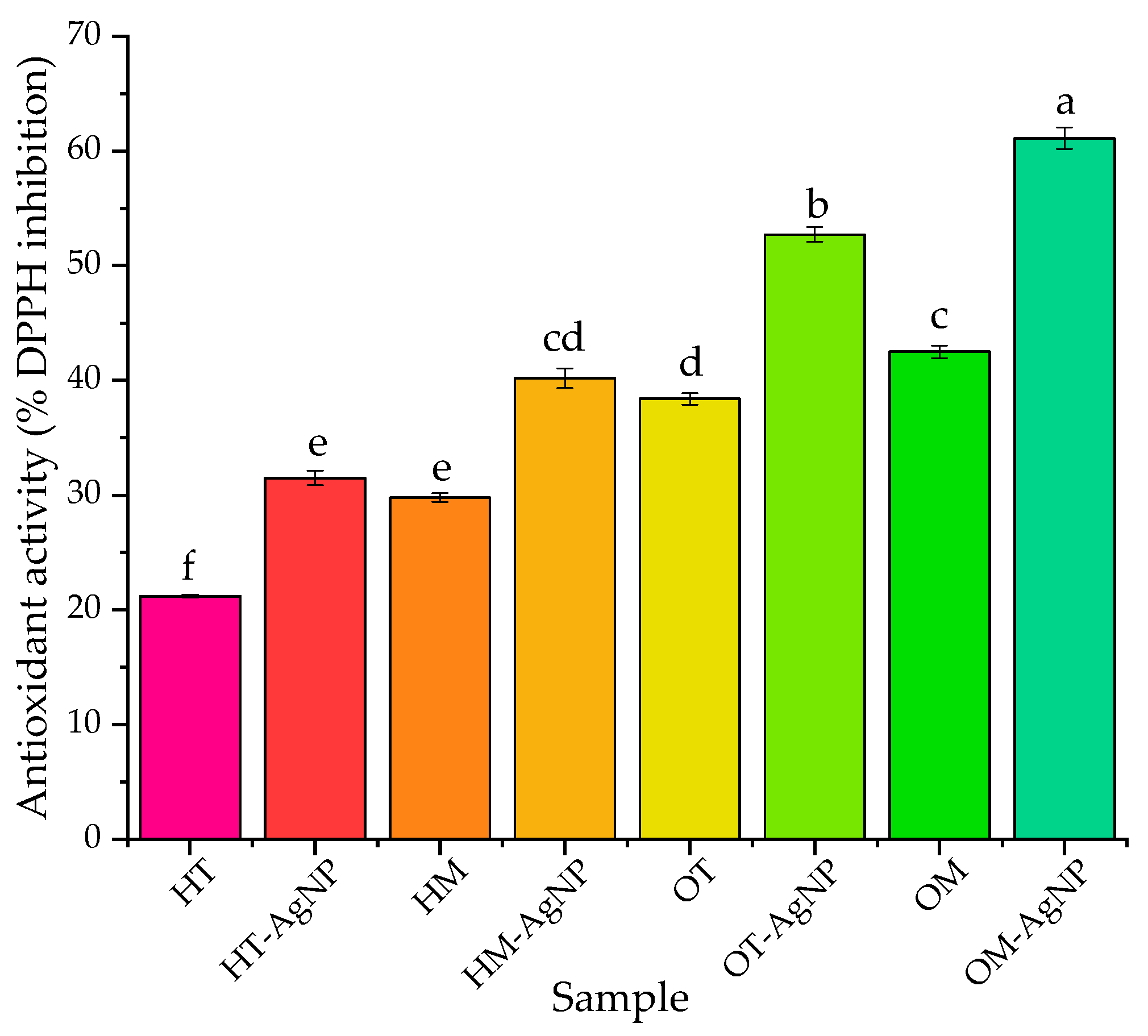
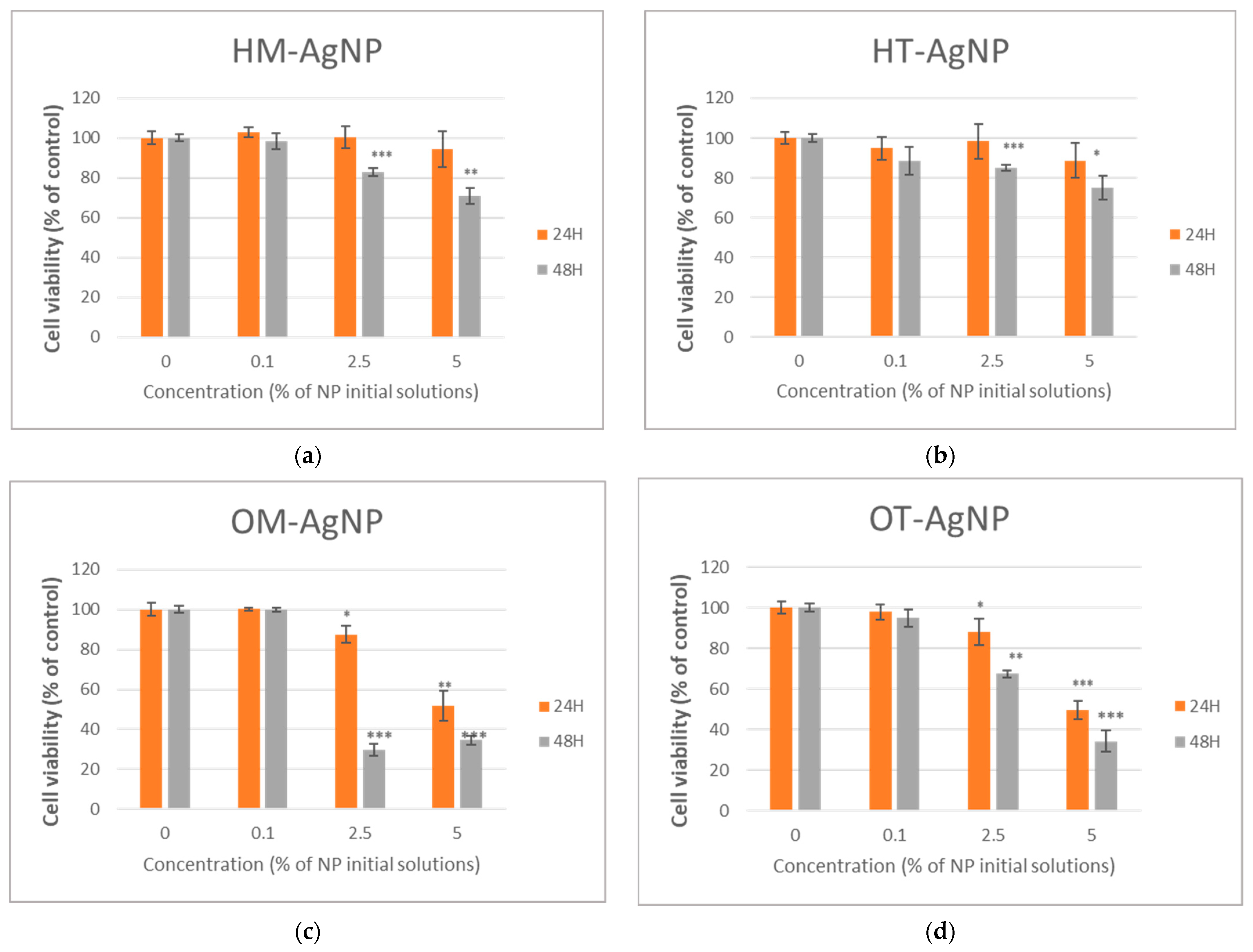
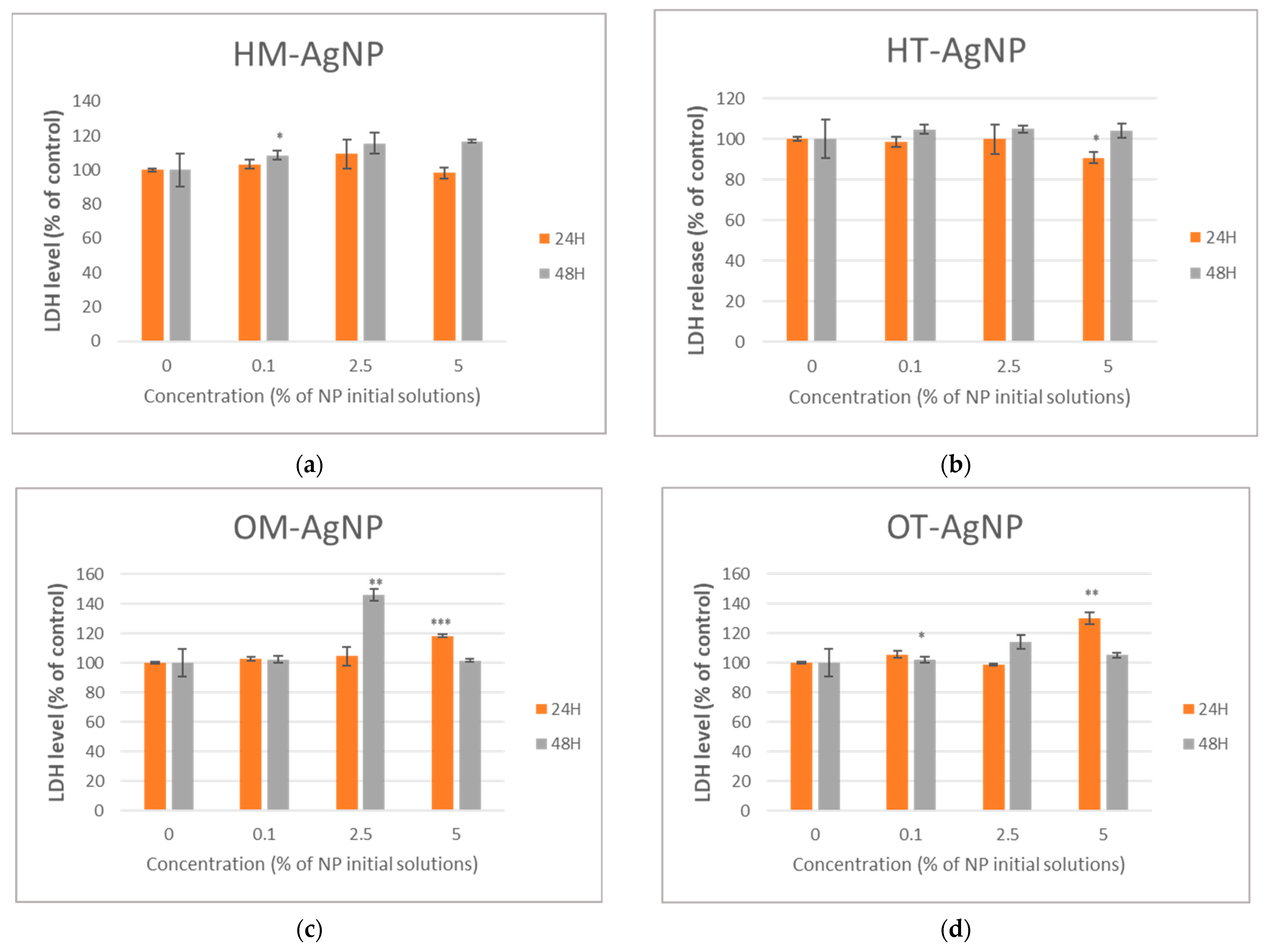
2.3. Evaluation of Biomedical Potential and Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization Methods
4.3. Evaluation of Biomedical Potential and Cytotoxicity
4.4. Statistical Interpretation and Data Representation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Mariappan, Y.; Viswanathan, V.; Baskaralingam, V. Culinary spices mediated biogenesis of nanoparticles for cancer and diabetes treatment. In Fundamentals of Bionanomaterials Micro and Nano Technologies; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 59–76. [Google Scholar]
- Lugasi, A.; Hovari, J.; Hagymasi, K.; Jakoczi, I.; Blazovics, A. Antioxidant properties of a mixture of Lamiaceae plants intended to use as a food additive. Acta Aliment. 2006, 35, 85–97. [Google Scholar] [CrossRef]
- Joulain, D. Study of the chemical composition of hyssop (Hyssopus officinalis Linnaeus) essential oil. Riv. Ital. Essenze 1976, 58, 479–485. [Google Scholar]
- Wang, N.; Yang, X. Two new flavonoid glycosides from the whole herbs of Hyssopus officinalis. J. Asian Nat. Prod. Res. 2010, 12, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, G.; D’Andrea, A.; Nicoletti, M. A Pinocamphone Poor Oil of Hyssopus officinalis L. var. decumbens from France (Banon). J. Essent. Oil Res. 1998, 10, 563–567. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Tipercius, B.; Popescu, H. Preliminary research of some polyphenolic compounds from Hyssopus officinalis L. (Lamiaceae). Farmacia 2002, 50, 54–57. [Google Scholar]
- Ahmadian, S.; Kenari, R.E.; Amiri, Z.R.; Sohbatzadeh, F.; Khodaparast, M.H.D. Effect of ultrasound-assisted cold plasma pretreatment on cell wall polysaccharides distribution and extraction of phenolic compounds from hyssop (Hyssopus officinalis L.). Int. J. Biol. Macromol. 2023, 233, 123557. [Google Scholar] [CrossRef]
- Fatahinezhad, N.; Lorigooini, Z.; Arabi, M.; Rabiei, Z.; Sheykhshabani, S.K.; Rafieian-Kopaei, M. Effects of Hyssopus Officinalis Hydroalcoholic Extract on Pentylenetetrazol-Induced Convulsive Seizures in Rat. Neurochem. Res. 2022, 47, 3792–3804. [Google Scholar] [CrossRef] [PubMed]
- Ozer, H.; Sokmen, M.; Gulluce, M.; Adiguzel, A.; Kilic, H.; Sahin, F.; Sokmen, A. In vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of Hyssopus officinalis L. ssp. Angustifolius. Ital. J. Food Sci. 2006, 18, 73–83. [Google Scholar]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Bahramian, F.; Bekhradnia, A.R. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 2010, 23, 29–34. [Google Scholar]
- Glamoålija, J.M.; Sokoviã, M.D.; Vukojeviã, J.B.; Milenkoviã, I.M.; Brkiã, D.D.; Van Griensven, L. Antifungal activity of essential oil Hyssopus officinalis L. against mycopathogen Mycogone perniciosa (MANG). Proc. Nat. Sci. 2005, 109, 123–128. [Google Scholar]
- Camele, I.; De Feo, V.; Altieri, L.; Mancini, E.; De Martino, L.; Luigi Rana, G. An attempt of postharvest orange fruit rot control using essential oils from mediterranean plants. J. Med. Food 2010, 13, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Insecticidal activity of certain medicinal plants. Fitoterapia 2004, 75, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.; Mohammad, K.S.; Zarei, M.; Shokoohi, M.; Oskoueian, E.; Poorbagher, M.R.M.; Karimi, E. Zinc oxide nanoparticles synthesized using Hyssopus officinalis L. Extract Induced oxidative stress and changes the expression of key genes involved in inflammatory and antioxidant Systems. Biol. Res. 2022, 55, 24. [Google Scholar] [CrossRef] [PubMed]
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. Cluj. Napoca 2019, 47, 545–552. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Parra, C.; Muñoz, P.; Bustos, L.; Parra, F.; Simirgiotis, M.J.; Escobar, H. UHPLC-DAD Characterization of Origanum vulgare L. from Atacama Desert Andean Region and Antioxidant, Antibacterial and Enzyme Inhibition Activities. Molecules 2021, 26, 2100. [Google Scholar] [CrossRef] [PubMed]
- Skoula, M.; Grayer, R.J.; Kite, G.C.; Veitch, N.C. Exudate flavones and flavanones in Origanum species and their interspecific variation. Biochem. Syst. Ecol. 2008, 36, 646–654. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Adler, A. The Lamiaceae Family: An Overview; Nova Science Pub Inc.: Hauppauge, NY, USA, 2020. [Google Scholar]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Shaik, M.R.; Khan, M.; Kuniyil, M.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H.; Shaik, J.P.; Ahamed, A.; Mahmood, A.; Khan, M.; et al. Plant-Extract-Assisted Green Synthesis of Silver Nanoparticles Using Origanum vulgare L. Extract and Their Microbicidal Activities. Sustainability 2018, 10, 913. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Puzeryte, V.; Radenkovs, V.; Krasnova, I.; Memvanga, P.B.; Viskelis, P.; Streimikyte, P.; Viskelis, J. Sustainable–Green Synthesis of Silver Nanoparticles Using Aqueous Hyssopus officinalis and Calendula officinalis Extracts and Their Antioxidant and Antibacterial Activities. Molecules 2022, 27, 7700. [Google Scholar] [CrossRef] [PubMed]
- Fathiazad, F.; Mazandarani, M.; Hamedeyazdan, S. Phytochemical analysis and antioxidant activity of Hyssopus officinalis L. from Iran. Adv. Pharm. Bull. 2011, 1, 63–67. [Google Scholar] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent Advances in the Analysis of Rosmarinic Acid from Herbs in the Lamiaceae Family. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Yuan, J.F.; Wang, T.T.; Wang, D.H.; Zhou, G.H.; Zou, G.X.; Wang, Y.; Gong, M.G.; Zhang, B. Effect of Microwave on Changes of Gallic Acid and Resveratrol in a Model Extraction Solution. Food Bioprocess Technol. 2020, 13, 1246–1254. [Google Scholar] [CrossRef]
- Skrypnik, L.; Feduraev, P.; Styran, T.; Golovin, A.; Katserov, D.; Nebreeva, S.; Maslennikov, P. Biomass, Phenolic Compounds, Essential Oil Content, and Antioxidant Properties of Hyssop (Hyssopus officinalis L.) Grown in Hydroponics as Affected by Treatment Type and Selenium Concentration. Horticulturae 2022, 8, 1037. [Google Scholar] [CrossRef]
- Jafari Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Bistgani, Z.E.; Maggi, F.; Khademi, A. Natural diversity in phenolic components and antioxidant properties of oregano (Origanum vulgare L.) accessions, grown under the same conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.C.; Fierascu, I.; Baroi, A.M.; Ungureanu, C.; Spinu, S.; Avramescu, S.M.; Somoghi, R.; Fierascu, R.C.; Dinu-Parvu, C.E. Phytosynthesis of Silver Nanoparticles Using Leonurus cardiaca L. Extracts. Materials 2023, 16, 3472. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Repajić, M.; Garofulić, I.E.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Dessi, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-step green synthesis and characterization of gold-conjugated polyphenol nanoparticles with antioxidant and biological activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar]
- Arunachalam, K.D.; Annamalai, S.K.; Hari, S. One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int. J. Nanomed. 2013, 8, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.D.; Annamalai, S.K. Chrysopogon zizanioides aqueous extract mediated synthesis, characterization of crystalline silver and gold nanoparticles for biomedical applications. Int. J. Nanomed. 2013, 8, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, N.; Ehsanfar, Z.; Shamsa, F.; Amin, G.; Shahverdi, H.R.; Esfahani, H.R.M.; Shamsaie, A.; Bazaz, R.D.; Shahverdi, A.R. Screening of Medicinal Plant Methanol Extracts for the Synthesis of Gold Nanoparticles by Their Reducing Potential. Z. Naturforschung B 2008, 63, 903–908. [Google Scholar] [CrossRef]
- Mubarak, A.D.; Thajuddin, N.; Jeganathanv, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Sobczak-Kupiec, A.; Malina, D.; Yathirajan, H.S.; Keerthi, V.R.; Chandrashekar, N.; Salman, D.; Liny, P. Plant mediated synthesis of gold nanoparticles using fruit extracts of Ananas comosus (L.) (Pineapple) and evaluation of biological activities. Adv. Mat. Lett. 2013, 4, 332–337. [Google Scholar] [CrossRef]
- Reddy, G.R.; Morais, A.B.; Gandhi, N.N. Green synthesis, characterization and in vitro antibacterial studies of gold nanoparticles by using Senna siamea plant seed aqueous extract at ambient conditions. Asian J. Chem. 2013, 25, 8541–8544. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Starnes, D.L.; Jain, A.; Sahi, S.V. In planta engineering of gold nanoparticles of desirable geometries by modulating growth conditions: An environment-friendly approach. Environ. Sci. Technol. 2010, 44, 7110–7115. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Toderas, F.; Iosin, M.; Stiufiuc, G. Anisotropic gold nanocrystals: Synthesis and characterization. Int. Mod. Phys. B 2010, 24, 753–761. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Cuibus, F.; Sevastre, B.; Stiufiuc, G.; Duma, M.; Hanganu, D.; Iacovita, C.; Stiufiuc, R.; Lucaciu, C.M. Origanum vulgare mediated green synthesis of biocompatible gold nanoparticles simultaneously possessing plasmonic, antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 1041–1058. [Google Scholar] [CrossRef] [PubMed]



| Extract/Parameter | HT | HM | OT | OM |
|---|---|---|---|---|
| TPC (mg GAE/g dried matter) | 10.9 ± 0.0224 d | 14.4 ± 0.0358 c | 23.4 ± 0.0626 b | 28.4 ± 0.0716 a |
| Tannic acid (mg/L) | N.D. | 9 ± 0.00635 a | 1.07 ± 0.000577 b | 0.902 ± 0.000577 c |
| Gallic acid (mg/L) | 0.166 ± 5.77 × 10−5 c | 0.239 ± 0.000173 a | 0.186 ± 0.000115 b | 0.154 ± 5.77 × 10−5 d |
| Protocatechuic acid (mg/L) | 4.88 ± 0.00346 a | 0.512 ± 0.000346 b | N.D. | N.D. |
| Catechin (mg/L) | N.D. | N.D. | 1.62 ± 0.00115 b | 10.4 ± 0.00693 a |
| Vanillic acid (mg/L) | 0.0709 ± 0.000115 b | N.D. | N.D. | 1.45 ± 0.000577 a |
| Caffeic acid (mg/L) | 28.9 ± 0.0173 a | 22.2 ± 0.0144 c | 15.2 ± 0.00577 d | 23.1 ± 0.0162 b |
| Ellagic acid (mg/L) | 5.44 ± 0.00346 b | 6.45 ± 0.00404 a | 3.2 ± 0.00173 c | 0.182 ± 0.000115 d |
| Chlorogenic acid (mg/L) | 2.76 ± 0.00173 c | 3.12 ± 0.00173 b | 0.15 ± 0.000115 d | 9.7 ± 0.00635 a |
| Syringic acid (mg/L) | 3.48 ± 0.00231 d | 4.03 ± 0.00289 c | 5.34 ± 0.00346 a | 4.94 ± 0.00346 b |
| Epicatechin (mg/L) | 5.62 ± 0.00404 c | 2.38 ± 0.00173 d | 21.8 ± 0.0139 b | 59.3 ± 0.0433 a |
| p-coumaric acid (mg/L) | 2.47 ± 0.00173 b | N.D. | N.D. | 4.77 ± 0.00289 a |
| Ferulic acid (mg/L) | 7.7 ± 0.0052 a | N.D. | 7.33 ± 0.0052 b | N.D. |
| Sinapic acid (mg/L) | 13.1 ± 0.0924 c | 13.8 ± 0.00866 b | N.D. | 29.5 ± 0.0202 a |
| o-coumaric acid (mg/L) | 1.52 ± 0.00115 d | 1.74 ± 0.00115 c | 2.38 ± 0.00115 a | 1.91 ± 0.00115 b |
| Isoquercitrin (mg/L) | 7.42 ± 0.00462 c | 12.2 ± 0.00866 b | N.D. | 95.2 ± 0.0658 a |
| Rutin (mg/L) | 94.4 ± 0.0635 c | 124 ± 0.086 b | 273 ± 0.191 a | N.D. |
| Hyperoside (mg/L) | N.D. | N.D. | 65.4 ± 0.0462 b | 243 ± 0.168 a |
| Naringin (mg/L) | 12.9 ± 0.00866 d | 16.7 ± 0.0115 c | 20.2 ± 0.0115 b | 28.7 ± 0.0289 a |
| Rosmarinic acid (mg/L) | 4380 ± 3.04 a | N.D. | 434 ± 0.3 c | 765 ± 0.538 b |
| Resveratrol (mg/L) | 83.8 ± 0.0577 a | 5.9 ± 0.00404 c | 47.5 ± 0.0346 b | N.D. |
| Luteolin (mg/L) | N.D. | N.D. | 49.8 ± 0.0341 b | 55.3 ± 0.0416 a |
| Naringenin (mg/L) | 4.19 ± 0.00289 b | 6.07 ± 0.00404 a | 1.3 ± 0.000924 d | 1.82 ± 0.00173 c |
| Sample | (111) Peak Position (Degrees) | (200) Peak Position (Degrees) | (220) Peak Position (Degrees) | (311) Peak Position (Degrees) | FWHM (Degrees) 1 | Crystallite Dimension (nm) 1 |
|---|---|---|---|---|---|---|
| HT | 38.0988 | 43.24 | 64.70 | 77.42 | 1.2738 | 6.89 |
| HM | 38.0264 | 43.15 | 64.41 | 77.54 | 2.7291 | 3.21 |
| OT | 38.0988 | 44.41 | 64.23 | 77.32 | 2.149 | 4.08 |
| OM | 38.0531 | 44.25 | 64.04 | 77.88 | 2.9862 | 2.94 |
| Vegetal Material | Extraction Method | Extraction Parameters | Extract Encoding | Phytosynthesized NPs |
|---|---|---|---|---|
| Hyssopus officinalis L., aerial parts | Temperature extraction | Shredded vegetal material, extracted for 3 h at 65 °C, solvent—hydroalcoholic mixture (ratio of ethanol:water = 1:1) | HT | HT-AgNP |
| Microwave-assisted extraction | Crushed vegetal material and hydroalcoholic solvent (ratio of ethanol:water = 1:1) heated using an Ethos Easy Advanced Microwave Digestion System (Milestone Srl, Sorisole, Italy), extraction time 25 min, extraction temperature 65 °C, microwave power 800 W | HM | HM-AgNP | |
| Origanum vulgare L., aerial parts | Temperature extraction | Shredded vegetal material, extracted for 3 h at 65 °C, solvent—hydroalcoholic mixture (ratio of ethanol:water = 1:1) | OT | OT-AgNP |
| Microwave-assisted extraction | Crushed vegetal material and hydroalcoholic solvent (ratio of ethanol:water = 1:1) heated using an Ethos Easy Advanced Microwave Digestion System (Milestone Srl, Sorisole, Italy), extraction time 25 min, extraction temperature 65 °C, microwave power 800 W | OM | OM-AgNP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupuliasa, A.I.; Baroi, A.-M.; Avramescu, S.M.; Vasile, B.S.; Prisada, R.M.; Fierascu, R.C.; Fierascu, I.; Sărdărescu, D.I.; Ripszky Totan, A.; Voicu-Bălășea, B.; et al. Application of Common Culinary Herbs for the Development of Bioactive Materials. Plants 2024, 13, 997. https://doi.org/10.3390/plants13070997
Lupuliasa AI, Baroi A-M, Avramescu SM, Vasile BS, Prisada RM, Fierascu RC, Fierascu I, Sărdărescu DI, Ripszky Totan A, Voicu-Bălășea B, et al. Application of Common Culinary Herbs for the Development of Bioactive Materials. Plants. 2024; 13(7):997. https://doi.org/10.3390/plants13070997
Chicago/Turabian StyleLupuliasa, Alina Ioana, Anda-Maria Baroi, Sorin Marius Avramescu, Bogdan Stefan Vasile, Răzvan Mihai Prisada, Radu Claudiu Fierascu, Irina Fierascu, Daniela Ionela Sărdărescu (Toma), Alexandra Ripszky Totan, Bianca Voicu-Bălășea, and et al. 2024. "Application of Common Culinary Herbs for the Development of Bioactive Materials" Plants 13, no. 7: 997. https://doi.org/10.3390/plants13070997
APA StyleLupuliasa, A. I., Baroi, A.-M., Avramescu, S. M., Vasile, B. S., Prisada, R. M., Fierascu, R. C., Fierascu, I., Sărdărescu, D. I., Ripszky Totan, A., Voicu-Bălășea, B., Pițuru, S.-M., Popa, L., Ghica, M. V., & Dinu-Pîrvu, C.-E. (2024). Application of Common Culinary Herbs for the Development of Bioactive Materials. Plants, 13(7), 997. https://doi.org/10.3390/plants13070997














