Abstract
Novel nanotechnology based on herbal products aspires to be a high-performing therapeutic platform. This study reports the development of an original engineering carrier system that jointly combines the pharmacological action of Chelidonium majus and AuNPs, with unique properties that ensure that the limitations imposed by low stability, toxicity, absorption, and targeted and prolonged release can be overcome. The metabolite profile of Romanian wild-grown Chelidonium majus contains a total of seventy-four phytochemicals belonging to eight secondary metabolite categories, including alkaloids, amino acids, phenolic acids, flavonoids, carotenoids, fatty acids, sterols, and miscellaneous others. In this study, various techniques (XRD, FTIR, SEM, DLS, and TG/DTG) were employed to investigate his new carrier system’s morpho-structural and thermal properties. In vitro assays were conducted to evaluate the antioxidant potential and release profile. The results indicate 99.9% and 94.4% dissolution at different pH values for the CG-AuNPs carrier system and 93.5% and 85.26% for greater celandine at pH 4 and pH 7, respectively. Additionally, three in vitro antioxidant assays indicated an increase in antioxidant potential (flavonoid content 3.8%; FRAP assay 24.6%; and DPPH 24.4%) of the CG-AuNPs carrier system compared to the herb sample. The collective results reflect the system’s promising perspective as a new efficient antimicrobial and anti-inflammatory candidate with versatile applications, ranging from target delivery systems, oral inflammation (periodontitis), and anti-age cosmetics to extending the shelf lives of products in the food industry.
1. Introduction
Chelidonium majus (Papaveraceae family) is the sole representative of the Chelidonium genus in the Romanian flora and, respectively, in that of Europe [1]. Its common names include greater celandine, swallow-wort, rostopască, negelarită in Romanian, and bai-qu-cai in Chinese. Greater celandine (GC) has been recognized as a plant with both medicinal and toxic properties since ancient times, with mentions dating back to ancient Europe and in Chinese traditional medicine [2]. From Dioscorides, Pliny the Elder, and Galenus until the XIV century, greater celandine was recommended for ocular ailments. In medieval Europe, the plant was used also for treating ulcers, cutaneous eczema, jaundice, and colic. Paracelsus mentioned the benefits of this plant for treating hepato-biliary conditions. Currently, greater celandine is appreciated in traditional European medicine, especially in the central and eastern regions, being recognized for its exceptional therapeutic properties, especially in dermatological conditions (e.g., eczema, verrucae, circumscribed cutaneous carcinomas), hepato-biliary (anti-jaundice) conditions, chole cytopathies, biliary lithiasis, gastrointestinal spasms, eye infections, and inflammation [3,4,5,6]. Modern research has reported the presence of a large variety of biomolecules (alkaloids, flavonoids, carotenoids, lectins, phenolic acids, volatile oils, and others) and, thus, remarkable biological activity (antibacterial, antimicrobial, antifungal, antiviral, anti-inflammatory, antitumoral, anti-spasmodic, hepato-protective, analgesic, and immunomodulatory) [7,8,9,10,11,12,13,14,15].
Cutting-edge nanotechnology-based phytochemical carriers have emerged as promising candidates with highly improved in vivo activity due to the overcoming of the drawbacks (low bioavailability, chemical, and thermally stability, and selectivity) of conventional herbal formulations [16]. Engineering phytochemical carriers are the most successful approaches, with highly improved in vivo activity. These carriers effectively overcome all the challenges posed by conventional herbal formulations, including low bioavailability, selectivity, and chemical and thermal stability [17].
Of all the metallic nanoparticles, gold nanoparticles are particularly well-suited for various biomedical applications thanks to their unique properties, such as versatile tailored surfaces, excellent stability, easy cellular uptake, and minimal toxicity. As a result, current research addresses the design of novel drug delivery systems that can mitigate drug resistance in cancer therapy, bacterial resistance antibiotics, etc. [18,19,20,21,22,23,24,25,26,27].
On the other hand, it is noteworthy that, despite the outstanding therapeutic activity of Chelidonium majus, its overdose due to self-medication with various market herbal supplements can induce severe outcomes on liver physiological function. Therefore, avoiding this liability requires advanced herbal formulations and safety and control of dosage, leading to increased efficacity [28,29].
Accordingly, in this study, our approach to plant-derived natural products moves to a different level, using the renowned medicinal plant Chelidonium majus and AuNPs to achieve an innovative engineering carrier system with unique pharmacological activity.
The chemical, morpho-structural, and thermal properties; antioxidant potential; and in vitro release profile were studied systematically.
2. Results and Discussion
Numerous research studies have been conducted on the chemical composition and pharmacological activity of Chelidonium majus. Most of these studies have focused on specific phytoconstituent categories found in certain parts of the plant [3,4,5,6,7,8,10,30,31,32].
However, the plant’s origin has an essential role in increasing the development of plants, as well as in triggering different defense mechanisms against various biotic factors in their environments. Among the most dominant defense systems of plants against environmental stress factors is a plant’s ability to produce varied secondary metabolites and signaling molecules. Accordingly, discrepancies occur in the metabolic profiles of particular plants of different origins [17,33]. Furthermore, extraction parameters, such as solvent polarity, temperature, and pH, have a decisive impact on the phytochemical composition [34,35,36].
To this end, establishing a correlation between the biomolecules found in a plant and its therapeutic activity is an arduous task.
Moreover, few studies have been performed, and only on the alkaloid or phenolic contents of the Romanian Chelidonium majus wild plant [37,38]. Therefore, this study investigates the low metabolic profile of greater celandine using gas-chromatography coupled with mass spectroscopy (GC-MS) and electrospray ionization–quadrupole time-of-flight mass spectrometry (ESI-QTOF-MS) analysis. The phytochemicals were identified on the retention indices, in the Mass Spectral Library 2.0 database, and in the literature.

Table 1.
Main biomolecules identified by GC–MS analysis of Chelidonium majus sample.
Table 1 shows the main phytoconstituents identified via GC-MS analysis from the greater celandine sample.
The GC-MS analysis displays thirteen compounds, accounting for about 87% of the total peak area in the greater celandine sample (Figure S1).
2.1. Mass Spectrometry Analysis of Chelidonium majus Sample
The MS spectra (Figure S2) indicate the presence of numerous molecules, some of which were detected and assigned to different chemical classes (alkaloids, amino acids, phenolic acids, flavonoids, carotenoids, organic acids, fatty acids, sterols, and others) that corroborate the literature results [6,7,11,15,31,33,38,40,42,43].
The phytoconstituents identified via ESI–QTOF–MS analysis are presented in Table 2.

Table 2.
Biomolecules identified in Chelidonium majus sample through MS analysis.
2.2. Screening and Classification of the Differential Phytoconstituents
A total of seventy-four biomolecules were identified and assigned to several categories of secondary metabolites: alkaloids (28.38%), amino acids (about 15%), phenolic acids (12.16%), flavonoids (6.75%), carotenoids (6.75%), fatty acids (5.4%), phytosterols (about 4%), terpenoids (1.75%), and miscellaneous others.
Figure 1 shows the phytochemical classification chart from the Chelidonium majus sample based on the data analysis reported in Table 2.
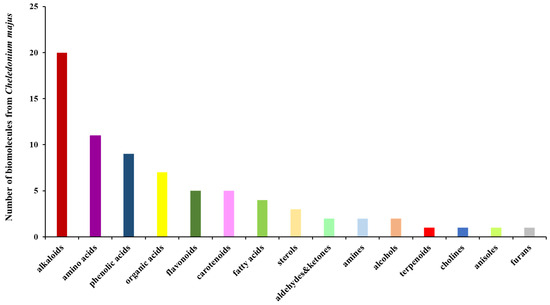
Figure 1.
Biomolecule classification bar chart for Chelidonium majus.
According to Figure 1, alkaloids, the largest category of phytoconstituents, exhibited outstanding therapeutic activities: sedative, analgesic, antitumoral, antimicrobial, antifungal, anti-inflammatory, antidiabetic, antiemetic, antioxidant, neuroprotective, etc. [44,45].
In the greater celandine sample, eleven amino acids were identified, of which the largest percent (72.7%) was represented by non-essential amino acids (glycine, alanine, serine, proline, asparagine, aspartic acid, glutamic acid, and tyrosine). Essential amino acids (isoleucine, valine, and threonine) were present in a minor proportion (27.3%). Various research has reported the exceptional pharmacological activities of these phytochemicals (anti-inflammatory, neuroprotective, antiproliferative, cytotoxic, and immunomodulating activities [46,47,48,49,50,51,52,53]).
Phenolic acids made up about 12% of the biomolecules from the greater celandine sample, being involved in antioxidant, antimicrobial, cardioprotective, anti-inflammatory, neuroprotective, antitumor, and antidiabetic mechanisms [54].
Flavonoids (hyperoside, luteolin, quercetol, isorhamnetin, quercetin) are another secondary metabolite class with significant beneficial effects on human health (antioxidant, antimicrobial, antiviral, anti-inflammatory, antitumoral, antidiabetic, cardioprotective, hepatoprotective, and neuroprotective). In addition, luteolin is involved in the management of pain caused by anti-inflammatory disorders [55,56,57,58,59,60].
Recent research has shown that carotenoids exhibit antioxidant, anti-inflammatory, neuroprotective, cardioprotective, skin and eye protection, anti-obesity, antitumoral, and antimutagen activities [61,62].
Fatty acids represented 5.4% of the total phytoconstituents from the greater celandine sample. These secondary metabolites act as anti-inflammatory, antioxidant, cardioprotective, and neuroprotective agents [63,64].
Phytosterols represented 4.05% of the total phytochemicals from the greater celandine sample. These are involved in antioxidant, anti-inflammatory, immunomodulatory, antiatherosclerotic, neuroprotective, and antitumoral mechanisms [65].
The terpenoid limonene displays antitumoral, antimicrobial, antifungal, antidiabetic, anti-inflammatory, antiallergenic, antitumoral (breast tumor), and neuroprotective activities [66,67].
2.3. Phyto-Nanocarrier System
Advanced nanotechnology is the key to overcoming the limitations of biomedical applications of medicinal plant preparations with high therapeutic activity. The reduced stability, permeability, and bioavailability of some specific secondary metabolites in biological environments pose significant challenges [16,17,32]. However, the engineering nanocarriers based on metallic nanoparticles offer a highly effective solution by improving biocompatibility, reducing harmful side effects, and exhibiting higher therapeutic efficiency through the synergistic effect of both components, namely, drugs and metallic nanoparticles. Moreover, these tailored nanocarriers enhance stability, permeability, targeting control, and release. Therefore, the engineering nanocarriers used in biomedical applications represent a significant advancement and are poised to have promising potential in personalized therapeutic strategies [16,68]. Accordingly, a novel phyto-carrier based on preparation of AuNPs will synergically merge the biological activities of the Chelidonium majus biomolecules and metallic nanoparticles, which will achieve a higher therapeutic yield.
2.4. FT-IR and Raman Spectroscopy
Fourier transform infrared (FTIR) spectroscopy is a widely analytical technique used to obtain findings regarding molecular structure and chemical composition from complex matrices.
Hence, the preparation of the GC-AuNPs carrier system was studied using FT-IR spectroscopy to emphasize the bonding mechanism between herbs and metallic nanoparticles. FT-IR spectra are presented in Figure 2.
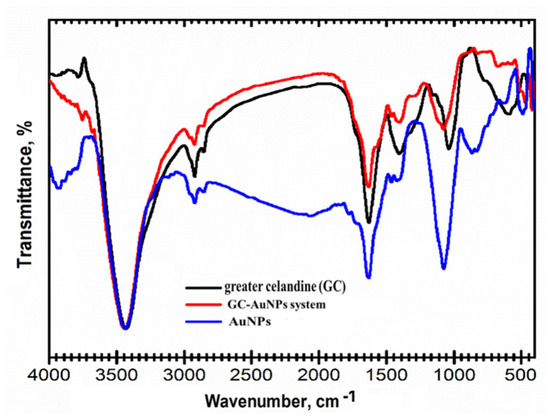
Figure 2.
FT-IR overlap spectra of the carrier system of greater celandine, AuNPs, and GC-AuNPs.
The functional groups assigned to Chelidonium majus phytochemicals are shown in Table 3.

Table 3.
The characteristic vibrational bands attributed to various biomolecule categories from the Chelidonium majus sample.
The FTIR spectra of the GC-AuNPs carrier system display the characteristic absorption bands of Chelidonium majus (3430 cm−1 (-OH group), 3293 cm−1 (O-H stretching carboxylic acid), 1709 cm−1 (C=O stretching vibration), 1609 cm−1 (C=C of carotenoids), 1601 cm−1 (C=C and C=N stretching vibrations of alkaloids), 1240 cm−1 (C-N of amine), 1032 cm−1 (NH stretching of amines), and 872 cm−1 (C-H bending vibration of aromatic rings)), as well as the AuNPs coated with trisodium citrate (2915 cm−1 (OH stretching vibration); 2848 cm−1 (corresponding to CH- asymmetric and symmetric stretching vibrations); 1596 cm−1 (COO- stretching vibration); and 1392 cm−1 (assigned to C–H bending)), thus pointing to the successful preparation of the CG-AuNPs carrier system [18,20,69,77].
Nonetheless, the changes in the intensity of the absorption bands and the shift toward higher wavenumbers in the corresponding regions (O-H, N-H, and C-H) are noticeable and indicate their involvement in the preparation of the GC-AuNPs carrier system [20,78,79].
2.5. Raman Spectroscopy
Raman spectroscopy is an important technique that is often used to study the vibrational modes of both molecules and hybrid nanomaterials.
Figure 3 shows the characteristic Raman spectrum of the GC-AuNPs carrier system.
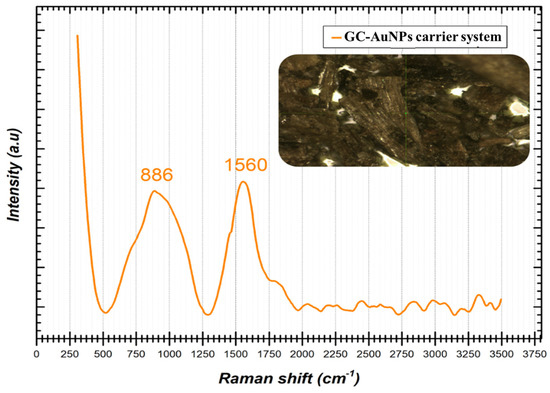
Figure 3.
Raman spectrum of GC-AuNPs carrier system.
The Raman spectrum displayed in Figure 3 indicates the presence of two peak shifts identified at 886 cm−1 and 1560 cm−1, respectively. When gold nanoparticles are subjected to Raman analysis, certain specific shifts are expected to appear in the spectrum in the ranges of 200–400 cm−1 and 500–580 cm−1 [80]. In the present case, no spectral information could be identified in the previously mentioned intervals, but the presence in the spectrum of the two Raman shifts of significantly high intensity at 886 cm−1 and 1560 cm−1, respectively, can most probably be attributed to the vibrational modes specific to the interactions and the strong bonds between the surfaces of the AuNPs and the phytochemicals from the greater celandine sample [7]. It is important to note that the precise shifts in the Raman spectra may vary depending on the size, morphology, and surface chemistry of the AuNPs (single or hybrid), as well as on the experimental conditions. In addition, Raman spectroscopy is often used in combination with other complementary characterization techniques, so that a more detailed understanding of the properties of gold nanoparticles and how they interact with compounds in the plant extract of celandine can be obtained.
2.6. X-Ray-Diffraction Spectroscopy (XRD)
X-Ray-Diffraction Spectroscopy (XRD) is a simple, fast, and non-destructive technique used to determine the phase composition and crystallographic data of materials [81].
Overlapped XRD patterns of the greater celandine, AuNPs, and GC-AuNPs carrier system are presented in Figure 4.
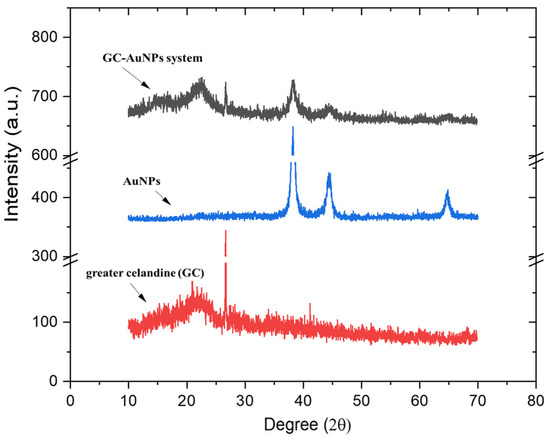
Figure 4.
Powder XRD patterns of greater celandine, AuNPs, and CG-AuNPs carrier system.
The XRD spectrum of AuNPs depicts defined peaks, evidencing a well-crystalline structure, with a crystallite mean size of 17 nm, as determined using the Scherrer equation [18].
The greater celandine pattern shows amorphous phases with poorly defined peaks in the (17–43°) range, associated with minerals and plant fibers. The XRD pattern of the GC-AuNPs carrier system exhibits, even if moderately weaker, peaks of herb components and AuNPs (Figure 4), thus confirming the formation of a new carrier system.
2.7. Scanning Electron Microscopy (SEM)
The comparative analysis of morpho-structural features was carried out using the SEM-EDX method.
It appears that the SEM image of the greater celandine (Figure 5A, low magnification (×850)) indicates the presence of a fibrous structure with large pores and irregular shape agglomerations of different-sized particles (average size: ~30 nm). The CG-AuNPs carrier system micrograph (Figure 5B,C low magnification (×850)) indicates that AuNPs and clusters of AuNPs (spherical shape, average size ~17 nm) were loaded in the herb particle pores.
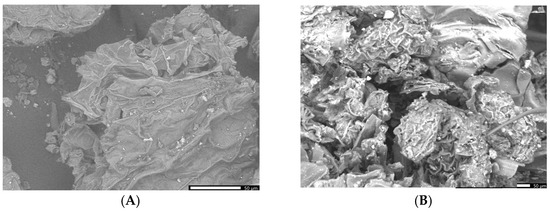
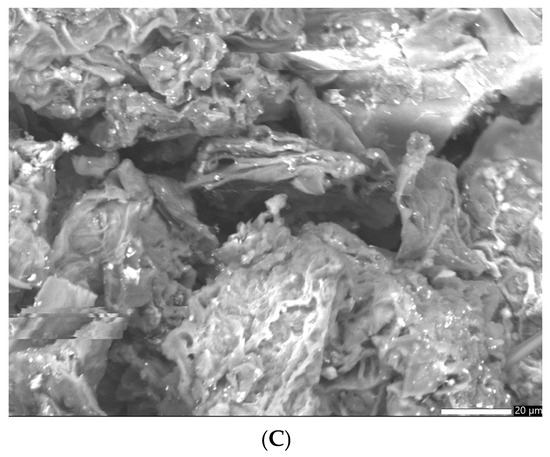
Figure 5.
SEM images of the greater celandine (A) and CG-AuNPs carrier system (B,C).
The EDX analysis was carried out comparatively on the greater celandine sample and the CG-AuNPs carrier system. The EDX spectra of the new carrier system are displayed beside the peaks corresponding to greater celandine (Figure 6A) and AuNPs (Figure 6B), indicating the achievement of the CG-AuNPs carrier system.
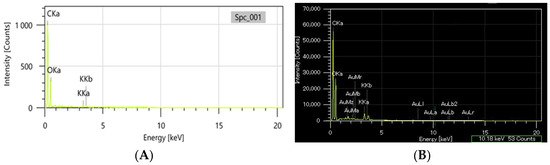
Figure 6.
EDX composition of the greater celandine (A) and CG-AuNPs carrier system (B).
2.8. Dynamic Light Scattering (DLS)
Dynamic light scattering is a routine, accurate analytic method for the mean and distribution determination of nano- and micro-scale particles in dispersion. The hydrodynamic size, distribution, and stability of the GC-AuNPs carrier system were investigated via the DLS technique. The obtained results are shown in Table 4.

Table 4.
The DLS mean hydrodynamic diameter values of the GC-AuNPs carrier system and components.
The distribution of particles in solution for all samples is presented in Figure 7.
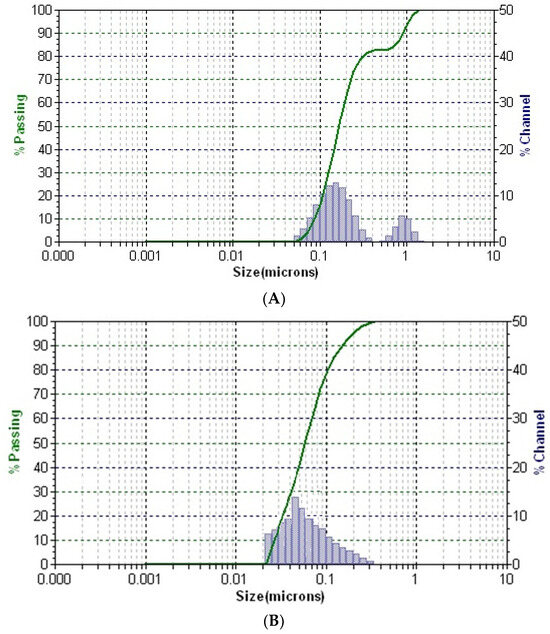
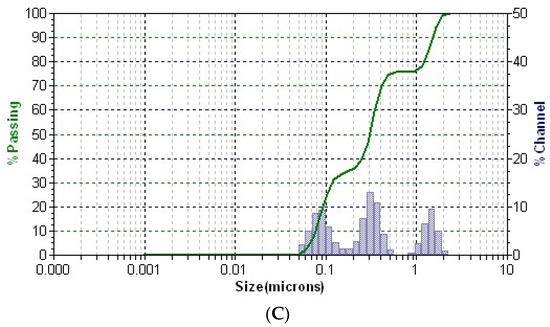
Figure 7.
DLS patterns of the greater celandine (A), AuNPs (B), and CG-AuNPs carrier system (C).
In the DLS pattern of the greater celandine sample (Figure 7A), there are two distinctive peaks corresponding to different hydrodynamic diameter values, which can be attributed to the fibrous structures and particles from the herb. According to the DLS analysis, the mean diameter of AuNPs is about 16 nm.
Conversely, the pattern of the CG-AuNPs carrier system (Figure 7C) exhibits three separate peaks, well-dispersed into a narrow range, indicating high stability. These peaks can be assigned to the presence of herb components (fibrous structures and particles) and AuNPs. It is worth noting that there was a significant shift in the sizes of AuNPs and herb components, which suggests that AuNPs were loaded into the herb pores. These findings agree with the results of the SEM study.
2.9. Thermal Properties
A comparative evaluation was conducted to determine the thermal stability of the novel carrier system and greater celandine and to identify the chemical changes. The results are presented in Figure 8.
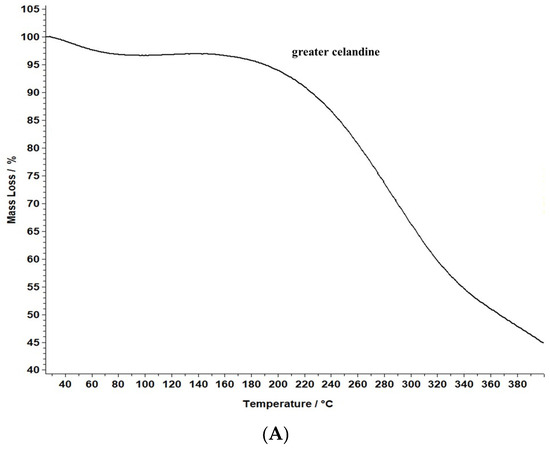
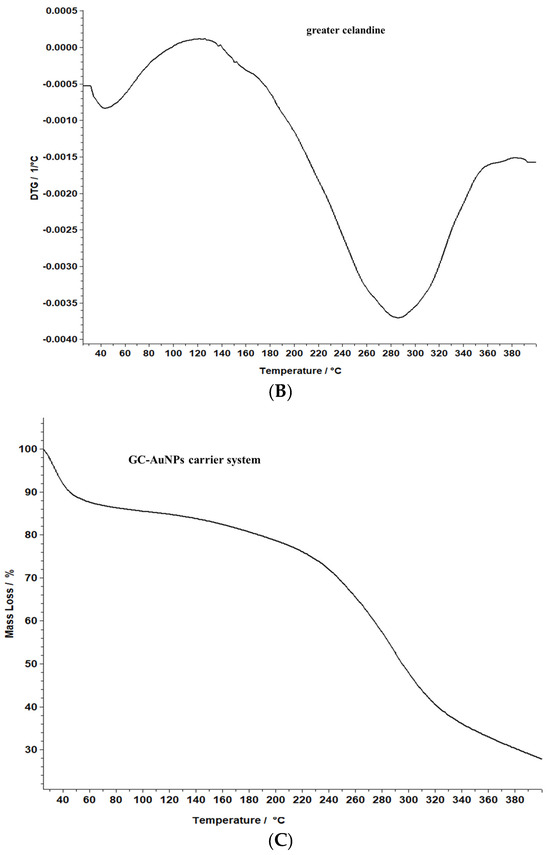
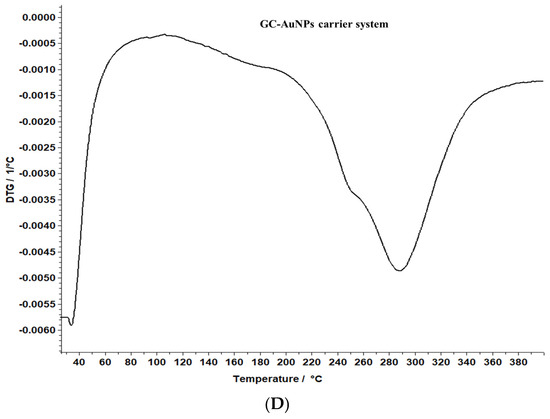
Figure 8.
TG/DTG thermograms of the greater celandine sample (A,B) and CG-AuNPs carrier system (C,D).
The data reveal that greater celandine demonstrated an endothermic process, resulting in a substantial weight loss (55%) in the temperature range of 150–180 °C due to moisture loss and decomposition of volatile compounds, carotenoids, alkaloids, and phenolics [82,83].
Similarly, the thermogravimetric curve of the novel carrier system indicated a noticeable weight loss (46%) at 190–220 °C, assigned to phytochemical decomposition. These changes in the differential thermogravimetric curve of the new carrier system may be linked to the loading of AuNPs in the herb particles, indicating a visible increase in the thermal stability of the CG-AuNPs carrier system. The findings provide valuable insight into the behavior of these samples, and could contribute to further research and development in this field.
2.10. In Vitro Dissolution Testing
In vitro dissolution assays are a ubiquitous technique in pharmacological development and quality control to predict the dissolution behavior and biological efficiency of drugs in the gastrointestinal tract [84,85]. However, due to the complex chemical composition, the bioavailability and performance assays for herbal formulations can be more challenging than for single compound products [84,85].
The pH value and time are key physiological factors with significant effects on the absorption of active biomolecules. Hence, a comparison study was performed between the dissolution profile of greater celandine and a newly prepared carrier system at pH values of 4 and 7 as a function of time.
The correlation between the pH value and dissolution rate is presented in Figure 9.
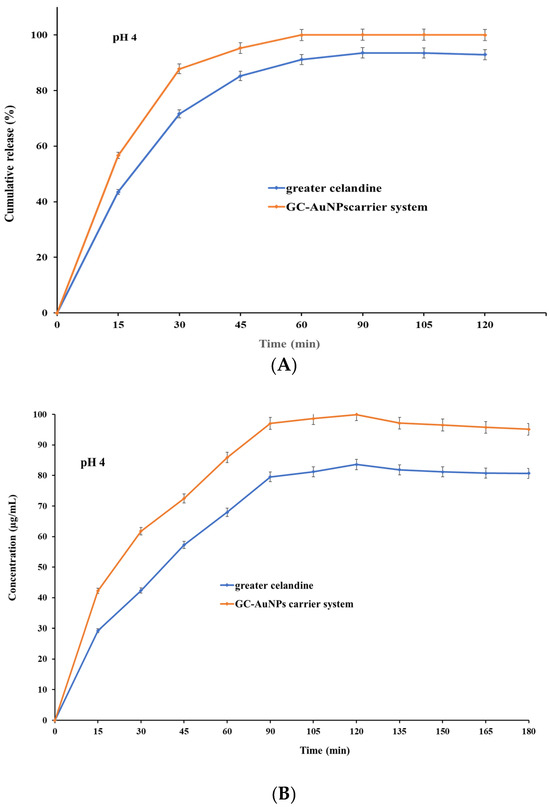
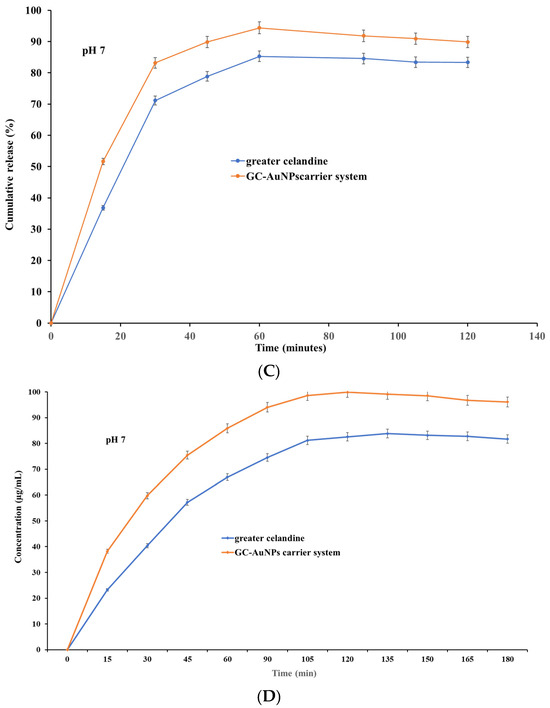
Figure 9.
The dissolution profile of the greater celandine and CG-AuNPs carrier system.
The results indicate that both samples exhibited, at pH 4, similar dissolution profiles and increased release (Figure 9 A) within 30 min (over 81% for GC and over 89% for carrier system), subsequently reaching a maximum value of 93.56% for the greater celandine (Figure 9B) and 99.99% for the CG-AuNPs carrier system at 60 min. Nevertheless, at pH 7, a notable difference appeared, specifically on the dissolution profile (Figure 9C). Furthermore, even though both samples still displayed a rapid release within 30 min, these values were significantly lower than at an acidic pH (about 71% for greater celandine and 83% for CG-AuNPs carrier system) (Figure 9D). The maximum release value was reached at 60 min for the greater celandine (85.26%) and the new carrier system (94.39%).
According to the results, the novel carrier system showed notably improved bioavailability compared to the greater celandine at both pH values investigated. This enhancement can be attributed to the specific surface modification under the employed experimental conditions. Additionally, a visible reduction in the dissolution rate (approximately 5% for the new carrier system and 8% for greater celandine) in a neutral environment (pH = 7) was observed. These unequivocal observations suggest that an acidic pH is more appropriate for the absorption of biomolecules. The findings of this study are highly significant and offer valuable insights that could significantly impact future studies on efficacy enhancement, as well as optimize the therapeutic outcome.
2.11. Screening of Antioxidant Activity
The evaluation of antioxidant potential for a specific herb formulation necessitates the selection of at least three appropriate antioxidant assays [86]. In vitro, non-competitive assays are widely acknowledged for their simplicity and accuracy in estimating the antioxidant potential of natural products [86,87].
The antioxidant activity of a novel carrier system is attributed to a combination of collective bioactive phytochemicals from the greater celandine and the biological activity of the AuNPs. Consequently, four assays, namely, total polyphenolic contents (TPCs)—Folin–Ciocalteu, flavonoid content, FRAP, and DPPH were deemed adequate for estimating the antioxidant potential of the CG-AuNPs carrier system. The results are presented in Table 5 and Figure 10.

Table 5.
The result of the selected antioxidant assay for the greater celandine and CG-AuNPs carrier system.
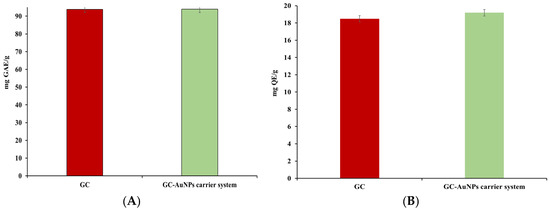
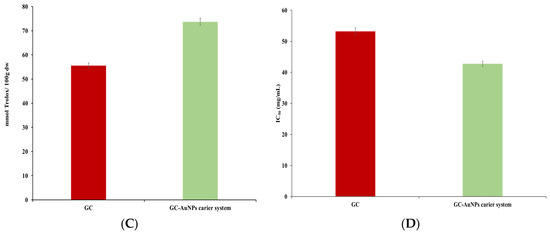
Figure 10.
Graphic representation of total phenolic (A), flavonoid content (B), FRAP (C), and DPPH (D) results.
No significant differences were found in the TPCs or flavonoid assays for the novel carrier system compared to the greater celandine. However, in FRAP and DPPH tests, the CG-AuNPs carrier system exhibited higher antioxidant activity than the herb plant sample. Thus, the maximum value of the FRAP assay indicated an increase (up to 24%) for the carrier system. Similarly, the IC50 value was lower than that of the greater celandine by over 24%. These results can be attributed to modifications in the surface electric charge of metallic nanoparticles loaded into herb particles, as well as the synergistic action of AuNPs and the bioactive phytoconstituents [88,89].
This assay selection offers a comprehensive evaluation of the antioxidant potential of the carrier system, which is vital for the development of effective and safe antioxidant formulations [20,90].
3. Materials and Methods
All reagents were of analytical grade, purchased from commercial sources (Merck Millipore (Darmstadt, Germany), Sigma-Aldrich (München, Germany)), and used without further purification.
Chelidonium majus (greater celandine) samples (whole plant) were harvested in August 2023 from the area of Hunedoara County, Romania (geographic coordinates 45°43′04″N 22°53′13″E), and taxonomically authenticated at the University of Medicine and Pharmacy Craiova, Romania.
3.1. Plant Samples’ Preparation for Chemical Screening
The greater celandine samples underwent milling via a planetary Fritsch Pulverisette mill (Idar-Oberstein, Germany) (700 rpm for 10 min at 22 °C), followed by sieving through ASTM sieves. Only particles that passed through a 0.25 mm mesh sift were used in this study. Then, the plant samples were subjected to ultrasonic-assisted extraction (Elmasonic, Singen, Germany) under specific temperature, time, and ratio conditions (25 min, at 40 °C and 50 Hz, in methanol: chloroform = 1:1, v/v). The resulting extract was concentrated using a rotary evaporator, dissolved in MeOH (10 mL), centrifuged, and filtered. Subsequently, the extract samples were stored in a freezer until further use. All experiments were prepared in triplicate.
3.1.1. GC-MS Analysis
Gas chromatography was carried out using a GCMS-QP2020NX Shimadzu apparatus with a ZB-5MS capillary column (30 m × 0.25 mm id × 0.25 µm) (Agilent Technologies, Santa Clara, CA, USA) and helium (flow rate of 1 mL/min.)
GC–MS Separation Conditions
The oven temperature was increased from 50 °C (kept for 2 min) to 300 °C (rate of 4 °C/min, hold for 5 min). The temperature of the injector was 290 °C, and the temperature at the interface was 217 °C. The mass of the compounds was registered at 70 eV of ionization energy. The mass spectrometer was source-heated at 225 °C, and the MS Quad was heated at 155 °C. Phytochemicals were identified based on their mass spectra compared to the NIST0.2 mass spectra library database (USA National Institute of Science and Technology software, (NIST, Gaithersburg, MD, USA) and the literature review.
3.1.2. Mass Spectrometry
The MS experiments were performed using an EIS-QTOF-MS (Bruker Daltonics, Bremen, Germany). The mass spectra were acquired in positive ion mode in a mass range of 50–3000 m/z. The scan speed was 2.1 scans/s, the collision energy was 10 ÷ 85 eV, and the temperature of the source block was 80 °C. Compounds were identified based on their mass spectra, then compared to the NIST 3.0 database mass spectra library database (USA National Institute of Science and Technology software) (NIST, Gaithersburg, MD, USA) and the literature review.
3.2. CG-AuNPs Carrier System Preparation
3.2.1. The synthesis of AuNPs was achieved according to the procedure described in our earlier publication [18]
3.2.2. CG-AuNPs Carrier System
The greater celandine sample (whole plant dried) and AuNPs solution were mixed (1:2.5 mass ratio) under continuous stirring for 6 h at room temperature (22 °C). The emerging suspension was centrifuged, filtered, and then dried at 40 °C for 6 h. Each experiment was repeated three times.
3.3. Characterization of Novel Carrier System and Raw Materials
3.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
FT-IR spectra of the CG-AuNPs carrier system and its components in the solid phase were recorded on a Fourier transform infrared spectrometer (Shimadzu AIM-9000 with ATR devices).
3.3.2. XRD Spectroscopy
Data on the phase composition were obtained on a Bruker AXS D8-Advance X-ray diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with a rotating sample stage; an Anton Paar TTK low-temperature cell (−180 °C ÷ 450 °C); a high-vacuum, inert atmosphere; and relative humidity control. The average crystallite size and phase content were determined using the whole-pattern profile-fitting method (WPPF).
3.3.3. Scanning Electron Microscopy (SEM)
Morpho-structural investigations were carried out using an SEM-EDS system (JEOL JSM-IT200 Field Emission, Nieuw-Vennep, The Netherlands) equipped with a high-resolution electron gun and an energy-dispersive X-ray spectrometer (EDS).
3.3.4. Dynamic Light Scattering (DLS) Particle Size Distribution Analysis
The DLS analysis was conducted with a scattering angle of 172 °C at room temperature (22 °C) using a Microtrac/Nanotrac 252 (Montgomeryville, PA, USA) instrument. Each experiment was repeated three times.
3.3.5. Thermal Analysis
The thermal stability study of the novel carrier system and herb sample was performed in a dynamic air atmosphere (20 mL/min, synthetic air) at a temperature range of 25 ÷ 400 °C and a heating rate of 10 ° C/min using a Thermal Analyzer produced by METTLER TOLEDO, model TGA/DSC3+ STARe System. The DSC analysis was performed in an air atmosphere (50 mL/min), at a temperature range of 25–400 °C, on a DSC 3+ Mettler Toledo.
3.4. Antioxidant Activity
In vitro antioxidant potential screening of the novel carrier system and herb sample were examined using four distinct tests: Folin–Ciocalteu assay; flavonoid content assay; 2,2-diphenyl-1-picrylhydrazyl; (DPPH) radical scavenging assay; and ferric reducing antioxidant power assay (Frap).
3.4.1. Folin–Ciocalteu Assay
The antioxidant activity of Chelidonium majus and GC-AuNPs was determined using UV-VIS spectrophotometry (DLAB SP-UV1000, Penjuru, Singapore), according to the experimental procedure described in the literature [91]. The results are expressed in gallic acid equivalents (mg GAE/g sample). Sample concentrations were calculated based on the linear equation obtained from the standard curve (y = 0.0015x + 0.2134) and the correlation coefficient (R2 = 0.9971).
3.4.2. The Flavonoid Content Assay
The flavonoid contents from both samples were determined according to the experimental procedure adapted from the literature [92].
The absorbance was measured at 510 nm using a UV-Vis spectrometer (DLAB SP-UV1000). The flavonoid content was expressed in quercetin equivalents (mg QE/g) using a quercetin standard calibration curve between 12.5 mg/mL and 100 mg/mL in methanol. Sample concentrations were calculated based on the linear equation obtained from the standard curve (y = 0.0083x + 0.1114) and the correlation coefficient (R2 = 0.9961).
3.4.3. Ferric Reducing Antioxidant Power Assay (FRAP)
The ferric reducing/antioxidant activity (FRAP) of the sample was determined spectrophotometrically using a Ferric Reducing Antioxidant Power (FRAP) Assay Kit (MAK369-1KT, Sigma-Aldrich). The absorbance was measured at 594 nm using a UV-Vis spectrometer (Elabscience®, Houston, TX, USA). The results were expressed in Trolox equivalents (mmol Trolox equivalents/100 g dry weight (dw)).
3.4.4. DPPH Radical Scavenging Assay
The radical scavenging properties of the novel carrier system and herb sample were assessed according to the procedure described in our earlier publication [93]. The results were used to calculate and obtain the IC50 (mg/mL).
All experiments for antioxidant activity screening were performed in triplicate.
3.5. In Vitro Dissolution Test
The dissolution profiles of the greater celandine (0.5 g ± 0.012) and CG-AuNps carrier system (0.5 g ± 0.016) were determined using a 708-DS Dissolution-Agilent Technologies (Santa Clara, CA, USA). The tests were conducted under strict conditions: a temperature of 37 ± 0.25 °C, a speed of 100 rpm, and two buffers of pH 4 and pH 7, respectively, to simulate the gastric and intestinal fluids [94].
Sink conditions were rigorously maintained throughout the test.
During the experiment, samples of dissolution medium (5 mL) were collected at different times (15–120 min). The cumulative drug released against time was determined spectrophotometrically (UV-Vis Perkin-Elmer Lambda 35 (Perkin Elmer, Waltham, MA, USA).
Triplicate samples were analyzed at each time point. The mean values of the samples and the standard deviation were calculated [95].
Preparation of the Curves of the Concentrations for the Compound Dissolution Profile
Different solution concentrations (in the range of 0.00 and 0.30 mg/mL) were prepared from each sample (greater celandine and CG-AuNP carrier system, respectively). Then, calibration curves were plotted. The amount of compound released was obtained from the standard curve of the concentration versus its absorbency. The correlation coefficients at pH = 4 were: R2 = 0.9978 (greater celandine) and R2 = 0.9986 (CG-Au NPs carrier system), and at pH = 7, R2 = 0.9991 (greater celandine) and R2 = 0.99984 (CG-Au NPs carrier system). This demonstrates the good linear relationship of the data.
The compound release was calculated according to Equation (1) [96]:
3.6. Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics 21.0 for Windows (SPSS Inc.). Each experimental set was performed in triplicate, using one-way analysis of variance (ANOVA) without replication with Scheffe’s post hoc test for comparison; p < 0.05 was taken as statistically significant. Data are presented as the means ± SD.
4. Conclusions
This study presents the low-molecular-mass-metabolite profiling of Chelidonium majus growing wild in Romania, followed by the development and in vitro evaluation of the antioxidant and release of a novel carrier system prepared using this medicinal plant and AuNPs. Various analytical methods, including FTIR, Raman, XRD, DLS, and SEM-EDX, were employed to confirm the preparation of the carrier system. The TG/DTG study results demonstrated that the GC-AuNPs carrier system had superior thermal behavior compared to the herb sample. The study indicates that this novel carrier system had significantly enhanced antioxidant activity and an improved release rate. These results suggest its auspicious potential as a promising candidate for various applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13050734/s1, Figure S1: TIC chromatogram of Chelidonium majus sample.; Figure S2: The mass spectra of Chelidonium majus sample.
Author Contributions
Conception and design of the study: A.-E.S.; methodology: A.-E.S., L.E.B., S.B., G.V., T.V., M.-V.C., and C.B.; analysis and interpretation of data: G.V., T.V., M.-V.C., A.D., G.B., and E.R.B.; writing—original draft preparation: S.B.; writing—review and editing: A.-E.S.; investigation: G.V. and T.V acquisition of data.; M.-V.C., A.D., G.B., and E.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmonson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1996; Volume 1. [Google Scholar]
- Biswas, S. Chelidonium majus L.—A Review on pharmacological activities and clinical effects. J. Med. Plants 2013, 2, 238–245. [Google Scholar]
- Dumitru, M.G.; Gănescu, A. Antioxidant activity of alcoholic extract of Chelidonium majus L. flowers. Ann. Univ. Craiova Chem. Ser. 2022, XLVIII, 63–69. [Google Scholar] [CrossRef]
- Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar]
- Dumitriu Buzia, O.; Ion, G.M.; Earar, K.; Gurau, G.; Mardare, N. Antimicrobial activity of Chelidonium species majus L. Med. Mater. 2022, 2, 31–38. [Google Scholar] [CrossRef]
- Krizhanovska, V.; Sile, I.; Kronberga, A.; Nakurte, I.; Mezaka, I.; Dambrova, M.; Pugovics, O.; Grinberga, S. The cultivation of Chelidonium majus L. increased the total alkaloid content and cytotoxic activity compared with those of wild-grown plants. Plants 2021, 10, 1971. [Google Scholar] [CrossRef]
- Musidlak, O.; Warowicka, A.; Broniarczyk, J.; Adamczyk, D.; Goździcka-Józefiak, A.; Nawrot, R. The activity of Chelidonium majus L. latex and its components on HPV reveal insights into the antiviral molecular mechanism. Int. J. Mol. Sci. 2022, 23, 9241. [Google Scholar] [CrossRef]
- Parsania, A.; Pouriayevali, M.H.; Parsania, M.; Ghorbani, M. Chelidonium majus L. alkaloid extract enhances TRAIL-induced apoptosis in HeLa cell line through death receptors 4 and 5 upregulation. Gene Rep. 2021, 25, 101311. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and antimicrobial-enhancing activity of Chelidonium majus and Corydalis cheilanthifolia extracts against multidrug-resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Arora, D.; Sharma, A. A review on phytochemical and pharmacological potential of genus Chelidonium. Pharmacogn. J. 2013, 5, 184–190. [Google Scholar] [CrossRef]
- Choi, H.A.; Ahn, S.O.; Lim, H.D.; Kim, G.J. Growth suppression of a gingivitis and skin pathogen Cutibacterium (Propionibacterium) acnes by medicinal plant extracts. Antibiotics 2021, 10, 1092. [Google Scholar] [CrossRef]
- Madjeed Haddao, K.; Dawood Saleem, H.; Hameed, N.M.; Mahdi Rheima, A.; Alkhafaje, W.K.; Abood, E.S.; Hussein, H.A.; Al-Aboudy, F.K.H.; Alwan, N.H.; Al-Dahy, L.B. Investigation of in vitro cytotoxicity of Chelidonium majus against Leishmania major. Arch. Razi Inst. 2022, 77, 1211–1214. [Google Scholar]
- Zielińska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater Celandine’s ups and downs—21 centuries of medicinal uses of Chelidonium majus from the viewpoint of today’s pharmacology. Front. Pharmacol. 2018, 9, 299. [Google Scholar] [CrossRef]
- Maji, A.; Banerji, P. Chelidonium majus L. (Greater celandine)—A review on its phytochemical and therapeutic perspectives. Int. J. Herb. Med. 2015, 3, 10–27. [Google Scholar] [CrossRef]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved activity of herbal medicines through nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Marin, C.N.; Herea, D.D.; Stanusoiu, I.; Muntean, C.; Grozescu, I. Romanian Viscum album L.—Untargeted low-molecular metabolomic approach to engineered viscum–AuNPs carrier assembly. Plants 2022, 11, 1820. [Google Scholar] [CrossRef]
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold nanoparticles: Construction for drug delivery and application in cancer immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef]
- Timoszyk, A.; Grochowalska, R. Mechanism and antibacterial activity of gold nanoparticles (AuNPs) functionalized with natural compounds from plants. Pharmaceutics 2022, 14, 2599. [Google Scholar] [CrossRef]
- Al-Fahham, B.; Mohamed, R.; Al-Talqani, J.; Fahad, A.; Haider, J. Evaluating antimicrobial effectiveness of gold nanoparticles against Streptococcus oralis. Int. J. Dent. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Marimuthu Prabhu, N.; Jeyakanthan, J.; Saravanan, M. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, Y.; Xu, S.; Chang, L.; Zhao, X.; Mei, X.; Gao, X. NIR-triggered tea polyphenol-modified gold nanoparticles-loaded hydrogel treats periodontitis by inhibiting bacteria and inducing bone regeneration. Mater. Des. 2023, 225, 111487. [Google Scholar] [CrossRef]
- Martínez-Cuazitl, A.; Gómez-García, M.d.C.; Pérez-Mora, S.; Rojas-López, M.; Delgado-Macuil, R.J.; Ocampo-López, J.; Vázquez-Zapién, G.J.; Mata-Miranda, M.M.; Pérez-Ishiwara, D.G. Polyphenolic compounds nanostructurated with gold nanoparticles enhance wound repair. Int. J. Mol. Sci. 2023, 24, 17138. [Google Scholar] [CrossRef]
- Chang, L.; Dong, S.; Wang, X.; Xu, H.; Liu, C.; Yang, X.; Wu, S.; Jiang, X.; Kan, M.; Xu, C. Research progress of polyphenols in nanoformulations for antibacterial application. Mater. Today Bio 2023, 21, 100729. [Google Scholar]
- Majeric, P.; Jovic, Z.; Švarc, T.; Jelen, Ž.; Horvat, A.; Koruga, D.; Rudolf, R. Physicochemical properties of gold nanoparticles for skin care creams. Materials 2023, 16, 3011. [Google Scholar] [CrossRef]
- Ahari, H.; Fakhrabadipour, M.; Paidari, S.; Goksen, G.; Xu, B. Role of AuNPs in active food packaging improvement: A review. Molecules 2022, 27, 8027. [Google Scholar] [CrossRef]
- Teschke, R.; Glass, X.; Schulze, J. Herbal hepatotoxicity by greater celandine (Chelidonium majus): Causality assessment of 22 spontaneous reports. Regul. Toxicol. Pharmacol. 2011, 61, 282–291. [Google Scholar] [CrossRef]
- Pantano, F.; Mannocchi, G.; Marinelli, E.; Gentili, S.; Graziano, S.; Busardò, F.; Luca, N. Hepatotoxicity induced by greater celandine (Chelidonium majus L.): A review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 46–52. [Google Scholar]
- Guo, Z.; Su, H.; Cai, R.; Ma, X. Determination of isoquinoline alkaloids in extracts of Chelidonium majus L. using emulsion liquid membrane extraction and GC/MS. Med. E Investig. 2016, 4, 3–9. [Google Scholar] [CrossRef]
- Fik, E.; Dalgalarrondo, M.; Haertlé, T.; Goździcka-Józefiak, A. Comparative biochemical analysis of lectin and nuclease from Chelidonium majus L. Acta Biochim. Pol. 2000, 47, 413–420. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Xu, M.; Xiao, Z.; Liu, C.; Du, B.; Xu, D.; Li, L. Signal molecules regulate the synthesis of secondary metabolites in the interaction between endophytes and medicinal plants. Processes 2023, 11, 849. [Google Scholar] [CrossRef]
- Zielińska, S.; Czerwińska, M.E.; Dryś, A.; Kucharski, M.; Płachno, B.J.; Matkowski, A. Modulatory effect of Chelidonium majus extract and its alkaloids on LPS-stimulated cytokine secretion in human neutrophils. Molecules 2020, 25, 842. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Damian, D.; Hulka, I.; Grozescu, I.; Salifoglou, A. A simple and rapid method for calixarene-based selective extraction of bioactive molecules from natural products. Amino Acids 2016, 48, 849–858. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Grozescu, I.; Sfirloaga, P. The influence of extraction process parameters of some biomaterials precursors from Helianthus annuus. Dig. J. Nanomater. Biostruct. 2013, 8, 1423–1433. [Google Scholar]
- Segneanu, A.E.; Grozescu, I.; Cziple, F.; Berki, D.; Damian, D.; Niculite, C.M.; Florea, A.; Leabu, M. Helleborus purpurascens—Amino acid and peptide analysis linked to the chemical and antiproliferative properties of the extracted compounds. Molecules 2015, 20, 22170–22218. [Google Scholar] [CrossRef]
- Harmănescu, M.A.; Moisuc, F.R.; Drăgan, S.; Gergen, I. Total polyphenols content determination in complex matrix of medicinal plants from Romania by NIR spectroscopy. Bull. UASVM Agric. 2008, 65, 123–128. [Google Scholar]
- Parvu, M.; Vlase, L.; Fodorpataki, L.; Parvu, O.; Rosca-Casian, O.; Bartha, C.; Barbu-Tudoran, L.; Parvu, A.E. Chemical composition of celandine (Chelidonium majus L.) extract and its effects on Botrytis tulipae (Lib.) Lind fungus and the tulip. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 414–426. [Google Scholar] [CrossRef]
- Omari, K.W.; Besaw, J.E.; Kerton, F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012, 14, 1480. [Google Scholar] [CrossRef]
- Hesami, S.; Safi, S.; Larijani, K.; Badi, H.N.; Abdossi, V.; Hadidi, M. Synthesis and characterization of chitosan nanoparticles loaded with greater celandine (Chelidonium majus L.) essential oil as an anticancer agent on MCF-7 cell line. Int. J. Biol. Macromol. 2022, 194, 974–981. [Google Scholar] [CrossRef]
- Pantami, H.A.; Ahamad Bustamam, M.S.; Lee, S.Y.; Ismail, I.S.; Mohd Faudzi, S.M.; Nakakuni, M.; Shaari, K. Comprehensive GC-MS and LC-MS/MS metabolite profiling of Chlorella vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Vargas Solis, R.; Gutierrez, G.D.; Martinez-Martinez, F.J. Identification of benzophenanthridine alkaloids from Bocconia arborea by gas chromatography-mass spectrometry. Phytochem. Anal. 2002, 13, 177–180. [Google Scholar] [CrossRef]
- Naglaa, R.A.; Kasem, F.A.; Mannaa, K.G.; Abdel-Wahhab, H.H.; Gomaa, H.F. Preventive efficiency of Chelidonium majus ethanolic extract against aflatoxin B1 induced neurochemical deteriorations in Rats. Pak. J. Biol. Sci. 2022, 25, 234–244. [Google Scholar]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Aryal, B.; Raut, B.K.; Bhattarai, S.; Bhandari, S.; Tandan, P.; Gyawali, K.; Sharma, K.; Ranabhat, D.; Thapa, R.; Aryal, D.; et al. Potential therapeutic applications of plant-derived alkaloids against inflammatory and neurodegenerative diseases. Evid. -Based Complement. Altern. Med. 2022, 2022, 7299778. [Google Scholar] [CrossRef]
- Gu, C.; Mao, X.; Chen, D.; Yu, B.; Yang, Q. Isoleucine plays an important role for maintaining immune function. Curr. Protein Pept. Sci. 2019, 20, 644–651. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M.; et al. Medicinal and therapeutic potential of herbs and plant metabolites/Extracts countering viral pathogens-current knowledge and future prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Pant, A.; Yang, Z. Asparagine: An Achilles heel of virus replication? ACS Infect. Dis. 2020, 6, 2301–2303. [Google Scholar] [CrossRef]
- Melano, I.; Kuo, L.; Lo, C.; Sung, W.; Tien, N.; Su, C. Effects of basic amino acids and their derivatives on SARS-CoV-2 and influenza-a virus infection. Viruses 2021, 13, 1301. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Blachier, F.; Wu, Z. Editorial: Amino acids in intestinal growth and health. Front. Nutr. 2023, 10, 1172548. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, J.; Pan, M.; Chen, S.; Zhao, D.; Zhang, Y.; Lei, R.X.; Wang, S.; Liu, A.C.; Chen, J.; et al. L-lysine confers neuroprotection by suppressing inflammatory response via microRNA-575/PTEN signaling after mouse intracerebral hemorrhage injury. Exp. Neurol. 2020, 327, 113214. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Antioxidant serine-(NSAID) hybrids with anti-inflammatory and hypolipidemic potency. Molecules 2021, 26, 4060. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Ntalouka, F.; Tsirivakou, A. Luteolin: A promising natural agent in the management of pain in chronic conditions. Front. Pain Res. 2023, 4, 1114428. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Melhim, S.B.; et al. Isorhamnetin reduces glucose level, inflammation, and oxidative stress in high-fat diet/Streptozotocin diabetic mice model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A review of its structure, synthesis, pharmacology, pharmacokinetics and toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the potential beneficial effects of carotenoids on consumer health and well-being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Das, K.; Gezici, S. Secondary plant metabolites, their separation and identification, and role in human disease prevention. Ann. Phytomed. Int. J. 2018, 7, 13–24. [Google Scholar] [CrossRef]
- He, M.; Ding, N. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Ribeiro, A.D.; Cardoso, M.N.A.; Freire, J.C.P.; Figueirêdo, E.C., Jr.; Costa, M.M.A.; Silva, P.G.; Gomes, D.Q.d.C.; de Brito, E.M.M.; Pereira, J.V. Antimicrobial activity of limonene: Integrative review. Bol. Latinoam. Y Del Caribe De Plantas Med. Aromat. 2023, 22, 581–593. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Zong, Z.; Tian, G.; Wang, J.; Fan, C.; Yang, F.; Guo, F. Recent advances in metal–organic-framework-based nanocarriers for controllable drug delivery and release. Pharmaceutics 2022, 14, 2790. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H. Chapter 4 Determination of alkaloids through infrared and Raman spectroscopy. Alkaloids Chem. Biol. 2008, 67, 217–255. [Google Scholar]
- Segneanu, A.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive molecules profile from natural compounds. In Amino Acid—New Insights and Roles in Plant and Animal; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Bensemmane, N.; Bouzidi, N.; Daghbouche, Y.; Garrigues, S.; Guardia, M.; El Hattab, M. Quantification of phenolic acids by partial least squares Fourier-transform infrared (PLS-FTIR) in extracts of medicinal plants. Phytochem. Anal. 2021, 32, 206–221. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef]
- Filopoulou, A.; Vlachou, S.; Boyatzis, S.C. Fatty acids and their metal salts: A review of their Infrared spectra in light of their presence in cultural heritage. Molecules 2021, 26, 6005. [Google Scholar] [CrossRef]
- Nirlipta, S.; Amit, S.; Surabhi, C.; Debjani, D. Characterization and antioxidant potential of a carotenoid from a newly isolated yeast. Food Sci. Biotechnol. 2015, 24, 117–124. [Google Scholar]
- Pang, M.; Jiang, S.; Cao, L.; Pan, L. Novel synthesis of steryl esters from phytosterols and amino acid. J. Agric. Food Chem. 2011, 59, 10732–10736. [Google Scholar] [CrossRef]
- Khichar, M.K.; Sharma, M.; Agrawal, R.D. Isolation and identification of phytosterols from leaves of Maytenus emarginata plant. Int. J. Educ. Mod. Manag. Appl. Sci. Soc. Sci. 2021, 3, 291–296. [Google Scholar]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Carbajal Morán, H. Raman Spectroscopy analysis of the morphology of gold nanoparticles produced by laser ablation in aqueous proteinogenic amino acid for the detection of mercury in water. J. Ecol. Eng. 2023, 24, 169–175. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray diffraction techniques for mineral characterization: A review for engineers of the fundamentals, applications, and research directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Vera-Baquero, F.L.; Morante-Zarcero, S.; Sierra, I. Evaluation of thermal degradation of tropane and opium alkaloids in gluten-free corn breadsticks samples contaminated with Stramonium seeds and baked with poppy seeds under different conditions. Foods 2022, 11, 2196. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; Macêdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2012, 113, 443–447. [Google Scholar] [CrossRef]
- Disch, L.; Drewe, J.; Fricker, G. Dissolution Testing of Herbal Medicines: Challenges and regulatory standards in Europe, the United States, Canada, and Asia. Dissolut. Technol. 2017, 5, 6–12. [Google Scholar] [CrossRef]
- Gusev, P.A.; Andrews, K.W.; Savarala, S.; Tey, P.-T.; Han, F.; Oh, L.; Pehrsson, P.R.; Dwyer, J.T.; Betz, J.M.; Kuszak, A.J.; et al. Disintegration and dissolution testing of green tea dietary supplements: Application and evaluation of United States Pharmacopeial standards. J. Pharm. Sci. 2020, 109, 1933–1942. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Milanezi, F.G.; Meireles, L.M.; de Christo Scherer, M.M.; de Oliveira, J.P.; da Silva, A.R.; de Araujo, M.L.; Endringer, D.C.; Fronza, M.; Guimarães, M.C.C.; Scherer, R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm. J. 2019, 27, 968–974. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Paczkowska, M.; Chanaj-Kaczmarek, J.; Romaniuk-Drapała, A.; Rubiś, B.; Szymanowska, D.; Kobus-Cisowska, J.; Szymańska, E.; Winnicka, K.; Cielecka-Piontek, J. Mucoadhesive chitosan delivery system with Chelidonii Herba lyophilized extract as a promising strategy for vaginitis treatment. J. Clin. Med. 2020, 9, 1208. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Vlase, G.; Lukinich-Gruia, A.T.; Herea, D.D.; Grozescu, I. Untargeted Metabolomic Approach of Curcuma longa to Neurodegenerative Phytocarrier System Based on Silver Nanoparticles. Antioxidants 2022, 11, 2261. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Manjusha, S.K.; Singh, N.; Vashishta, B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008, 58, 215–220. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Vlase, G.; Vlase, T.; Sicoe, C.A.; Ciocalteu, M.V.; Herea, D.D.; Ghirlea, O.F.; Grozescu, I.; Nanescu, V. Wild-grown Romanian Helleborus purpurascens approach to novel chitosan phyto-nanocarriers—Metabolite profile and antioxidant properties. Plants 2023, 12, 3479. [Google Scholar] [CrossRef]
- Surat, P. pH in the Human Body. News-Medical, 10 October 2022. Available online: https://www.news-medical.net/health/pH-in-the-Human-Body.aspx (accessed on 16 November 2023).
- Anumolu, P.D.; Gurrala, S.; Venkata Satya, S.C.; Polisetty, S.V.; Ravindran, A.; Achanta, R. Development of a discriminative and biorelevant dissolution test method for atorvastatin/fenofibrate combination with appliance of derivative spectrophotometry. Turk. J. Pharm. Sci. 2019, 16, 62–68. [Google Scholar] [CrossRef]
- Mureşan, M.; Olteanu, D.; Filip, G.A.; Clichici, S.; Baldea, I.; Jurca, T.; Pallag, A.; Marian, E.; Frum, A.; Gligor, F.G.; et al. Comparative study of the pharmacological properties and biological effects of Polygonum aviculare L. herba extract-entrapped liposomes versus quercetin-wntrapped liposomes on doxorubicin-induced toxicity on HUVECs. Pharmaceutics 2021, 13, 1418. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).