The Impact of Nanomaterials on Photosynthesis and Antioxidant Mechanisms in Gramineae Plants: Research Progress and Future Prospects
Abstract
1. Introduction
2. The Impact of Carbon-Based Nanomaterials on Gramineae Plants
2.1. Graphene
2.2. Carbon Nanotubes
2.3. Nano-Biochar
3. The Impact of Metal Nanoparticles on Gramineae Plants
3.1. Effect of Nanocerium on Gramineae Plants
3.2. Impact of Iron-Based Nanomaterials on Gramineae Plants
3.3. Impact of Titanium-Containing Nanomaterials on Gramineae Plants
3.4. The Impact of Nanozinc on Gramineae Plants
3.5. The Impact of Nanocopper on Gramineae Plants
3.6. The Impact of Nanomaterials on the Environment
4. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nair, R. Effects of nanoparticles on plant growth and development. Plant Nanotechnol. Princ. Pract. 2016, 95–118. [Google Scholar]
- Tiwari, P.K.; Shweta; Singh, A.K.; Singh, V.P.; Prasad, S.M.; Ramawat, N.; Tripathi, D.K.; Chauhan, D.K.; Rai, A.K. Liquid assisted pulsed laser ablation synthesized copper oxide nanoparticles (CuO-NPs) and their differential impact on rice seedlings. Ecotoxicol. Environ. Saf. 2019, 176, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ. Int. 2020, 142, 105831. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-Q.; White, J.C.; Xing, B.-S. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. A 2014, 15, 552–572. [Google Scholar] [CrossRef]
- Vaughan, R.G.; Calvin, W.M.; Taranik, J.V. SEBASS hyperspectral thermal infrared data: Surface emissivity measurement and mineral mapping. Remote Sens. Environ. 2003, 85, 48–63. [Google Scholar] [CrossRef]
- Yin, J.J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes--generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.R.; Hasan, M.R.; Ahommed, M.S.; Bacchu, M.S.; Ali, M.R.; Khan, M.Z.H. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Pawlett, M.; Ritz, K.; Dorey, R.A.; Rocks, S.; Ramsden, J.; Harris, J.A. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ. Sci. Pollut. Res. 2013, 20, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Xu, P.; Yan, M.; Liu, Z.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Li, H.; Qu, X.; Yang, S.-T.; Chang, X.-L. Bioaccumulation and Toxicity of 13C-Skeleton Labeled Graphene Oxide in Wheat. Environ. Sci. Technol. 2017, 51, 10146–10153. [Google Scholar] [CrossRef] [PubMed]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem.-Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tan, H.; Li, J.; Li, Z.; Qin, F.; Luo, H.; Qin, D.; Weng, H.; Zhang, C. Unveiling the Effects of Carbon-Based Nanomaterials on Crop Growth: From Benefits to Detriments. J. Agric. Food Chem. 2023, 71, 11860–11874. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wei, H.M.; Liu, S.J.; Xu, Y.C.; Zhu, Z.Y.; Yan, H.; Li, J.X.; Tian, Z.H. Growth response of Oryza sativa seedlings to graphene oxide and its variability among genotypes. Biol. Plant. 2021, 65, 39–46. [Google Scholar] [CrossRef]
- He, Y.; Hu, R.; Zhong, Y.; Zhao, X.; Chen, Q.; Zhu, H. Graphene oxide as a water transporter promoting germination of plants in soil. Nano Res. 2018, 11, 1928–1937. [Google Scholar] [CrossRef]
- Vochita, G.; Oprica, L.; Gherghel, D.; Mihai, C.T.; Boukherroub, R.; Lobiuc, A. Graphene oxide effects in early ontogenetic stages of Triticum aestivum L. seedlings. Ecotoxicol. Environ. Saf. 2019, 181, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, R.; Fang, X.; Song, T.; Cai, X.; Liu, H.; Du, S. Toxic effects of graphene on the growth and nutritional levels of wheat (Triticum aestivum L.): Short- and long-term exposure studies. J. Hazard. Mater. 2016, 317, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Song, R.-R.; Wu, Q.; Wu, X.; Yun, Z.-Y. Effect of graphene oxide on seedling growth and physiological characteristics of maize. J. Agro-Environ. Sci. 2021, 40, 1167–1173. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Hu, X.; Ouyang, S.; Mu, L.; An, J.; Zhou, Q. Effects of Graphene Oxide and Oxidized Carbon Nanotubes on the Cellular Division, Microstructure, Uptake, Oxidative Stress, and Metabolic Profiles. Environ. Sci. Technol. 2015, 49, 10825–10833. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, Z.; Wang, S.; Xu, W.; Bao, H. Effects of Graphene Oxide and/or Cd2+ on Seed Germination, Seedling Growth, and Uptake to Cd2+ in Solution Culture. Water Air Soil Pollut. 2018, 229, 151. [Google Scholar] [CrossRef]
- Younes, N.A.; Dawood, M.F.A.; Wardany, A.A. Biosafety assessment of graphene nanosheets on leaf ultrastructure, physiological and yield traits of Capsicum annuum L. and Solanum melongena L. Chemosphere 2019, 228, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Tiwari, D.K.; Tripathi, D. Interaction of carbon nanotubes with plant system: A review. Carbon Lett. 2020, 31, 167–176. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, C.; Wu, Y.; Dai, Z.; Tang, Q.; Cheng, C.; Xu, Y.; Hu, R.; Liu, C.; Chen, X. Insights into the mechanism of multi-walled carbon nanotubes phytotoxicity in Arabidopsis through transcriptome and m6A methylome analysis. Sci. Total Environ. 2021, 787, 147510. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Sharma, L.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Plant Nanobionic Effect of Multi-walled Carbon Nanotubes on Growth, Anatomy, Yield and Grain Composition of Rice. BioNanoScience 2020, 10, 430–445. [Google Scholar] [CrossRef]
- Wang, X.; Han, H.; Liu, X.; Gu, X.; Chen, K.; Lu, D. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanoparticle Res. 2012, 14, 841. [Google Scholar] [CrossRef]
- Tan, X.-M.; Lin, C.; Fugetsu, B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 2009, 47, 3479–3487. [Google Scholar] [CrossRef]
- Tan, X.-M.; Fugetsu, B. Multi-walled carbon nanotubes interact with cultured rice cells: Evidence of a self-defense response. J. Biomed. Nanotechnol. 2007, 3, 285–288. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, P.; Zhang, X.; Liu, Y.; Feng, S.; Guo, D.; Nadezhda, T.; Song, Z.; Dang, X. Multi-Wall Carbon Nanotubes Promote the Growth of Maize (Zea mays) by Regulating Carbon and Nitrogen Metabolism in Leaves. J. Agric. Food Chem. 2021, 69, 4981–4991. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free. Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xing, B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cao, Y.; Ma, C.; Yan, W. Nano-biochar as a potential amendment for metal(loid) remediation: Implications for soil quality improvement and stress alleviation. J. Environ. Manag. 2024, 351, 119658. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Ahmed, B.; Singh, V.K.; Mandzhieva, S.; Sushkova, S.; Bauer, T.; Verma, K.K.; Shan, S.; van Hullebusch, E.D.; et al. Nano-biochar: A novel solution for sustainable agriculture and environmental remediation. Environ. Res. 2022, 210, 112891. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, F.; Anwar, S.; Bareen, F.E.; Zhang, L.; Ashraf, M. Nano-biochar: Properties and prospects for sustainable agriculture. Land Degrad. Dev. 2023, 34, 2445–2463. [Google Scholar] [CrossRef]
- Yue, L.; Lian, F.; Han, Y.; Bao, Q.; Wang, Z.; Xing, B. The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: Implications for agronomic benefits and potential risk. Sci. Total Environ. 2019, 656, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tang, H.; Wu, W.; Shang, H.; Zhang, D.; Zhan, X.; Xing, B. Role of nano-biochar in attenuating the allelopathic effect from Imperata cylindrica on rice seedlings. Environ. Sci. Nano 2020, 7, 116–126. [Google Scholar] [CrossRef]
- Samart, S.; Chutipaijit, S. Nanocarbon induced modifications in morpho-physiopgical characteristics in rice plants [Oryza sativa l. cv. black jasmine rice (hom-nin)]. Suranaree J. Sci. Technol. 2020, 27, 030024. [Google Scholar]
- Pérez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Lu, F.; Liu, Y.; Song, Y.; Sun, Y.; Zhong, J.; Huang, H.; Wang, Y.; Li, S. Impacts of carbon dots on rice plants: Boosting the growth and improving the disease resistance. ACS Appl. Bio Mater. 2018, 1, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Q.; Lin, X.; Shang, Y.; Cui, X.; Guo, L.; Huang, Y.; Wu, M.; Song, K. Potential Effects of Metal Oxides on Agricultural Production of Rice: A Mini Review. Plants 2023, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Zhu, J.; Peralta-Videa, J.R.; Guo, H. Physiological and Biochemical Changes Imposed by CeO2 Nanoparticles on Wheat: A Life Cycle Field Study. Environ. Sci. Technol. 2015, 49, 11884–11893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.; Liu, S.; Wang, G.; Zhang, J.; He, X.; Zhang, J.; Rui, Y.; Zhang, Z. Phytotoxicity, uptake and transformation of nano-CeO(2) in sand cultured romaine lettuce. Environ. Pollut. 2017, 220, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Saadatmand, S.; Mahmoodzadeh, H.; Khavari-Nejad, R.A. Effect of foliar application of cerium oxide nanoparticles on growth, photosynthetic pigments, electrolyte leakage, compatible osmolytes and antioxidant enzymes activities of Calendula officinalis L. Biologia 2019, 74, 1063–1075. [Google Scholar] [CrossRef]
- Schwabe, F.; Schulin, R.; Limbach, L.K.; Stark, W.; Burge, D.; Nowack, B. Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere 2013, 91, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, W.; Zhang, S.; Sharifan, H.; Ma, X. Elucidating the Effects of Cerium Oxide Nanoparticles and Zinc Oxide Nanoparticles on Arsenic Uptake and Speciation in Rice (Oryza sativa) in a Hydroponic System. Environ. Sci. Technol. 2018, 52, 10040–10047. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Dong, Z. Transfer, transportation, and accumulation of cerium-doped carbon quantum dots: Promoting growth and development in wheat. Ecotoxicol. Environ. Saf. 2021, 226, 112852. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Rizwan, M.; Adrees, M.; Ali, S.; Ur Rehman, M.Z.; Qayyum, M.F.; Hussain, A. Effect of gibberellic acid on growth, biomass, and antioxidant defense system of wheat (Triticum aestivum L.) under cerium oxide nanoparticle stress. Environ. Sci. Pollut. Res. Int. 2020, 27, 33809–33820. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of iron oxide nanoparticles (Fe3O4) on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Shirsat, S.; Suthindhiran, K. Iron oxide nanoparticles as iron micronutrient fertilizer—Opportunities and limitations. J. Plant Nutr. Soil Sci. 2023. [Google Scholar] [CrossRef]
- Al-Amri, N.; Tombuloglu, H.; Slimani, Y.; Akhtar, S.; Barghouthi, M.; Almessiere, M.; Alshammari, T.; Baykal, A.; Sabit, H.; Ercan, I.; et al. Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2020, 194, 110377. [Google Scholar] [CrossRef] [PubMed]
- Taran, S.; Garip, A.K.; Arslan, H. Theoretical study of the structures and chemical ordering of CoPd nanoalloys supported on MgO (001). Int. J. Mod. Phys. C 2016, 27, 1650146. [Google Scholar] [CrossRef]
- Kokina, I.; Plaksenkova, I.; Galek, R.; Jermalonoka, M.; Kirilova, E.; Gerbreders, V.; Krasovska, M.; Sledevskis, E. Genotoxic Evaluation of Fe(3)O(4) Nanoparticles in Different Three Barley (Hordeum vulgare L.) Genotypes to Explore the Stress-Resistant Molecules. Molecules 2021, 26, 6710. [Google Scholar] [CrossRef] [PubMed]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Almessiere, M.; Baykal, A. Uptake and translocation of magnetite (Fe(3)O(4)) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere 2019, 226, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Cheng, W.; Tsang, P.E.; Zhao, D. Ageing decreases the phytotoxicity of zero-valent iron nanoparticles in soil cultivated with Oryza sativa. Ecotoxicology 2016, 25, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kang, Y.-G.; Chang, Y.-S.; Kim, J.-H. Effects of zerovalent iron nanoparticles on photosynthesis and biochemical adaptation of soil-grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, A.; Du, W.; Mao, L.; Wei, Z.; Wang, S.; Yuan, H.; Ji, R.; Zhao, L. Insight into the interaction between Fe-based nanomaterials and maize (Zea mays) plants at metabolic level. Sci. Total Environ. 2020, 738, 139795. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Ullah, S.; Nafees, M.; Bibi, F.; Ullah, R. Morphological assessment of glutamate zerovalent iron nanoparticles by scanning electron microscopy and its combined effect with indole acetic acid on amelioration of lead toxicity in maize (Zea mays L.). Microsc. Res. Tech. 2020, 83, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Tombuloglu, H.; Slimani, Y.; AlShammari, T.M.; Bargouti, M.; Ozdemir, M.; Tombuloglu, G.; Akhtar, S.; Sabit, H.; Hakeem, K.R.; Almessiere, M.; et al. Uptake, translocation, and physiological effects of hematite (alpha-Fe(2)O(3)) nanoparticles in barley (Hordeum vulgare L.). Environ. Pollut. 2020, 266, 115391. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Shen, D.; Liu, X.; Dong, S.; Jing, X.; Wu, W.; Tong, Y.; Gao, S.; Mao, L. Uptake of iron oxide nanoparticles inhibits the photosynthesis of the wheat after foliar exposure. Chemosphere 2020, 259, 127445. [Google Scholar] [CrossRef]
- Petcu, E.; Lazăr, C.; Predoi, D.; Cîmpeanu, C.; Predoi, G.; Bartha, S.; Ioana, V.; Partal, E. The effect of hydroxyapatite and irone oxide nanoparticles on maize and winter wheat plants. Sci. Pap. 2023, 515–519. [Google Scholar]
- Iannone, M.F.; Groppa, M.D.; de Sousa, M.E.; Fernández van Raap, M.B.; Benavides, M.P. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ. Exp. Bot. 2016, 131, 77–88. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021, 269, 116134. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Ali, S. Combined Role of Fe Nanoparticles (Fe NPs) and Staphylococcus aureus L. in the Alleviation of Chromium Stress in Rice Plants. Life 2022, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Dong, L.; Li, J.; Wu, Y.; Chen, W. Endogenous TGF-beta activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25- T cells. Retrovirology 2007, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.; Rui, Y. Magnetic (Fe3O4) Nanoparticles Reduce Heavy Metals Uptake and Mitigate Their Toxicity in Wheat Seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Hrubý, M.; Cígler, P.; Kuzel, S. Contribution To Understanding the Mechanism of Titanium Action in Plant. J. Plant Nutr. 2002, 25, 577–598. [Google Scholar] [CrossRef]
- Chahardoli, A.; Sharifan, H.; Karimi, N.; Kakavand, S.N. Uptake, translocation, phytotoxicity, and hormetic effects of titanium dioxide nanoparticles (TiO(2)NPs) in Nigella arvensis L. Sci. Total Environ. 2022, 806, 151222. [Google Scholar] [CrossRef] [PubMed]
- Feher, M.; Lukacs, D.; Vamos-Vigyazo, L.; Pais, I. Role of titanium in the life of plants. IV: Effect of titanium on the germinative ability of wheat, maize and sunflower seeds and on the growth of seedlings. Acta Agron. Acad. Sci. Hung. 1984, 33, 95–100. [Google Scholar]
- Samart, S.; Chutipaijit, S. Modifications of morphological and physiological characteristics of pigmented-rice seedlings by application of titanium dioxide nanoparticles. In Proceedings of the AIP Conference Proceedings, Chonburi, Thailand, 18–20 July 2018. [Google Scholar] [CrossRef]
- Dumon, J.C.; Ernst, W.H.O. Titanium in Plants. J. Plant Physiol. 1988, 133, 203–209. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Dias, M.C.; Santos, C.; Pinto, G.; Silva, A.M.S.; Silva, S. Titanium dioxide nanoparticles impaired both photochemical and non-photochemical phases of photosynthesis in wheat. Protoplasma 2019, 256, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Zhu, Y.G.; Gu, K.H.; Zhu, J.G.; Yin, Y.; Ji, R.; Du, W.C.; Guo, H.Y. Transcriptome Reveals the Rice Response to Elevated Free Air CO(2) Concentration and TiO(2) Nanoparticles. Environ. Sci. Technol. 2019, 53, 11714–11724. [Google Scholar] [CrossRef] [PubMed]
- Faraji, J.; Sepehri, A. Exogenous Nitric Oxide Improves the Protective Effects of TiO2 Nanoparticles on Growth, Antioxidant System, and Photosynthetic Performance of Wheat Seedlings Under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Khalofah, A.; Kilany, M.; Migdadi, H. Phytostimulatory influence of Comamonas testosteroni and silver nanoparticles on Linum usitatissimum L. under salinity stress. Plants 2021, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Patni, B.; Shankhdhar, D.; Shankhdhar, S.C. Zinc—An indispensable micronutrient. Physiol. Mol. Biol. Plants 2013, 19, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 Nanoparticles on Germinating Rice Seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 282–285. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, R.; Yang, Z.; You, T.; Gao, X.; Wang, L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 130, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, J.; Rui, M.; Yang, L.; Shen, J.; Chu, H.; Song, S.; Chen, Y. OsFTIP7 determines metallic oxide nanoparticles response and tolerance by regulating auxin biosynthesis in rice. J. Hazard. Mater. 2021, 403, 123946. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D.; Luzi, F.; Tolisano, C.; Puglia, D.; Di Michele, A. Synthesis of a Lignin/Zinc Oxide Hybrid Nanoparticles System and Its Application by Nano-Priming in Maize. Nanomaterials 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef] [PubMed]
- Sagardoy, R.; Morales, F.; Lopez-Millan, A.F.; Abadia, A.; Abadia, J. Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biol. 2009, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: A review. Biomater. Res. 2020, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.G.; Gong, J.; Zeng, G. Phytotoxicity, uptake and bio-transport of metal semiconductor nanoparticles in rice seedlings. Acta Sctentiae Circumstantiae 2017, 37, 395–404. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 2014, 21, 12709–12722. [Google Scholar] [CrossRef] [PubMed]
- Tamez, C.; Morelius, E.W.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J. Biochemical and physiological effects of copper compounds/nanoparticles on sugarcane (Saccharum officinarum). Sci. Total Environ. 2019, 649, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Yang, D.; Jin, Q.; Wu, C.; Cui, J. Multifunctional molybdenum disulfide-copper nanocomposite that enhances the antibacterial activity, promotes rice growth and induces rice resistance. J. Hazard. Mater. 2020, 394, 122551. [Google Scholar] [CrossRef] [PubMed]
- Jaskulski, D.; Jaskulska, I.; Majewska, J.; Radziemska, M.; Bilgin, A.; Brtnicky, M. Silver Nanoparticles (AgNPs) in Urea Solution in Laboratory Tests and Field Experiments with Crops and Vegetables. Materials 2022, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.; Seabra, A.B.; Kondak, S.; Adedokun, O.P.; Kolbert, Z. Multilevel approach to plant-nanomaterial relationships: From cells to living ecosystems. J. Exp. Bot. 2023, 74, 3406–3424. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. Int. J. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Collin, B.; Tsyusko, O.V.; Starnes, D.L.; Unrine, J.M. Effect of natural organic matter on dissolution and toxicity of sulfidized silver nanoparticles to Caenorhabditis elegans. Environ. Sci. Nano 2016, 3, 728–736. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Zhang, Z.; Fu, H.; White, J.C.; Lynch, I. Nanomaterial transformation in the soil–plant system: Implications for food safety and application in agriculture. Small 2020, 16, 2000705. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Zahra, N.; Lang, T.; Zain, M.; Raza, M.; Shakoor, N.; Adeel, M.; Zhou, H. Integrating nanotechnology with plant microbiome for next-generation crop health. Plant Physiol. Biochem. 2023, 196, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Moll, J.; Klingenfuss, F.; Widmer, F.; Gogos, A.; Bucheli, T.D.; Hartmann, M.; van der Heijden, M.G. Effects of titanium dioxide nanoparticles on soil microbial communities and wheat biomass. Soil Biol. Biochem. 2017, 111, 85–93. [Google Scholar] [CrossRef]
- Grün, A.-L.; Manz, W.; Kohl, Y.L.; Meier, F.; Straskraba, S.; Jost, C.; Drexel, R.; Emmerling, C. Impact of silver nanoparticles (AgNP) on soil microbial community depending on functionalization, concentration, exposure time, and soil texture. Environ. Sci. Eur. 2019, 31, 15. [Google Scholar] [CrossRef]
- Johansen, A.; Pedersen, A.L.; Jensen, K.A.; Karlson, U.; Hansen, B.M.; Scott-Fordsmand, J.J.; Winding, A. Effects of C60 fullerene nanoparticles on soil bacteria and protozoans. Environ. Toxicol. Chem. 2008, 27, 1895–1903. [Google Scholar] [CrossRef]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Colloids Surf. B Biointerfaces 2016, 146, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, V.K.; Chitara, M.K.; Mishra, D.; Jha, M.N.; Jaiswal, A.; Kumari, G.; Ghosh, S.; Patel, V.K.; Naitam, M.G.; Singh, A.K.; et al. Synergistic impact of nanomaterials and plant probiotics in agriculture: A tale of two-way strategy for long-term sustainability. Front. Microbiol. 2023, 14, 1133968. [Google Scholar] [CrossRef] [PubMed]
- Noor, R.S.; Wang, Z.; Umair, M.; Ameen, M.; Misaal, M.A.; Sun, Y. Long-term application effects of organic and chemical fertilizers on soil health and productivity of taramira (Eruca sativa L.) under rainfed conditions. J. Anim. Plant Sci. 2020, 30, 970–987. [Google Scholar]
- Picasso, V.D.; Berti, M.; Cassida, K.; Collier, S.; Fang, D.; Finan, A.; Krome, M.; Hannaway, D.; Lamp, W.; Stevens, A.W.; et al. Diverse perennial circular forage systems are needed to foster resilience, ecosystem services, and socioeconomic benefits in agricultural landscapes. Grassl. Res. 2022, 1, 123–130. [Google Scholar] [CrossRef]
- Feintrenie, L.; Roda, J.-M.; Rival, A. Industrial investments in agriculture in Central Africa. Establishing the conditions for sustainability and equity. Perspective 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
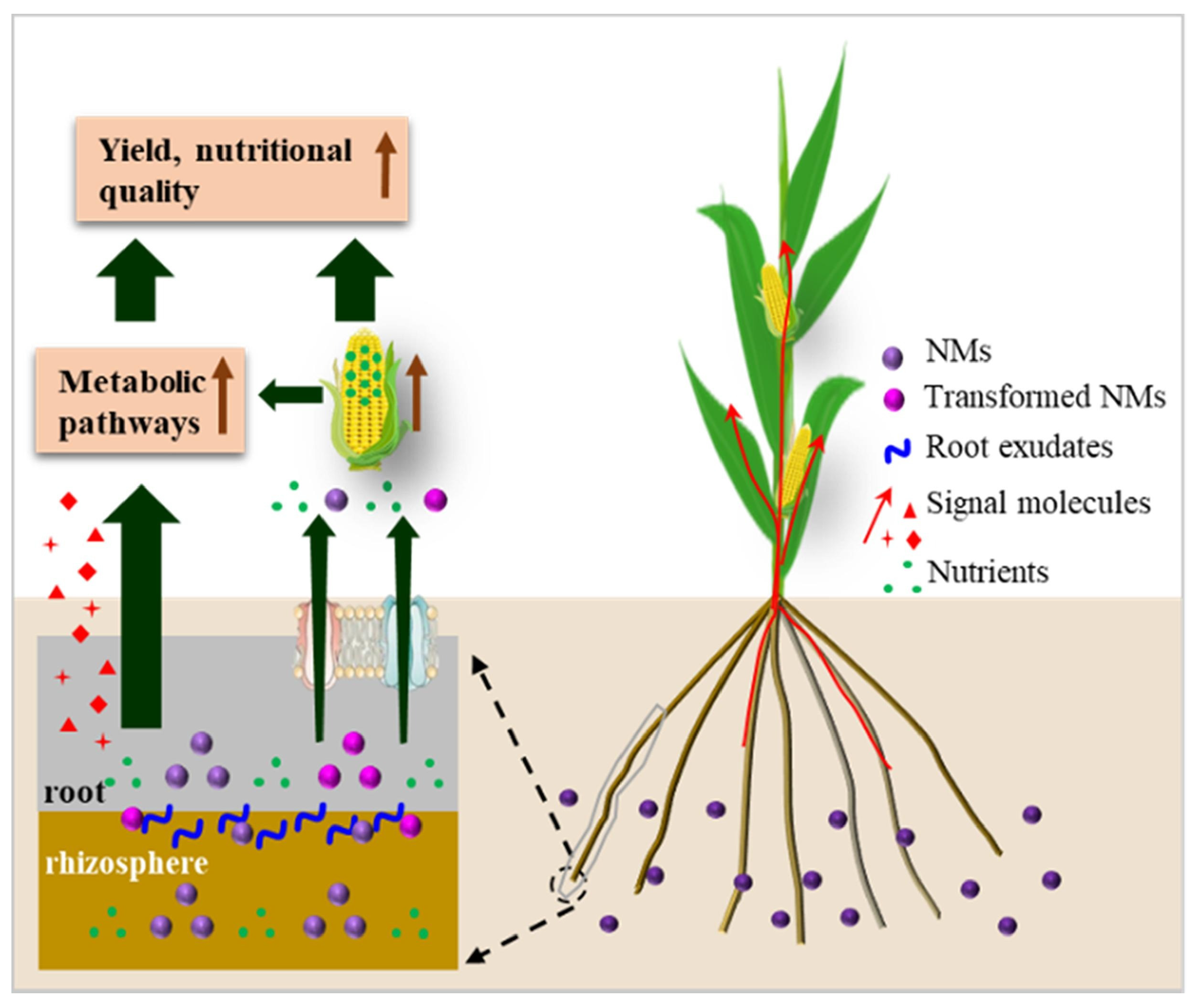



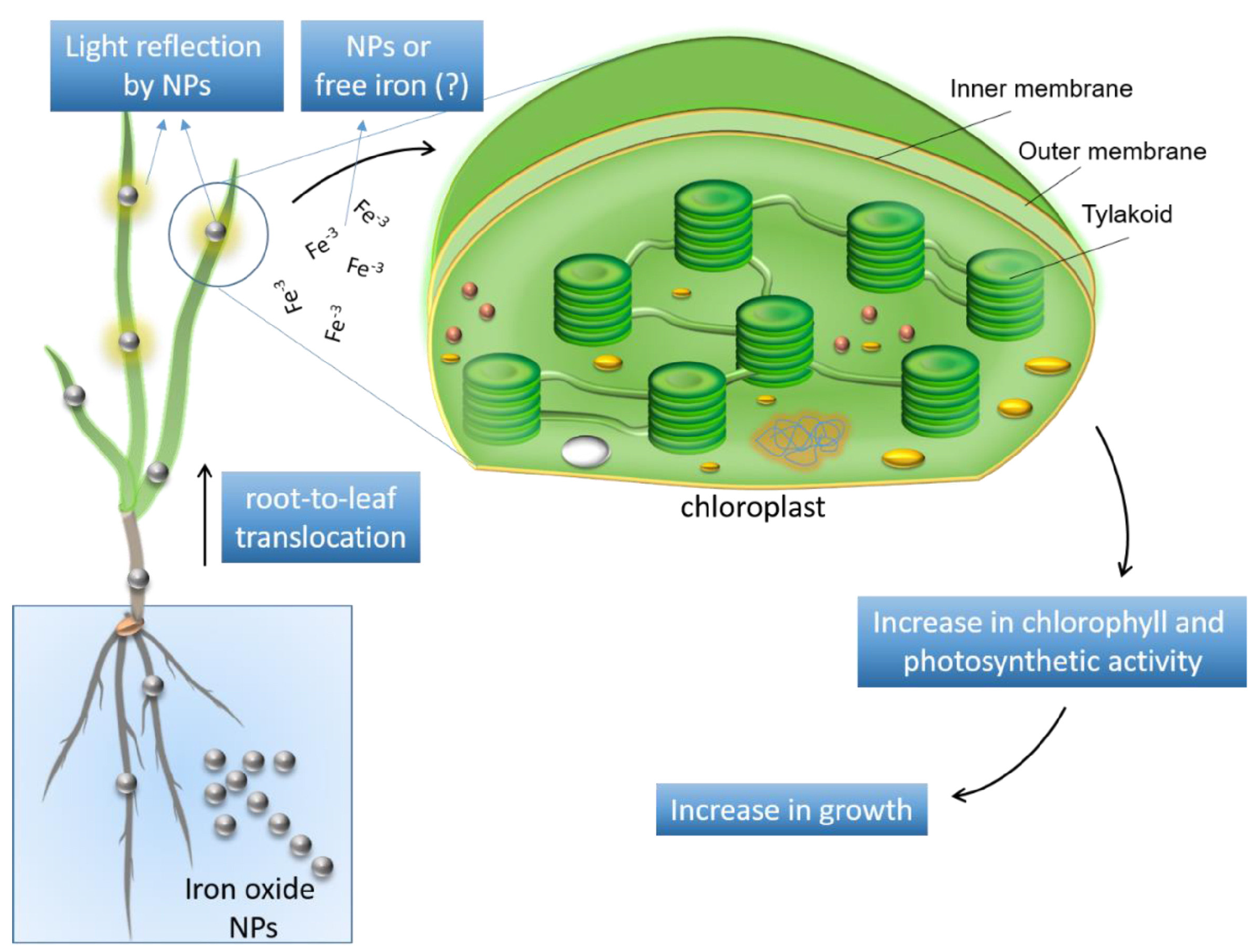
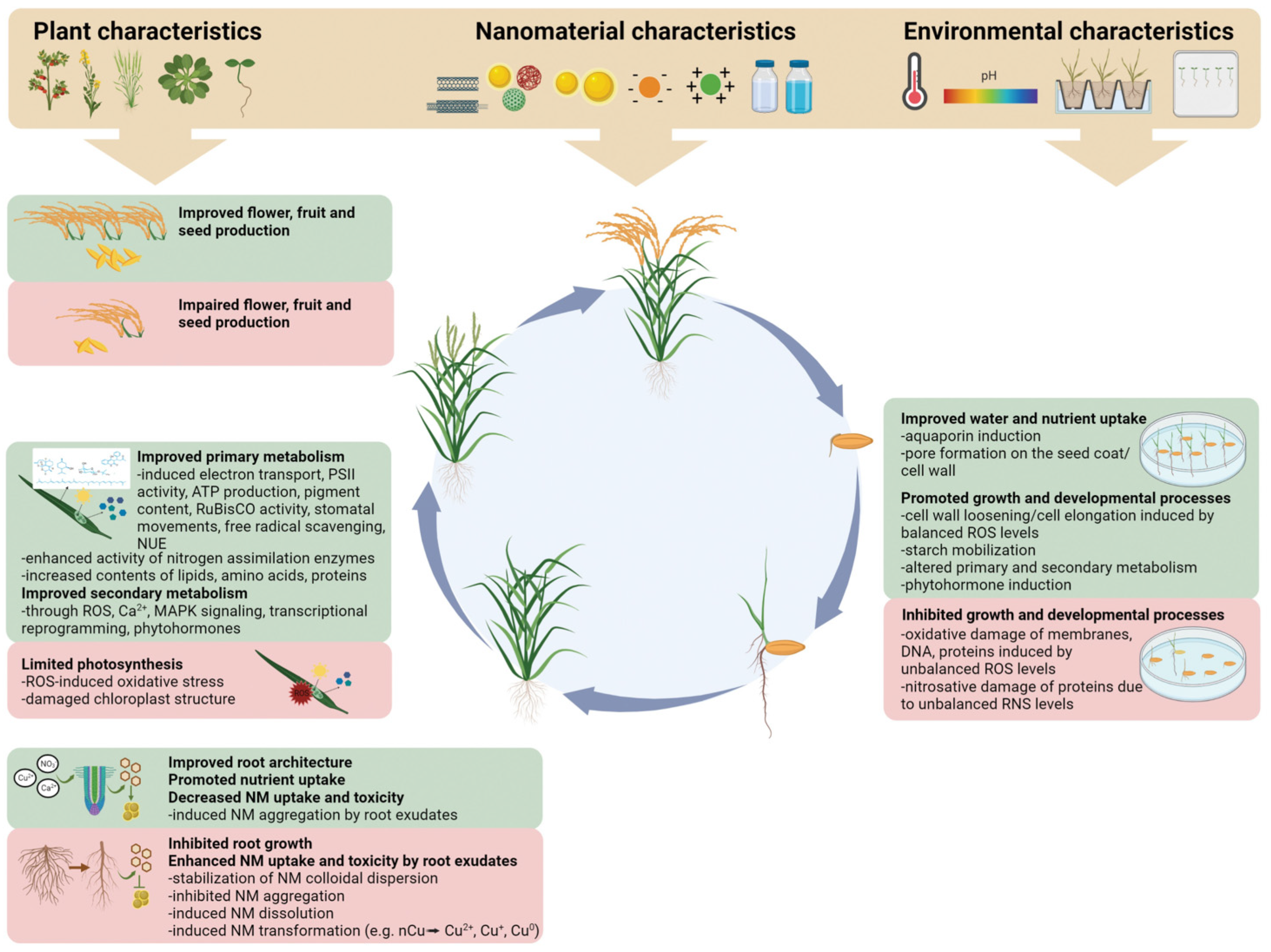
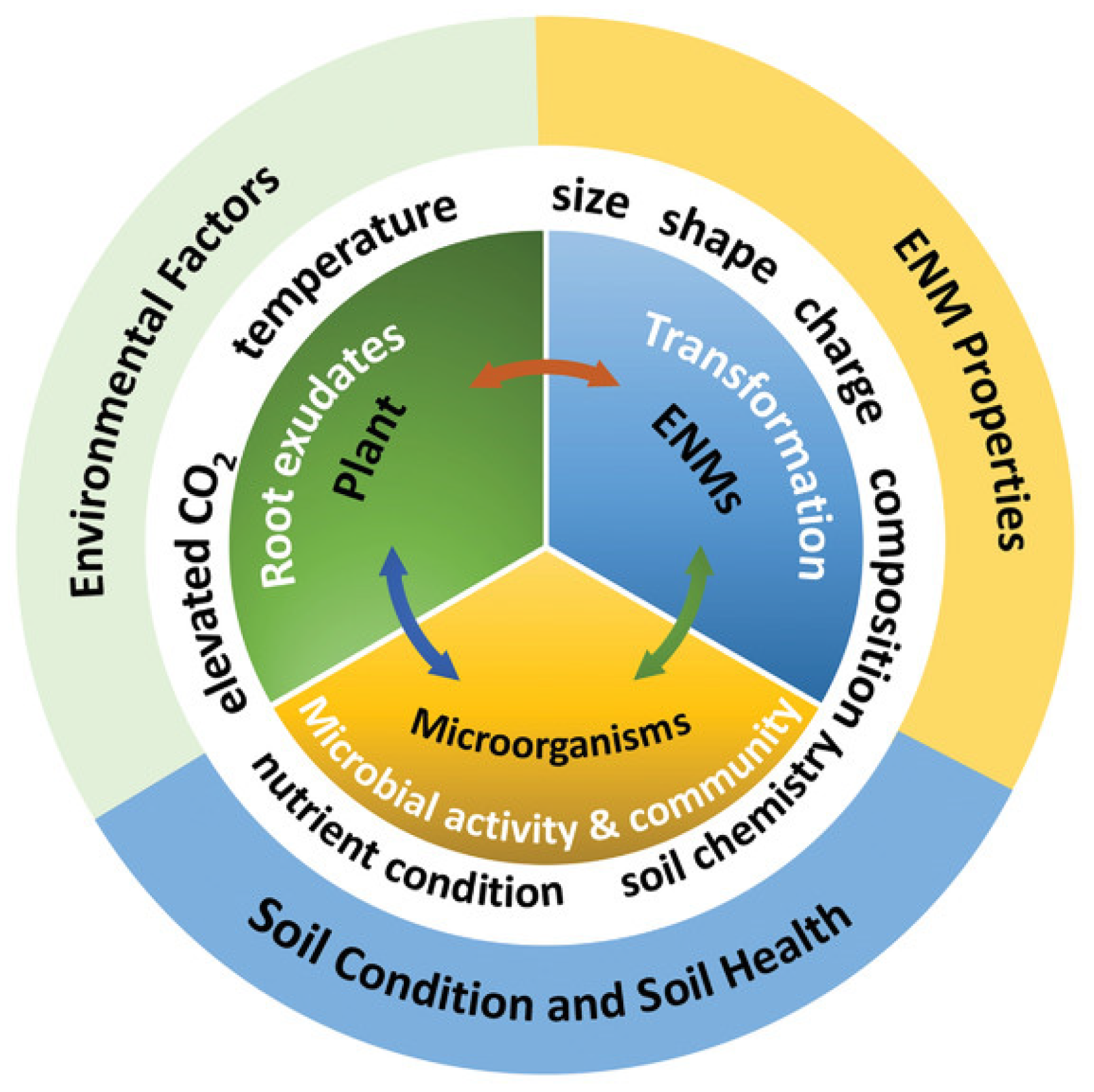
| Material | Plant | Application Form | Effect | Proposed Mechanism of Action | Reference |
|---|---|---|---|---|---|
| nZVI, size of 33.8 ±3.59 nm | Rice | Soaking of seeds for 3 days in 20 mg/L suspension | Increased seedling growth | Chlorophyll content, NADPH dehydrogenase activity, and root metabolism significantly increased | [71] |
| nZVI or Fe3O4 NPs, size of 20 nm | Rice | Seedlings treated with 50 mg/L suspension for 14 days | Promoted seedling growth | Chlorophyll content and POD enzyme activity increased | [72] |
| Fe3O4 NPs, size of 50–100 nm | Rice | Spraying with 0–20 mg/L suspension for four months | Promoted growth of rice | Reduced chromium absorption and accumulation, chlorophyll content, and SOD enzyme activity | [73] |
| Fe2O3 NPs, size of 20–40 nm | Wheat | Seedlings treated hydroponically for 21 days | Root length, plant height, biomass, and chlorophyll content of wheat increased | NPs supported chlorophyll synthesis | [74] |
| Fe3O4 NPs, size of 6.85 ±1.70 nm | Wheat | Seeds treated with 2000 mg/L suspension for five days | Alleviation of heavy metal-induced oxidative stress in wheat seedlings | Absorption of cadmium, lead, copper, and zinc decreased, and antioxidant enzyme activities of SOD and POD increased | [75] |
| Fe3O4 NPs, size of 80–110 nm | Wheat | Treatment with 200–500 mg/L suspension for three weeks | Photosynthetic pigment content and SOD enzyme activity increased | Improved plant photosynthetic performance and iron and phosphorus utilization rates promoted plant growth | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Xia, Y.; Song, K.; Liu, D. The Impact of Nanomaterials on Photosynthesis and Antioxidant Mechanisms in Gramineae Plants: Research Progress and Future Prospects. Plants 2024, 13, 984. https://doi.org/10.3390/plants13070984
Li P, Xia Y, Song K, Liu D. The Impact of Nanomaterials on Photosynthesis and Antioxidant Mechanisms in Gramineae Plants: Research Progress and Future Prospects. Plants. 2024; 13(7):984. https://doi.org/10.3390/plants13070984
Chicago/Turabian StyleLi, Ping, Yunfei Xia, Kai Song, and Duo Liu. 2024. "The Impact of Nanomaterials on Photosynthesis and Antioxidant Mechanisms in Gramineae Plants: Research Progress and Future Prospects" Plants 13, no. 7: 984. https://doi.org/10.3390/plants13070984
APA StyleLi, P., Xia, Y., Song, K., & Liu, D. (2024). The Impact of Nanomaterials on Photosynthesis and Antioxidant Mechanisms in Gramineae Plants: Research Progress and Future Prospects. Plants, 13(7), 984. https://doi.org/10.3390/plants13070984






