Safe Farming: Ultrafine Bubble Water Reduces Insect Infestation and Improves Melon Yield and Quality
Abstract
1. Introduction
2. Results
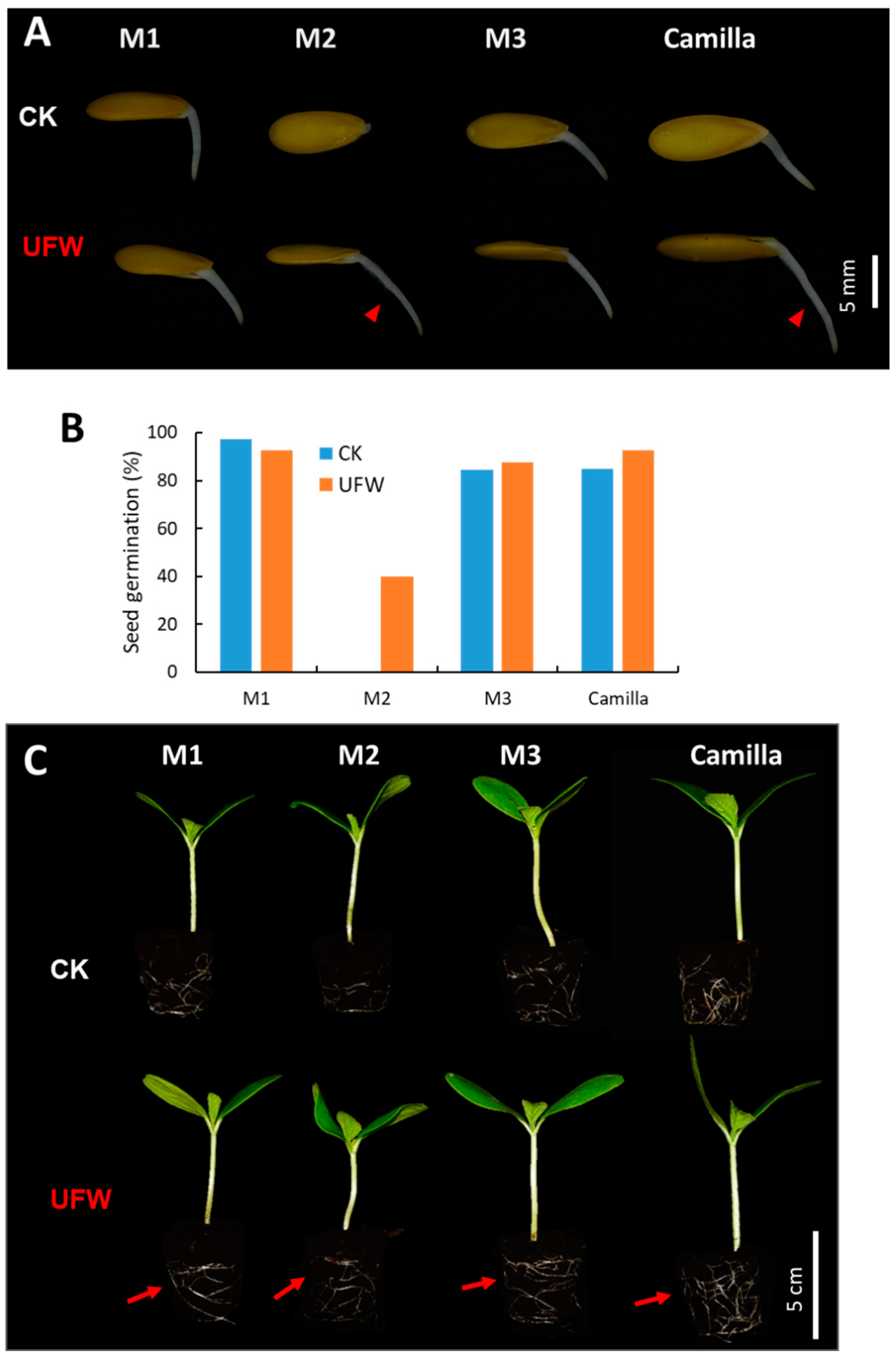
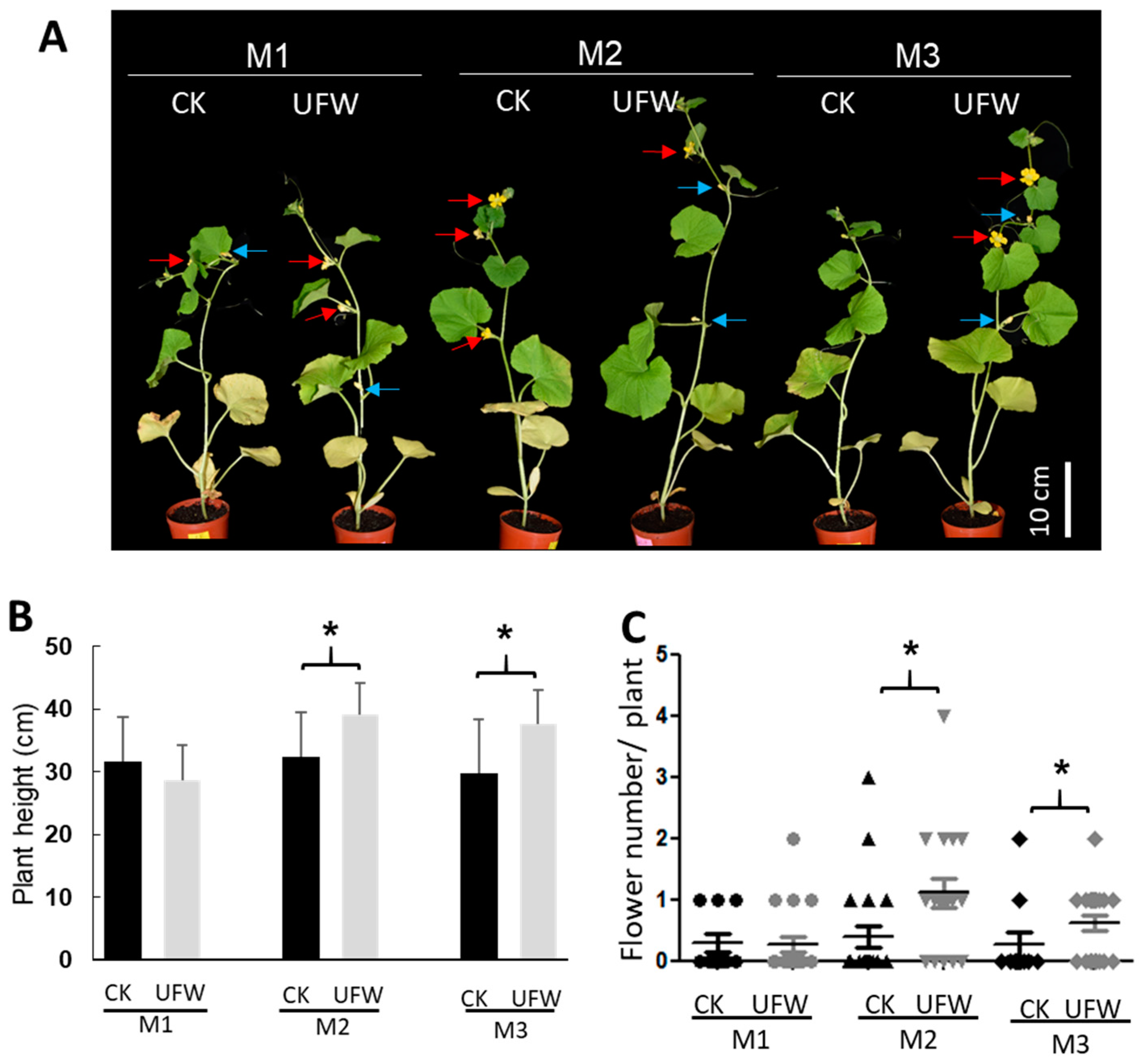
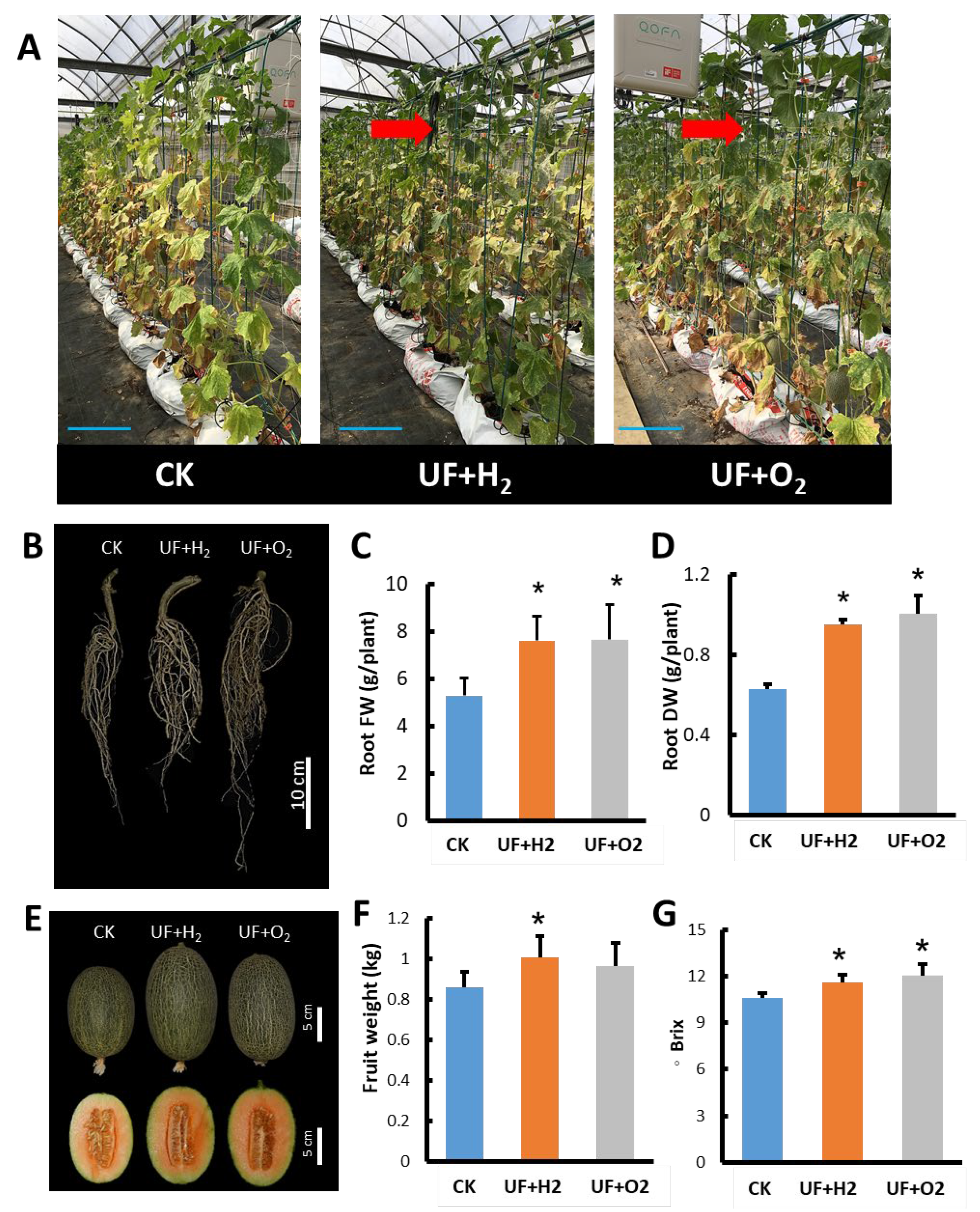
2.1. UFW Treatment Improved the Growth of Melon Seedlings
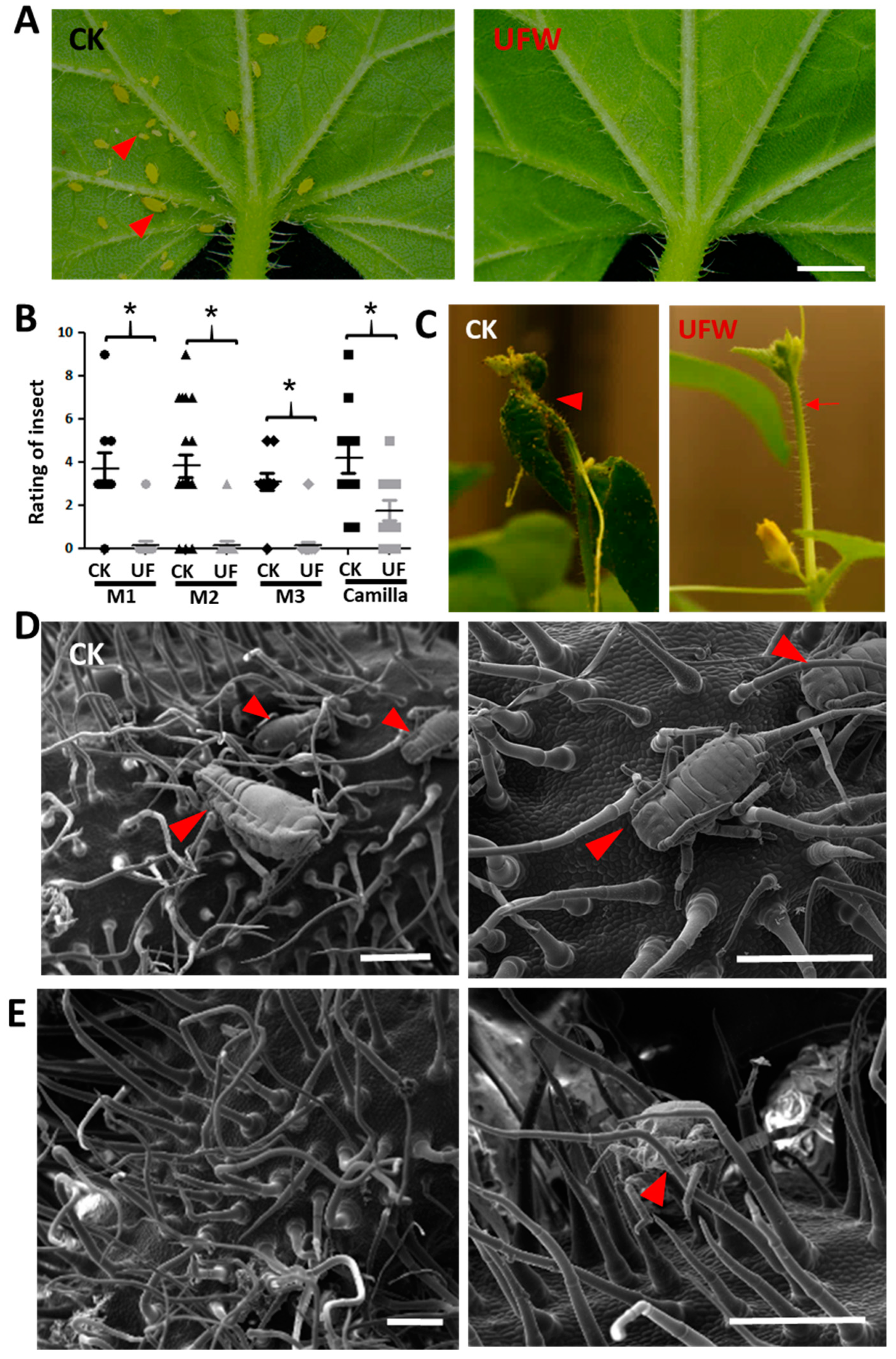
2.2. UFW Reduced Aphid Infestation of Seedlings
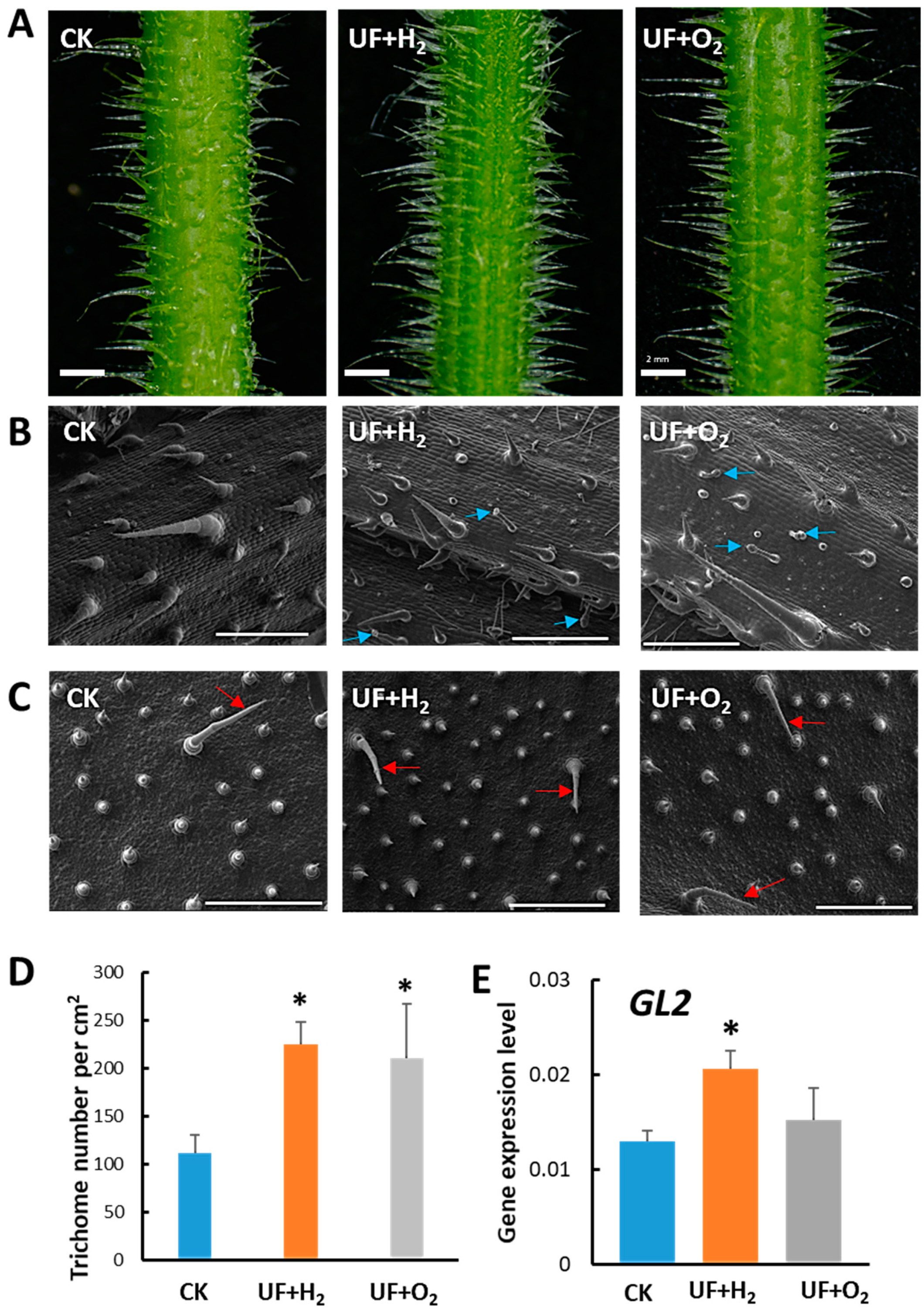
2.3. Effect of Hydrogen-Rich (UF+H2) or Oxygen-Rich (UF+O2) Ultrafine Water on Trichome Development
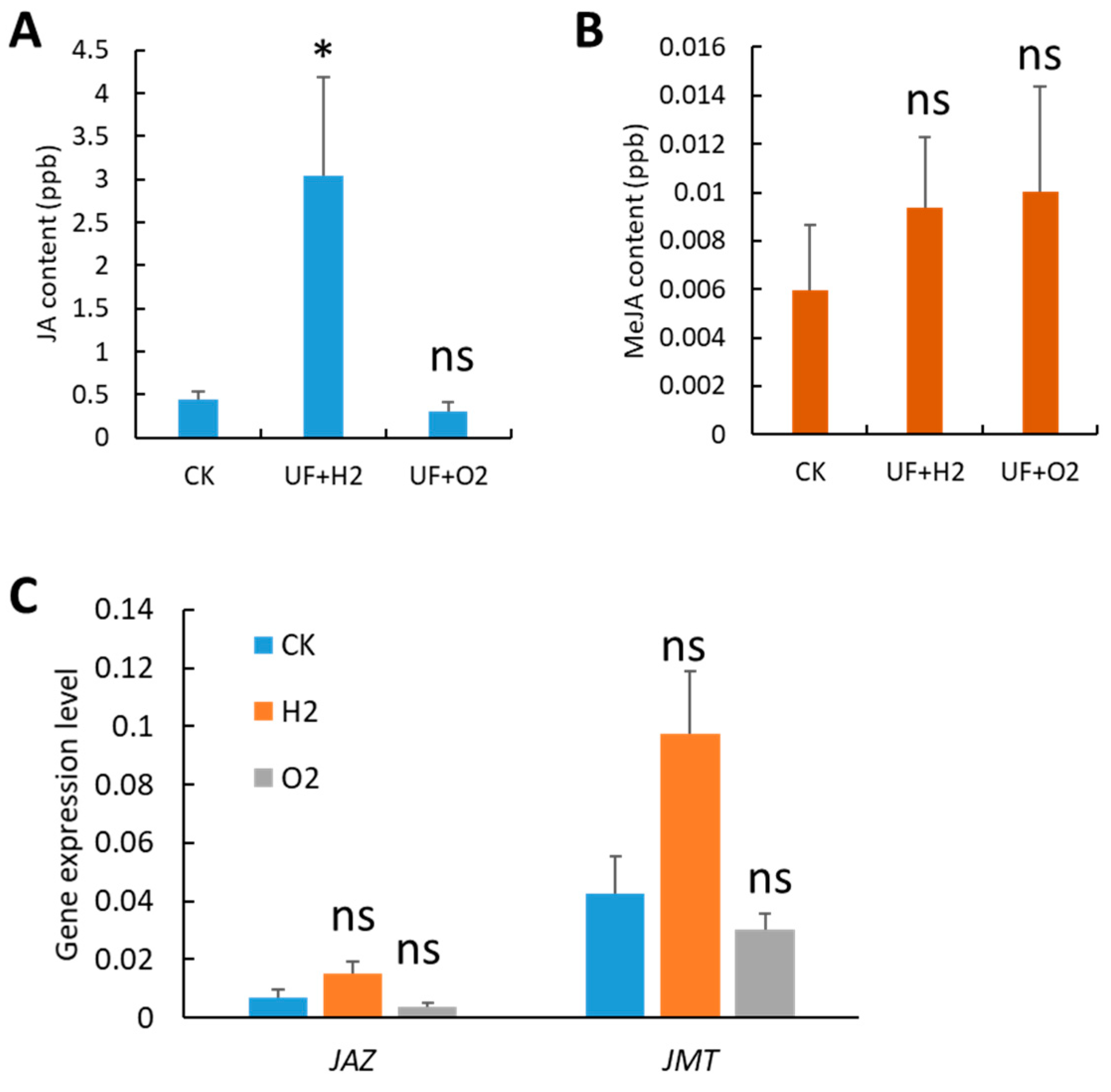
2.4. Enrichment of Hydrogen-Induced Jasmonic Acid Accumulation
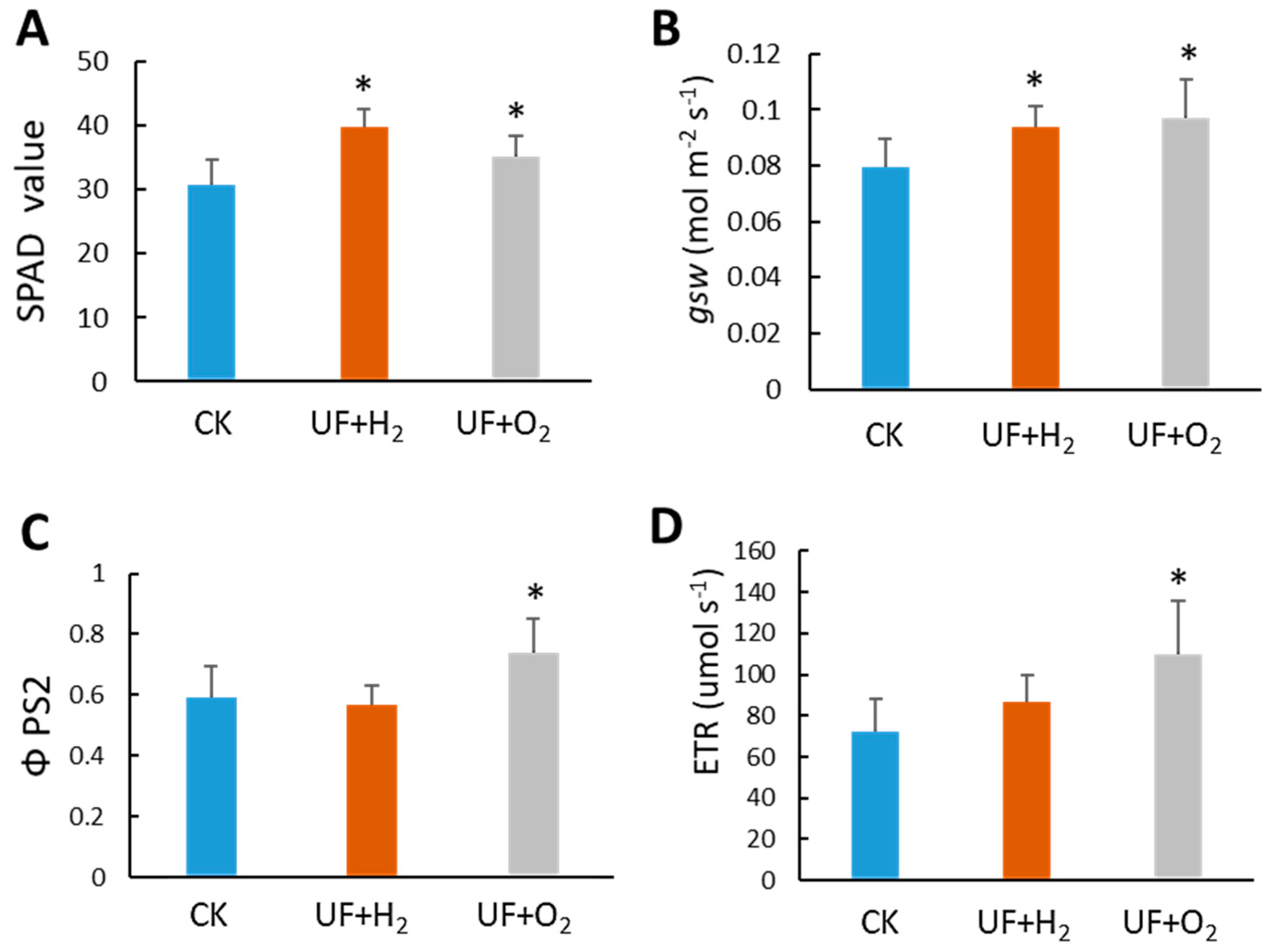
2.5. Effect of UF Water on Photosynthesis Parameters, Fruit Yield and Quality
3. Discussion
4. Materials and Methods
4.1. Ultrafine Water Preparation
4.2. Plant Materials and Growth Conditions
4.3. Insect Materials
4.4. Observation of Trichome Density
4.5. Cryo-Scanning Electron Microscopy
4.6. Total RNA Isolation and Real-Time PCR
4.7. Detected JA and Methyl-JA Content
4.8. SPAD Value and Photosynthesis Rate of Melon
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Li, L.N.; Zeng, Y.; Cheng, X.; Shen, W.B. The applications of molecular hydrogen in horticulture. Horticulturae 2021, 7, 513. [Google Scholar] [CrossRef]
- Takahata, J.; Takaki, K.; Satta, N.; Takahashi, K.; Fujio, T.; Sasaki, Y. Improvement of growth rate of plants by bubble discharge in water. Jpn. J. Appl. Phys. 2014, 54, 01AG07. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, S.; Cho, A.R.; Shim, M.S.; Chung, Y.K.; Kim, Y.J. Germination and seedling growth response of sprouts and leafy vegetables after applying oxygen nanobubble water. J. People Plants Environ. 2021, 24, 607–617. [Google Scholar] [CrossRef]
- Oshita, S.; Boerzhijin, S.; Kameya, H.; Yoshimura, M.; Sotome, I. Promotion effects of ultrafine bubbles/ nanobubbles on seed germination. Nanomaterials 2023, 13, 1677. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and its effect on seed germination. ACS Sustain. Chem. Eng. 2015, 4, 1347–1353. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W. A positive role for hydrogen gas in adventitious root development. Plant Signal Behav. 2016, 11, e1187359. [Google Scholar] [CrossRef]
- Xue, S.; Marhaba, T.; Zhang, W. Nanobubble Watering Affects Nutrient Release and Soil Characteristics. ACS Agric. Sci. Technol. 2022, 2, 453–461. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Sun, J.; Dai, H.; Zhang, B.; Xiang, W.; Hu, Z.; Li, P.; Yang, J.; Zhang, W. Nanobubbles promote nutrient utilization and plant growth in rice by upregulating nutrient uptake genes and stimulating growth hormone production. Sci. Total Environ. 2021, 800, 149627. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kurata, K. Application of microbubbles to hydroponics solution promotes lettuce growth. Horttechnology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Xue, S.; Gao, J.; Liu, C.; Marhaba, T.; Zhang, W. Unveiling the potential of nanobubbles in water: Impacts on tomato’s early growth and soil properties. Sci. Total Environ. 2023, 903, 166499. [Google Scholar] [CrossRef]
- Marcelino, K.R.; Ling, L.; Wongkiew, S.; Nhan, H.T.; Surendra, K.; Shitanaka, T.; Lu, H.; Khanal, S.K. Nanobubble technology applications in environmental and agricultural systems: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1378–1403. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Qi, F.; Cui, W.; Xie, Y.; Shen, W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol. 2014, 171, 1–8. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.Z.; Wang, T.Z.; Chen, W.J.; Liu, B.; Zhou, Y.P.; Li, Y.K. Effects of nanobubble in subsurface drip irrigation on the yield, quality, irrigation water use efficiency and nitrogen partial productivity of watermelon and muskmelon. Int. Agrophysics 2022, 36, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Jiang, K.; Kuang, Y.; Zeng, Y.; Cheng, X.; Liu, Y.; Wang, S.; Shen, W. Preharvest application of hydrogen nanobubble water enhances strawberry flavor and consumer preferences. Food Chem. 2022, 377, 131953. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Q.; Zhang, T.; Cheng, P.; Xu, S.; Shen, W. Hydrogen nanobubble water delays petal senescence and prolongs the vase life of cut carnation (Dianthus caryophyllus L.) flowers. Plants 2021, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.J.; Jin, X.; Liao, W.B.; Wang, M.; Niu, L.J.; Li, X.P.; Xu, X.T.; Zhu, Y.C. Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic. Environ. Biotechnol. 2017, 58, 576–584. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Wang, Y.; Gu, R. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014, 156, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.W.; Liu, Z.Y.; Chen, G.M.; Li, L.N.; Zeng, Y.; Cheng, X.; Pathier, D.; Xu, G.Y.; Shen, W.B. Molecular hydrogen-based irrigation extends strawberry shelf life by improving the synthesis of cell wall components in fruit. Postharvest Biol. Technol. 2023, 206, 112551. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Shen, W. H2 enhances arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Zulfiqar, F.; Hancock, J.T. Hydrogenases and the role of molecular hydrogen in plants. Plants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Iijima, M.; Yamashita, K.; Hirooka, Y.; Ueda, Y.; Yamane, K.; Kamimura, C. Ultrafine bubbles alleviated osmotic stress in soybean seedlings. Plant Prod. Sci. 2022, 25, 218–223. [Google Scholar] [CrossRef]
- Webb, S.E. Insect Management for Cucurbits (Cucumber, Squash, Cantaloupe, and Watermelon): ENY-460/IN168, Rev. 9/2005; EDIS: Graz, Austria, 2005. [Google Scholar]
- Luis Alonso-Prados, J.; Luis-Arteaga, M.; Alvarez, J.M.; Moriones, E.; Batlle, A.; Laviña, A.; García-Arenal, F.; Fraile, A. Epidemics of aphid-transmitted viruses in melon crops in Spain. Eur. J. Plant Pathol. 2003, 109, 129–138. [Google Scholar] [CrossRef]
- Van Cauwenbergh, N.; Biala, K.; Bielders, C.; Brouckaert, V.; Franchois, L.; Cidad, V.G.; Hermy, M.; Mathijs, E.; Muys, B.; Reijnders, J. SAFE—A hierarchical framework for assessing the sustainability of agricultural systems. Agric. Ecosyst. Environ. 2007, 120, 229–242. [Google Scholar] [CrossRef]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf trichome formation and plant resistance to herbivory. In Induced Plant Resistance to Herbivory; Springer: Berlin/Heidelberg, Germany, 2008; pp. 89–105. [Google Scholar]
- Handley, R.; Ekbom, B.; Ågren, J. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecol. Entomol. 2005, 30, 284–292. [Google Scholar] [CrossRef]
- Lin, S.Y.; Trumble, J.T.; Kumamoto, J. Activity of volatile compounds in glandular trichomes of Lycopersicon species against two insect herbivores. J. Chem. Ecol. 1987, 13, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Chang, J.; Wu, M.; Xu, Z.; Zhang, F.; Yang, M.; Xu, H.; Wang, L.J.; Chen, X.Y.; Wu, S. Mediation of JA signalling in glandular trichomes by the woolly/SlMYC1 regulatory module improves pest resistance in tomato. Plant Biotechnol. J. 2021, 19, 375–393. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 2005, 31, 2211–2216. [Google Scholar] [CrossRef]
- Pattanaik, S.; Patra, B.; Singh, S.K.; Yuan, L. An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Front. Plant Sci. 2014, 5, 259. [Google Scholar] [CrossRef]
- Ohashi, Y.; Oka, A.; Rodrigues-Pousada, R.; Possenti, M.; Ruberti, I.; Morelli, G.; Aoyama, T. Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 2003, 300, 1427–1430. [Google Scholar] [CrossRef]
- Oppenheimer, D.G.; Herman, P.L.; Sivakumaran, S.; Esch, J.; Marks, M.D. A Myb Gene Required for Leaf Trichome Differentiation in Arabidopsis Is Expressed in Stipules. Cell 1991, 67, 483–493. [Google Scholar] [CrossRef]
- Yoshida, Y.; Sano, R.; Wada, T.; Takabayashi, J.; Okada, K. Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 2009, 136, 1039–1048. [Google Scholar] [CrossRef]
- Zhao, M.; Morohashi, K.; Hatlestad, G.; Grotewold, E.; Lloyd, A. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development 2008, 135, 1991–1999. [Google Scholar] [CrossRef]
- Rerie, W.G.; Feldmann, K.A.; Marks, M.D. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev. 1994, 8, 1388–1399. [Google Scholar] [CrossRef]
- Morohashi, K.; Zhao, M.; Yang, M.; Read, B.; Lloyd, A.; Lamb, R.; Grotewold, E. Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiol. 2007, 145, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, C.; Lee, M.M.; Gonzalez, A.; Zhang, F.; Lloyd, A.; Schiefelbein, J. The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 2003, 130, 6431–6439. [Google Scholar] [CrossRef]
- Schellmann, S.; Schnittger, A.; Kirik, V.; Wada, T.; Okada, K.; Beermann, A.; Thumfahrt, J.; Jurgens, G.; Hulskamp, M. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J. 2002, 21, 5036–5046. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cui, L.; Liu, X.; Cui, Y.; Wang, Z.; Zhang, H.; Chen, L.; Cui, H. NbJAZ3 is required for jasmonate-meditated glandular trichome development in Nicotiana benthamiana. Physiol. Plant 2022, 174, e13666. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Shang, L.; Li, Y.; Zhang, Q.; Chu, Z.; He, S.; Yang, W.; Ding, X. Ectopic Expression of OsJAZs Alters Plant Defense and Development. Int. J. Mol. Sci. 2022, 23, 4581. [Google Scholar] [CrossRef] [PubMed]
- English, N.J. Environmental Exploration of Ultra-Dense Nanobubbles: Rethinking Sustainability. Environments 2022, 9, 33. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.N.; Huang, X.; Ling, X.P.; Yu, M.; Cui, J.; Shabala, S. Hydrogen-rich water promotes elongation of hypocotyls and roots in plants through mediating the level of endogenous gibberellin and auxin. Funct. Plant Biol. 2020, 47, 771–778. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W.; Wang, M.; Niu, L.; Xu, Q.; Jin, X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016, 195, 50–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Liu, X.; Wang, K.; Muhammad, T. Synergistic improvement in spring maize yield and quality with micro/nanobubbles water oxygation. Sci. Rep. 2019, 9, 5226. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Bastida, F.; Liu, Y.Z.; Zhou, Y.P.; He, J.; Song, P.; Kuang, N.K.; Li, Y.K. Nanobubble oxygenated increases crop production via soil structure improvement: The perspective of microbially mediated effects. Agric. Water Manag. 2023, 282, 108263. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Lyu, T.; Pan, G.; Li, P. Aquatic macrophytes in morphological and physiological responses to the nanobubble technology application for water restoration. ACS EST Water 2020, 1, 376–387. [Google Scholar] [CrossRef]
- Wang, F.; Park, Y.L.; Gutensohn, M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (Solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; LeBaron, T.W.; May, J.; Thomas, A.; Russell, G. Molecular Hydrogen: Is This a Viable New Treatment for Plants in the UK? Plants 2021, 10, 2270. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, J.-C.; Li, N.-J.; Peng, C.-Y.; Yang, C.-C.; Ko, S.-S. Safe Farming: Ultrafine Bubble Water Reduces Insect Infestation and Improves Melon Yield and Quality. Plants 2024, 13, 537. https://doi.org/10.3390/plants13040537
Hung J-C, Li N-J, Peng C-Y, Yang C-C, Ko S-S. Safe Farming: Ultrafine Bubble Water Reduces Insect Infestation and Improves Melon Yield and Quality. Plants. 2024; 13(4):537. https://doi.org/10.3390/plants13040537
Chicago/Turabian StyleHung, Jo-Chi, Ning-Juan Li, Ching-Yen Peng, Ching-Chieh Yang, and Swee-Suak Ko. 2024. "Safe Farming: Ultrafine Bubble Water Reduces Insect Infestation and Improves Melon Yield and Quality" Plants 13, no. 4: 537. https://doi.org/10.3390/plants13040537
APA StyleHung, J.-C., Li, N.-J., Peng, C.-Y., Yang, C.-C., & Ko, S.-S. (2024). Safe Farming: Ultrafine Bubble Water Reduces Insect Infestation and Improves Melon Yield and Quality. Plants, 13(4), 537. https://doi.org/10.3390/plants13040537






