The Mechanism of Ovule Abortion in Self-Pollinated ‘Hanfu’ Apple Fruits and Related Gene Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Pollination Methods
2.3. Seed Transparent Treatment and DIC Observation
2.4. Seed Dissection and Observation
2.5. Paraffin Sections and LAM
2.6. RNA Extraction and Transcriptome Analysis
3. Results
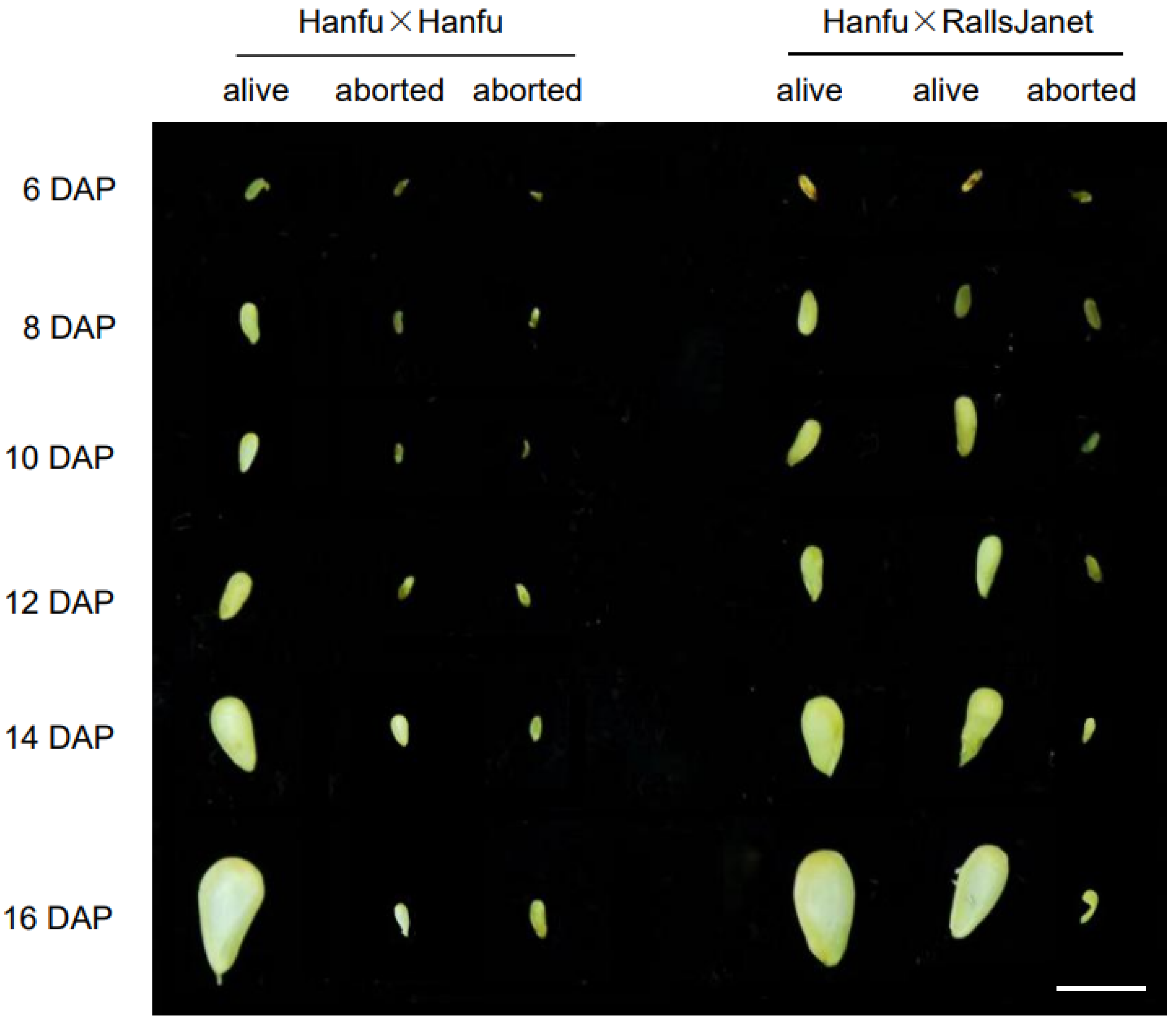
3.1. Abortion of Apple Ovules Due to Failure of Embryo Formation
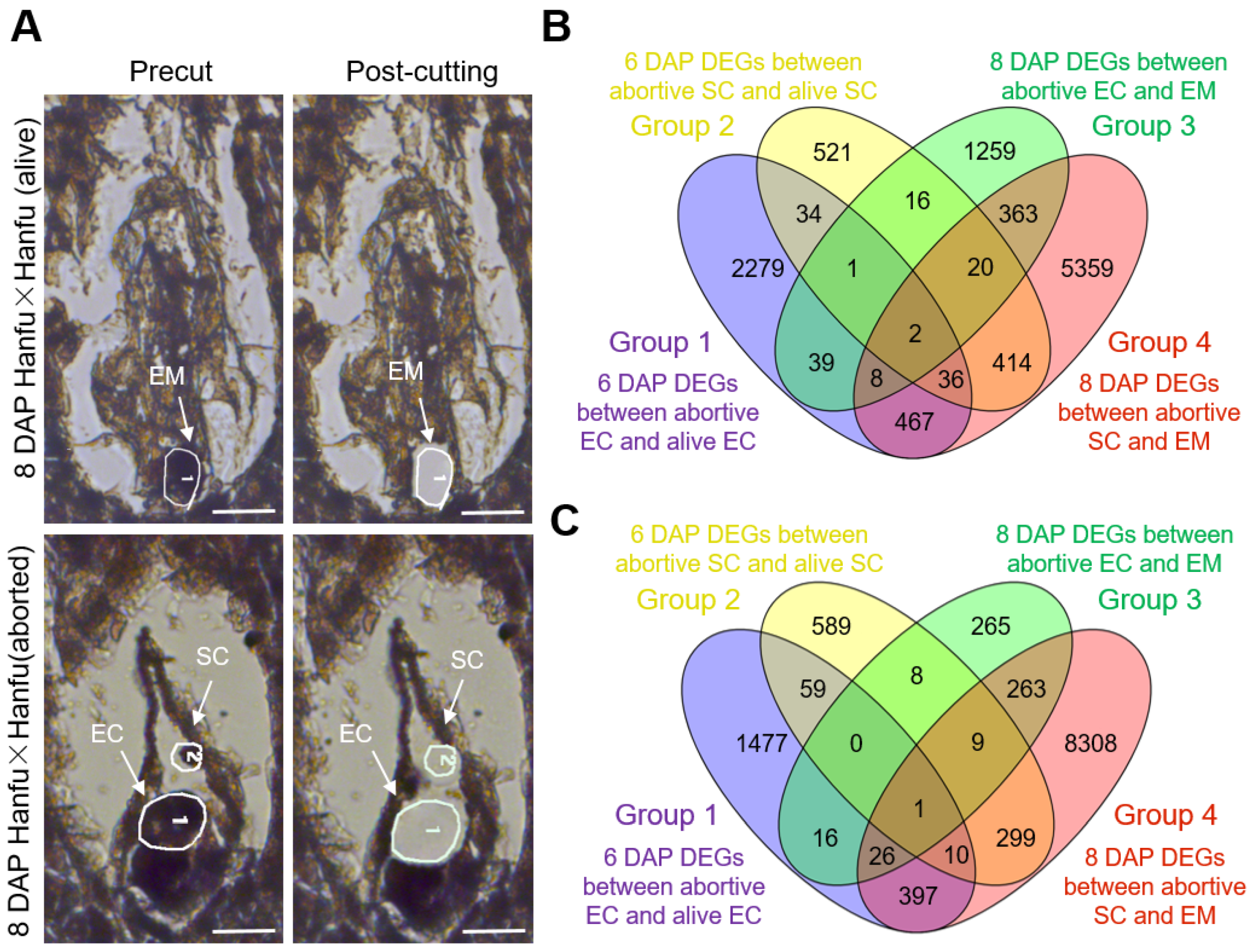
3.2. Sperm–Egg Cell Fusion Failure Results in Failure of Embryo Formation
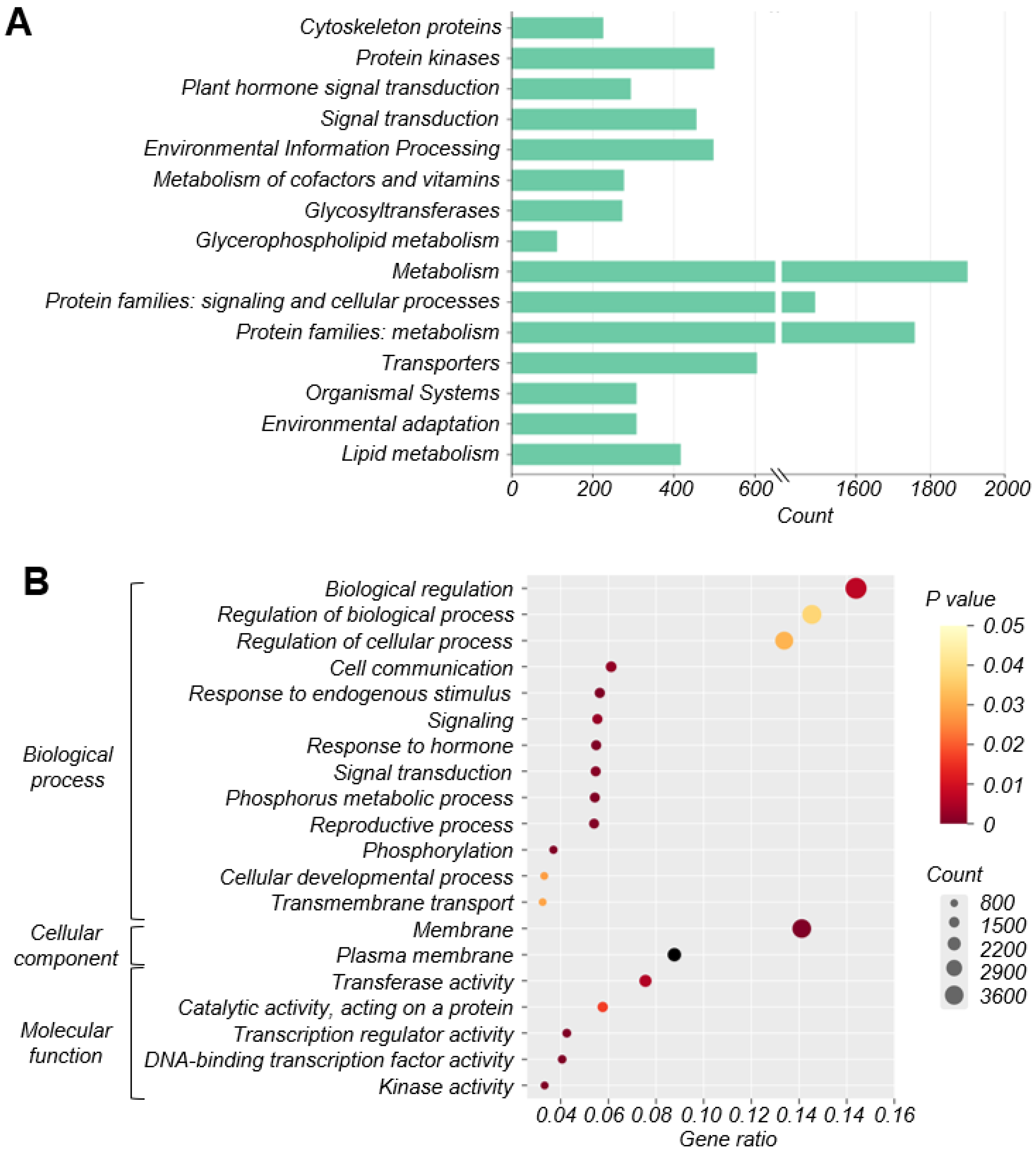
3.3. Screening of Genes Related to Ovule Abortion in Apple
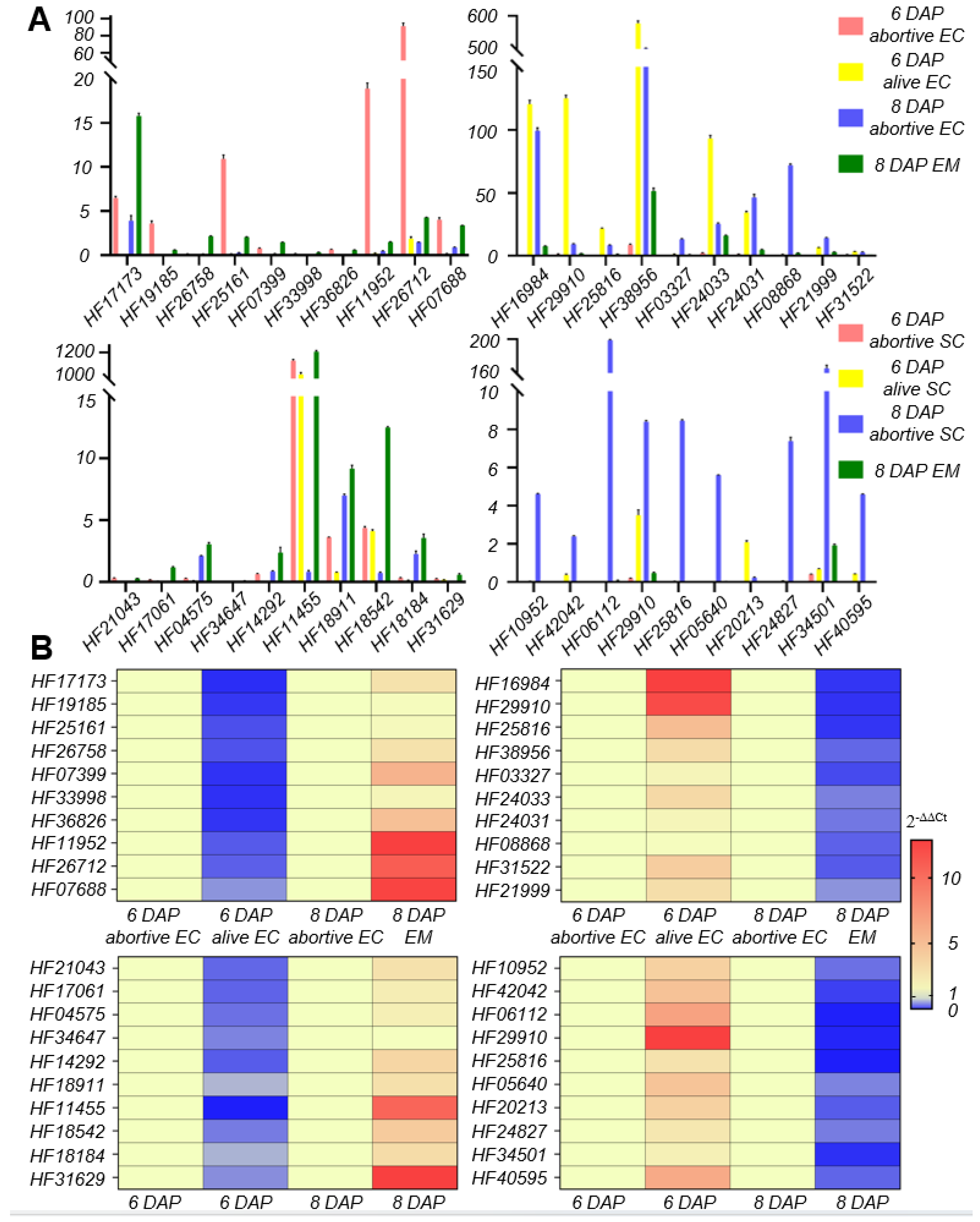
3.4. Functional Analysis and Validation of Genes Related to Ovule Abortion in Apple
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fichant, T.; Ledent, A.; Collart, F.; Vanderpoorten, A. Dispersal Capacities of Pollen, Seeds and Spores: Insights from Comparative Analyses of Spatial Genetic Structures in Bryophytes and Spermatophytes. Front. Plant Sci. 2023, 14, 1289240. [Google Scholar] [CrossRef] [PubMed]
- Rudall, P.J. Evolution and Patterning of the Ovule in Seed Plants. Biol. Rev. 2021, 96, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Katoh, N.; Goto, K.; Asano, J.; Fukushima, K.; Yamada, K.; Kasai, A.; Li, T.Z.; Takanoha, M.; Miyairi, K.; Okuno, T. S-RNases from Self-Incompatible and -Compatible Apple Cultivars: Purification, Cloning, Enzymic Properties, and Pollen Tube Growth Inhibitory Activity. Biosci. Biotechnol. Biochem. 2002, 66, 1185–1195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Xiao, P.; Jiang, F.; Wang, S.; Liu, Z.; Song, G.; Li, W.; Lv, T.; Li, J.; Wang, D.; et al. Exogenous Gibberellin Treatment Improves Fruit Quality in Self-Pollinated Apple. Plant Physiol. Biochem. 2022, 174, 11–21. [Google Scholar] [CrossRef]
- Li, W.; Yang, Q.; Gu, Z.; Wu, C.; Meng, D.; Yu, J.; Chen, Q.; Li, Y.; Yuan, H.; Wang, D.; et al. Molecular and Genetic Characterization of a Self-Compatible Apple Cultivar, ‘CAU-1’. Plant Sci. 2016, 252, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, B.; Fan, W.; Fan, S.; Xu, Y.; Liu, C.; Lv, T.; Liu, W.; Wu, L.; Xian, L.; et al. Polyamines Involved in Regulating Self-Incompatibility in Apple. Genes 2021, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gu, Z.; Li, T.; Yu, J.; Liu, C.; Fan, W.; Wang, B.; Jiang, F.; Zhang, Q.; Li, W. The Apple MdPTI1L Kinase is Phosphorylated by MdOXI1 during S-RNase-Induced Reactive Oxygen Species Signaling in Pollen Tubes. Plant Sci. 2021, 305, 110824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Meng, D.; Gu, Z.; Li, W.; Wang, A.; Yang, Q.; Zhu, Y.; Li, T. A Novel Gene, MdSSK1, as a Component of the SCF Complex Rather than MdSBP1 can Mediate the Ubiquitination of S-RNase in Apple. J. Exp. Bot. 2014, 65, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Li, W.; Doughty, J.; Meng, D.; Yang, Q.; Yuan, H.; Li, Y.; Chen, Q.; Yu, J.; Liu, C.S.; et al. A Gamma-thionin Protein from Apple, MdD1, is Required for Defence against S-RNase-induced Inhibition of Pollen Tube Prior to Self/Non-self Recognition. Plant Biotechnol. J. 2019, 17, 2184–2198. [Google Scholar] [CrossRef]
- Meng, D.; Gu, Z.; Li, W.; Wang, A.; Yuan, H.; Yang, Q.; Li, T. Apple MdABCF Assists in the Transportation of S-RNAse into Pollen Tubes. Plant J. 2014, 78, 990–1002. [Google Scholar] [CrossRef]
- Yang, Q.; Meng, D.; Gu, Z.; Li, W.; Chen, Q.; Li, Y.; Yuan, H.; Yu, J.; Liu, C.; Li, T. Apple S-RNAse Interacts with an Actin-binding Protein, Md MVG, to Reduce Pollen Tube Growth by Inhibiting Its Actin-severing Activity at the Early Stage of Self-pollination Induction. Plant J. 2018, 95, 41–56. [Google Scholar] [CrossRef]
- Raghavan, V. Some Reflections on Double Fertilization, from Its Discovery to the Present. New Phytol. 2003, 159, 565–583. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. Pollen-Stigma Interaction and Cross-Incompatibility in the Grasses. Sci. New Ser. 1982, 215, 1358–1364. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y.; Heslop-Harrison, J.; Shivanna, K.R. Heterostyly in Primula. 1. Fine-Structural and Cytochemical Features of the Stigma and Style in Primula vulgaris Huds. Protoplasma 1981, 107, 171–187. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. The Pollen-Stigma Interaction in the Grasses: 4. An Interpretation of the Self-Incompatibility Response. Acta Bot. Neerl. 1982, 31, 429–439. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. An Interpretation of the Hydrodynamics of Pollen. Am. J. Bot. 1979, 66, 737–743. [Google Scholar] [CrossRef]
- Taylor, L.P.; Hepler, P.K. Pollen Germination and Tube Growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 461–491. [Google Scholar] [CrossRef]
- Edlund, A.F. Pollen and Stigma Structure and Function: The Role of Diversity in Pollination. Plant Cell 2004, 16, S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Lord, E. Adhesion and Cell Movement during Pollination: Cherchez La Femme. Trends Plant Sci. 2000, 5, 368–373. [Google Scholar] [CrossRef]
- Higashiyama, T.; Kuroiwa, H.; Kuroiwa, T. Pollen-Tube Guidance: Beacons from the Female Gametophyte. Curr. Opin. Plant Biol. 2003, 6, 36–41. [Google Scholar] [CrossRef]
- Higashiyama, T. The Synergid Cell: Attractor and Acceptor of the Pollen Tube for Double Fertilization. J. Plant Res. 2002, 115, 149–160. [Google Scholar] [CrossRef]
- Okuda, S.; Tsutsui, H.; Shiina, K.; Sprunck, S.; Takeuchi, H.; Yui, R.; Kasahara, R.D.; Hamamura, Y.; Mizukami, A.; Susaki, D.; et al. Defensin-like Polypeptide LUREs Are Pollen Tube Attractants Secreted from Synergid Cells. Nature 2009, 458, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Hou, S.; Wang, L.; Huang, Q.; Gu, H.; Dresselhaus, T.; Zhong, S.; Qu, L.-J. AtLURE1/PRK6-Mediated Signaling Promotes Conspecific Micropylar Pollen Tube Guidance. Plant Physiol. 2021, 186, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Tavormina, P.; De Coninck, B.; Nikonorova, N.; De Smet, I.; Cammue, B.P.A. The Plant Peptidome: An Expanding Repertoire of Structural Features and Biological Functions. Plant Cell 2015, 27, 2095–2118. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Brownfield, L.; Twell, D. Male Gametophyte Development: A Molecular Perspective. J. Exp. Bot. 2009, 60, 1465–1478. [Google Scholar] [CrossRef]
- McCue, A.D.; Cresti, M.; Feijo, J.A.; Slotkin, R.K. Cytoplasmic Connection of Sperm Cells to the Pollen Vegetative Cell Nucleus: Potential Roles of the Male Germ Unit Revisited. J. Exp. Bot. 2011, 62, 1621–1631. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Liang, F.; Cong, L.; Song, L.; Li, X.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; et al. PbEIL1 Acts Upstream of PbCysp1 to Regulate Ovule Senescence in Seedless Pear. Hortic. Res. 2021, 8, 59. [Google Scholar] [CrossRef]
- Sprunck, S.; Rademacher, S.; Vogler, F.; Gheyselinck, J.; Grossniklaus, U.; Dresselhaus, T. Egg Cell–Secreted EC1 Triggers Sperm Cell Activation During Double Fertilization. Science 2012, 338, 1093–1097. [Google Scholar] [CrossRef]
- Rademacher, S.; Sprunck, S. Downregulation of Egg Cell-Secreted EC1 Is Accompanied with Delayed Gamete Fusion and Polytubey. Plant Signal. Behav. 2013, 8, e27377. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, H.; Cyprys, P.; Malka, R.; Flores-Tornero, M.; Zhao, P.; Peng, X.; Sprunck, S.; Sun, M.-X. DMP8 and 9 Regulate HAP2/GCS1 Trafficking for the Timely Acquisition of Sperm Fusion Competence. Proc. Natl. Acad. Sci. USA 2022, 119, e2207608119. [Google Scholar] [CrossRef]
- Cyprys, P.; Lindemeier, M.; Sprunck, S. Gamete Fusion Is Facilitated by Two Sperm Cell-Expressed DUF679 Membrane Proteins. Nat. Plants 2019, 5, 253–257. [Google Scholar] [CrossRef]
- Takahashi, T.; Mori, T.; Ueda, K.; Yamada, L.; Nagahara, S.; Higashiyama, T.; Sawada, H.; Igawa, T. The Male Gamete Membrane Protein DMP9/DAU2 Is Required for Double Fertilization in Flowering Plants. Development 2018, 145, dev170076. [Google Scholar] [CrossRef] [PubMed]
- Fédry, J.; Liu, Y.; Péhau-Arnaudet, G.; Pei, J.; Li, W.; Tortorici, M.A.; Traincard, F.; Meola, A.; Bricogne, G.; Grishin, N.V.; et al. The Ancient Gamete Fusogen HAP2 Is a Eukaryotic Class II Fusion Protein. Cell 2017, 168, 904–915.e10. [Google Scholar] [CrossRef] [PubMed]
- Von Besser, K.; Frank, A.C.; Johnson, M.A.; Preuss, D. Arabidopsis HAP2 (GCS1) Is a Sperm-Specific Gene Required for Pollen Tube Guidance and fertilization. Development 2006, 133, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Takahashi, T.; Ohashi, Y.; Ueda, M.; Mimuro, A.; Sugimoto, J.; Noguchi, Y.; Igawa, T. Behavior of Male Gamete Fusogen GCS1/HAP2 and the Regulation in Arabidopsis Double Fertilization. Biomolecules 2023, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Igawa, T.; Tamiya, G.; Miyagishima, S.; Berger, F. Gamete Attachment Requires GEX2 for Successful Fertilization in Arabidopsis. Curr. Biol. 2014, 24, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pinello, J.F.; Snell, W.J. Plant Sperm Need a Little Help. Nat. Plants 2019, 5, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.L.; Holmes-Davis, R.; McCormick, S. Green Sperm. Identification of Male Gamete Promoters in Arabidopsis. Plant Physiol. 2005, 138, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Geng, X.; Chen, S.; Liu, K.; Yu, S.; Wang, X.; Zhang, C.; Zhang, J.; Wen, Y.; Luo, Q.; et al. The Co-Expression of Genes Involved in Seed Coat and Endosperm Development Promotes Seed Abortion in Grapevine. Planta 2021, 254, 87. [Google Scholar] [CrossRef]
- Lu, L.; Yang, H.; Xu, Y.; Zhang, L.; Wu, J.; Yi, H. Laser Capture Microdissection-based Spatiotemporal Transcriptomes Uncover Regulatory Networks during Seed Abortion in Seedless Ponkan (Citrus reticulata). Plant J. 2023, 115, 642–661. [Google Scholar] [CrossRef]
- Wuest, S.E.; Vijverberg, K.; Schmidt, A.; Weiss, M.; Gheyselinck, J.; Lohr, M.; Wellmer, F.; Rahnenführer, J.; Von Mering, C.; Grossniklaus, U. Arabidopsis Female Gametophyte Gene Expression Map Reveals Similarities between Plant and Animal Gametes. Curr. Biol. 2010, 20, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Florez Rueda, A.M.; Grossniklaus, U.; Schmidt, A. Laser-Assisted Microdissection (LAM) as a Tool for Transcriptional Profiling of Individual Cell Types. JoVE 2016, e53916. [Google Scholar] [CrossRef]
- Hamamura, Y.; Saito, C.; Awai, C.; Kurihara, D.; Miyawaki, A.; Nakagawa, T.; Kanaoka, M.M.; Sasaki, N.; Nakano, A.; Berger, F.; et al. Live-Cell Imaging Reveals the Dynamics of Two Sperm Cells during Double Fertilization in Arabidopsis thaliana. Curr. Biol. 2011, 21, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Houston, N.L.; Fan, C.; Xiang, Q.-Y.; Schulze, J.-M.; Jung, R.; Boston, R.S. Phylogenetic Analyses Identify 10 Classes of the Protein Disulfide Isomerase Family in Plants, Including Single-Domain Protein Disulfide Isomerase-Related Proteins. Plant Physiol. 2005, 137, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.Y.L.; Wang, P.; Kang, B.-H.; Matsumoto, K.; Christopher, D.A. A Non-Classical Member of the Protein Disulfide Isomerase Family, PDI7 of Arabidopsis thaliana, Localizes to the Cis-Golgi and Endoplasmic Reticulum Membranes. Plant Cell Physiol. 2017, 58, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B. Protein Disulfide Isomerase. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2004, 1699, 35–44. [Google Scholar] [CrossRef]
- Li, C.P.; Larkins, B.A. Expression of Protein Disulfide Isomerase is Elevated in the Endosperm of the Maize Floury-2 Mutant. Plant Mol. Biol. 1996, 30, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Stacey, M.G.; Osawa, H.; Patel, A.; Gassmann, W.; Stacey, G. Expression Analyses of Arabidopsis Oligopeptide Transporters during Seed Germination, Vegetative Growth and Reproduction. Planta 2006, 223, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Osawa, H.; Stacey, G.; Gassmann, W. ScOPT1 and AtOPT4 Function as Proton-Coupled Oligopeptide Transporters with Broad but Distinct Substrate Specificities. Biochem. J. 2006, 393, 267–275. [Google Scholar] [CrossRef]
- Lubkowitz, M.A.; Hauser, L.; Breslav, M.; Naider, F.; Becker, J.M. An Oligopeptide Transport Gene from Candida albicans. Microbiology 1997, 143, 387–396. [Google Scholar] [CrossRef]
- Pineda-Hernández, E.; Cruz-Valderrama, J.E.; Gómez-Maqueo, X.; Martínez-Barajas, E.; Gamboa-deBuen, A. BIIDXI, a DUF642 Cell Wall Protein that Regulates Pectin Methyl Esterase Activity, is Involved in Thermotolerance Processes in Arabidopsis thaliana. Plants 2022, 11, 3049. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Wang, B.; Xu, Y.; Fan, W.; Zhang, M.; Huang, F.; Shi, C.; Li, T.; Wang, S.; Wang, S. The Mechanism of Ovule Abortion in Self-Pollinated ‘Hanfu’ Apple Fruits and Related Gene Screening. Plants 2024, 13, 996. https://doi.org/10.3390/plants13070996
Wei H, Wang B, Xu Y, Fan W, Zhang M, Huang F, Shi C, Li T, Wang S, Wang S. The Mechanism of Ovule Abortion in Self-Pollinated ‘Hanfu’ Apple Fruits and Related Gene Screening. Plants. 2024; 13(7):996. https://doi.org/10.3390/plants13070996
Chicago/Turabian StyleWei, Haiyang, Baoan Wang, Ya Xu, Wenqi Fan, Manyu Zhang, Fuli Huang, Chenxi Shi, Tianzhong Li, Shengnan Wang, and Shengyuan Wang. 2024. "The Mechanism of Ovule Abortion in Self-Pollinated ‘Hanfu’ Apple Fruits and Related Gene Screening" Plants 13, no. 7: 996. https://doi.org/10.3390/plants13070996
APA StyleWei, H., Wang, B., Xu, Y., Fan, W., Zhang, M., Huang, F., Shi, C., Li, T., Wang, S., & Wang, S. (2024). The Mechanism of Ovule Abortion in Self-Pollinated ‘Hanfu’ Apple Fruits and Related Gene Screening. Plants, 13(7), 996. https://doi.org/10.3390/plants13070996






