Appropriately Reduced Nitrogen and Increased Phosphorus in Ratooning Rice Increased the Yield and Reduced the Greenhouse Gas Emissions in Southeast China
Abstract
1. Introduction
2. Results
2.1. Effects of Different Nitrogen and Phosphorus Fertilization Treatments in Ratooning Rice on the Yield
2.2. Effects of Different Nitrogen and Phosphorus Fertilization Treatments in Ratooning Rice on Greenhouse Gas Emissions
2.3. Effects of Different Nitrogen and Phosphorus Fertilization Treatments in Ratooning Rice on the Carbon Footprint
2.4. Effects of Appropriately Reduced Nitrogen and Increased Phosphorus Treatments in Ratooning Rice on Rhizosphere Soil Microbial Communities
2.5. Effects of Appropriately Reduced Nitrogen and Increased Phosphorus Treatments in Ratooning Rice on Rhizosphere Soil Microbial Community Assembly
2.6. Effects of Appropriately Reduced Nitrogen and Increased Phosphorus in Ratooning Rice on Dominant Rhizosphere Soil Microbial Genera
2.7. Prediction of Rhizosphere Soil Microbial Community Functional Genes
3. Discussion
3.1. Effects of Different Nitrogen and Phosphorus Fertilization Treatments in Ratooning Rice on Yield and Greenhouse Gas Emissions
3.2. Effects of Different Nitrogen and Phosphorus Fertilization Treatments in Ratooning Rice on Rhizosphere Microbial Communities
4. Materials and Methods
4.1. Experimental Site Overview
4.2. Experimental Design
4.3. Measurement Items and Methods
4.3.1. Field Growth Meteorological Data Measurement
4.3.2. Yield Measurement of the Ratooning Crop
4.3.3. Collection and Measurement of Greenhouse Gases
4.3.4. Calculation of Global Warming Potential and Greenhouse Gas Emission Intensity
4.3.5. Carbon Footprint Study Boundary and Estimation Method
4.3.6. Rhizosphere Soil Sample Collection
4.3.7. Extraction and PCR Amplification of Total DNA from Rhizosphere Soil Microorganisms
4.4. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Gu, W.; Liu, B.; Zhang, C.; Wang, C.; Yang, Y.; Zhuang, M. Closing Greenhouse Gas Emission Gaps of Staple Crops in China. Environ. Sci. Technol. 2022, 56, 9302–9311. [Google Scholar] [CrossRef]
- Yan, X.; Akimoto, H.; Ohara, T. Estimation of nitrous oxide, nitric oxide and ammonia emissions from croplands in East, Southeast and South Asia: NITROGENOUS GAS EMISSIONS FROM CROPLANDS. Glob. Chang. Biol. 2003, 9, 1080–1096. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Zhang, Y.; Liu, C.; Wang, R.; Lin, S.; Zuo, Q.; Butterbach-Bahl, K. Urea deep placement reduces yield-scaled greenhouse gas (CH4 and N2O) and NO emissions from a ground cover rice production system. Sci. Rep. 2017, 7, 11415. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, L.; Zhang, J. Ratoon rice production in central China: Environmental sustainability and food production. Sci. Total Environ. 2021, 764, 142850. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, P.; Xiao, J.; Jiang, Y.; Cai, M.; Wang, J.; Li, C.; Zhan, M.; Cao, C. Dry cultivation with ratoon system impacts rice quality using rice flour physicochemical traits, fatty and amino acids contents. Food Res. Int. 2021, 150, 110764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, Q.; Li, L.; Xu, H.; Zheng, H.; Wang, J.; Hua, Y.; Ren, L.; Tang, J. Ratoon rice with direct seeding improves soil carbon sequestration in rice fields and increases grain quality. J. Environ. Manag. 2022, 317, 115374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Shi, X.; Wang, T.; Chen, Y.; Yang, R.; Mi, J.; Zhang, Y.; Zhang, Y. Identification of QTNs, QTN-by-environment interactions, and their candidate genes for grain size traits in main crop and ratoon rice. Front. Plant Sci. 2023, 14, 1119218. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhang, G.; Yu, H.; Huang, Q.; Zhu, X.; Wang, T.; Xu, H.; Lv, S.; Ma, J. Evaluation of methane and nitrous oxide emissions in a three-year case study on single rice and ratoon rice paddy fields. J. Clean. Prod. 2021, 297, 126650. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, L.; Wang, B.; Xiang, J.; Gao, M.; Fu, Z.; Long, P.; Luo, H.; Huang, C. Conversion from double-season rice to ratoon rice paddy fields reduces carbon footprint and enhances net ecosystem economic benefit. Sci. Total Environ. 2022, 813, 152550. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, X.; He, X. A global meta-analysis of crop yield and agricultural greenhouse gas emissions under nitrogen fertilizer application. Sci. Total Environ. 2022, 831, 154982. [Google Scholar] [CrossRef]
- Tie, L.; Hu, J.; Peñuelas, J.; Sardans, J.; Wei, S.; Liu, X.; Zhou, S.; Huang, C. The amounts and ratio of nitrogen and phosphorus addition drive the rate of litter decomposition in a subtropical forest. Sci. Total Environ. 2022, 833, 155163. [Google Scholar] [CrossRef]
- Kim, S.Y.; Veraart, A.J.; Meima-Franke, M.; Bodelier, P.L.E. Combined effects of carbon, nitrogen and phosphorus on CH4 production and denitrification in wetland sediments. Geoderma 2015, 259–260, 354–361. [Google Scholar] [CrossRef]
- Lu, Y.; Wassmann, R.; Neue, H.U.; Huang, C. Impact of phosphorus supply on root exudation, aerenchyma formation and methane emission of rice plants. Biogeochemistry 1999, 47, 203–218. [Google Scholar] [CrossRef]
- He, Z.; Xu, C.; Zhou, B.B.; Xue, L.H.; Wang, Y.; Shen, M.X.; Yang, L.Z. Effects of long-term fertilization without phosphorus on greenhouse gas emissions from paddy fields. Chin. J. Appl. Ecol. 2021, 32, 942–950. [Google Scholar] [CrossRef]
- Datta, A.; Santra, S.C.; Adhya, T.K. Effect of inorganic fertilizers (N, P, K) on methane emission from tropical rice field of India. Atmos. Environ. 2013, 66, 123–130. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.; Ridoutt, B.G.; Wang, X.; Ren, P. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Thomas, A.S.S.; Pongprayoon, W.; Cheenkachorn, K.; Sriariyanun, M. Plant-microbe interactions—Insights and views for applications in sustainable agriculture. Appl. Sci. Eng. Prog. 2022, 15, 5286. [Google Scholar] [CrossRef]
- Wang, L.; Huang, D. Soil microbial community composition in a paddy field with different fertilization managements. Can. J. Microbiol. 2021, 67, 864–874. [Google Scholar] [CrossRef]
- Liu, D.; Ishikawa, H.; Nishida, M.; Tsuchiya, K.; Takahashi, T.; Kimura, M.; Asakawa, S. Effect of Paddy-Upland Rotation on Methanogenic Archaeal Community Structure in Paddy Field Soil. Microb. Ecol. 2015, 69, 160–168. [Google Scholar] [CrossRef]
- Kharitonov, S.; Semenov, M.; Sabrekov, A.; Kotsyurbenko, O.; Zhelezova, A.; Schegolkova, N. Microbial Communities in Methane Cycle: Modern Molecular Methods Gain Insights into Their Global Ecology. Environments 2021, 8, 16. [Google Scholar] [CrossRef]
- MR, A.; TK, N.; JB, M.; NM, S.; LH, H. Muskoxen homogenise soil microbial communities and affect the abundance of methanogens and methanotrophs. Sci. Total Environ. 2022, 827, 153877. [Google Scholar] [CrossRef]
- Ma, K.; Conrad, R.; Lu, Y. Dry/Wet Cycles Change the Activity and Population Dynamics of Methanotrophs in Rice Field Soil. Appl. Environ. Microb. 2013, 79, 4932–4939. [Google Scholar] [CrossRef]
- Schrier-Uijl, A.P.; Veraart, A.J.; Leffelaar, P.A.; Berendse, F.; Veenendaal, E.M. Release of CO2 and CH4 from lakes and drainage ditches in temperate wetlands. Biogeochemistry 2011, 102, 265–279. [Google Scholar] [CrossRef]
- Yu, X.; Tao, X.; Liao, J.; Liu, S.; Xu, L.; Yuan, S.; Zhang, Z.; Wang, F.; Deng, N.; Huang, J.; et al. Predicting potential cultivation region and paddy area for ratoon rice production in China using Maxent model. Field Crop Res. 2022, 275, 108372. [Google Scholar] [CrossRef]
- Ren, M.; Wang, J. A few dominant bacteria and their genomic basis in mediating distinct ecosystem functions. Environ. Microbiol. 2021, 23, 4478–4488. [Google Scholar] [CrossRef] [PubMed]
- Coll, C.; Bier, R.; Li, Z.; Langenheder, S.; Gorokhova, E.; Sobek, A. Association between Aquatic Micropollutant Dissipation and River Sediment Bacterial Communities. Environ. Sci. Technol. 2020, 54, 14380–14392. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yin, Y.; Zhu, W.; Zhou, Y. Variations in Soil Bacterial Community Diversity and Structures Among Different Revegetation Types in the Baishilazi Nature Reserve. Front. Microbiol. 2018, 9, 2874. [Google Scholar] [CrossRef] [PubMed]
- Zi, H.; Jiang, Y.; Cheng, X.; Li, W.; Huang, X. Change of rhizospheric bacterial community of the ancient wild tea along elevational gradients in Ailao mountain, China. Sci. Rep. 2020, 10, 9203. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Y.L.; Zhou, Z.A.; Wu, F.; Wang, H.F.; Zong, X. Response of sediment microbial communities to different levels of PAC contamination and exposure time. Sci. Total Environ. 2023, 861, 160683. [Google Scholar] [CrossRef]
- Guo, S.; Toth, C.R.A.; Luo, F.; Chen, X.; Xiao, J.; Edwards, E.A. Transient Oxygen Exposure Causes Profound and Lasting Changes to a Benzene-Degrading Methanogenic Community. Environ. Sci. Technol. 2022, 56, 13036–13045. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Musa, N.; Manzoor, M. Mineralization of soluble P fertilizers and insoluble rock phosphate in response to phosphate-solubilizing bacteria and poultry manure and their effect on the growth and P utilization efficiency of chilli (Capsicum annuum L.). Biogeosciences 2015, 12, 4607–4619. [Google Scholar] [CrossRef]
- Dai, X.; Ouyang, Z.; Li, Y.; Wang, H. Variation in yield gap induced by nitrogen, phosphorus and potassium fertilizer in North China Plain. PLoS ONE 2013, 8, e82147. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, E.; Ahouanton, K.; Mwakasege, L.; Mujuni, S.; Mujawamariya, G.; Kamanda, J.; Senthilkumar, K.; Saito, K. Phosphorus micro-dosing as an entry point to sustainable intensification of rice systems in sub-Saharan Africa. Field Crop Res. 2018, 222, 39–49. [Google Scholar] [CrossRef]
- Wei, S.; Tie, L.; Liao, J.; Liu, X.; Du, M.; Lan, S.; Li, X.; Li, C.; Zhan, H.; Huang, C. Nitrogen and phosphorus co-addition stimulates soil respiration in a subtropical evergreen broad-leaved forest. Plant Soil 2020, 450, 171–182. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, C.; Han, S.; Li, X.; Kan, Z.; Zhao, X.; Zhang, H. Strategic tillage achieves lower carbon footprints with higher carbon accumulation and grain yield in a wheat-maize cropping system. Sci. Total Environ. 2021, 798, 149220. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Lei, X.; Wang, B.; Cui, J.; Xu, Y.; Chen, Y.; Sui, P. Reducing carbon footprint without compromising grain security through relaxing cropping rotation system in the North China Plain. J. Clean. Prod. 2021, 318, 128465. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Lu, X.; Wang, M.; Chu, Q.; Wen, X.; Chen, F. Lowering carbon footprint of winter wheat by improving management practices in North China Plain. J. Clean. Prod. 2016, 112, 149–157. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Dong, H.; Fan, S.; Sun, H.; Chen, C.; Wang, A.; Jiang, L.; Ma, D. Rhizosphere-Associated Microbiomes of Rice (Oryza sativa L.) Under the Effect of Increased Nitrogen Fertilization. Front. Microbiol. 2021, 12, 730506. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Larmola, T.; Leppänen, S.M.; Tuittila, E.; Aarva, M.; Merilä, P.; Fritze, H.; Tiirola, M. Methanotrophy induces nitrogen fixation during peatland development. Proc. Natl. Acad. Sci. USA 2014, 111, 734–739. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.Y.; Su, S.L.; Li, C.S. Quantifying methane emissions from rice paddies in Northeast China by integrating remote sensing mapping with a biogeochemical model. Biogeosciences 2011, 8, 1225–1235. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Claus, P. Phosphate inhibits acetotrophic methanogenesis on rice roots. Appl. Environ. Microbiol. 2000, 66, 828–831. [Google Scholar] [CrossRef]
- Robertson, G.P.; Paul, E.A.; Harwood, R.R. Greenhouse Gases in Intensive Agriculture: Contributions of Individual Gases to the Radiative Forcing of the Atmosphere. Science 2000, 289, 1922–1925. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; Del Giorgio, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–491. [Google Scholar] [CrossRef]
- Liu, X.J.; Jin, X.B.; Luo, X.L.; Zhou, Y.K. Multi-scale variations and impact factors of carbon emission intensity in China. Sci. Total Environ. 2023, 857, 159403. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhong, Y.; Yang, J.; Wu, Y.; Li, H.; Zheng, L. Effect of nitrogen fertilizer rates on carbon footprint and ecosystem service of carbon sequestration in rice production. Sci. Total Environ. 2019, 670, 210–217. [Google Scholar] [CrossRef]

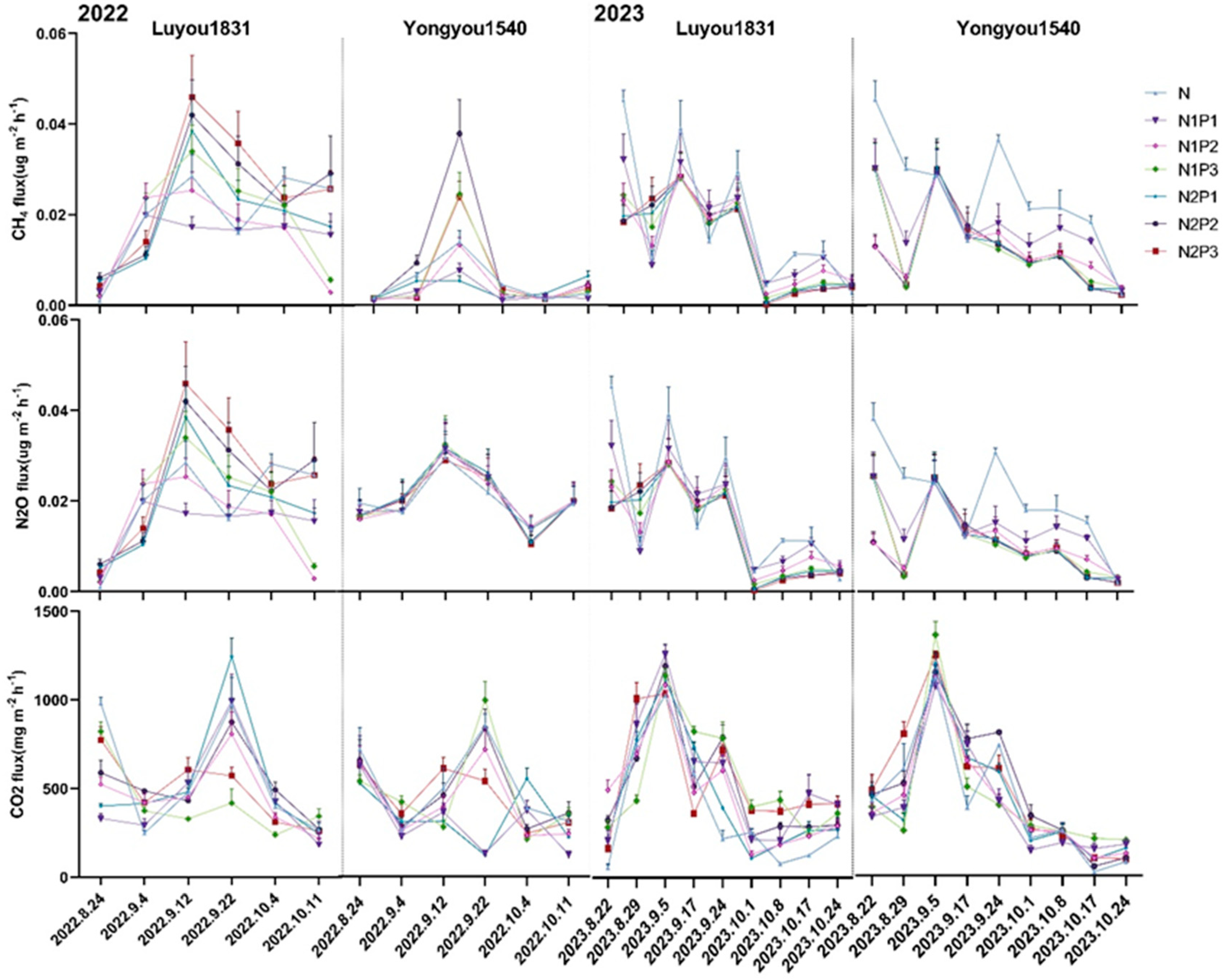
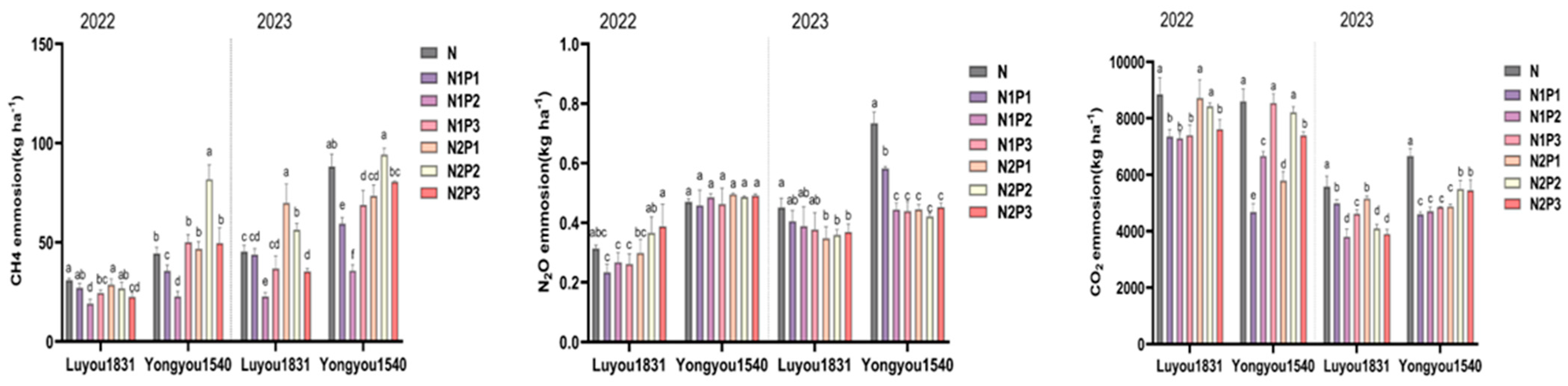


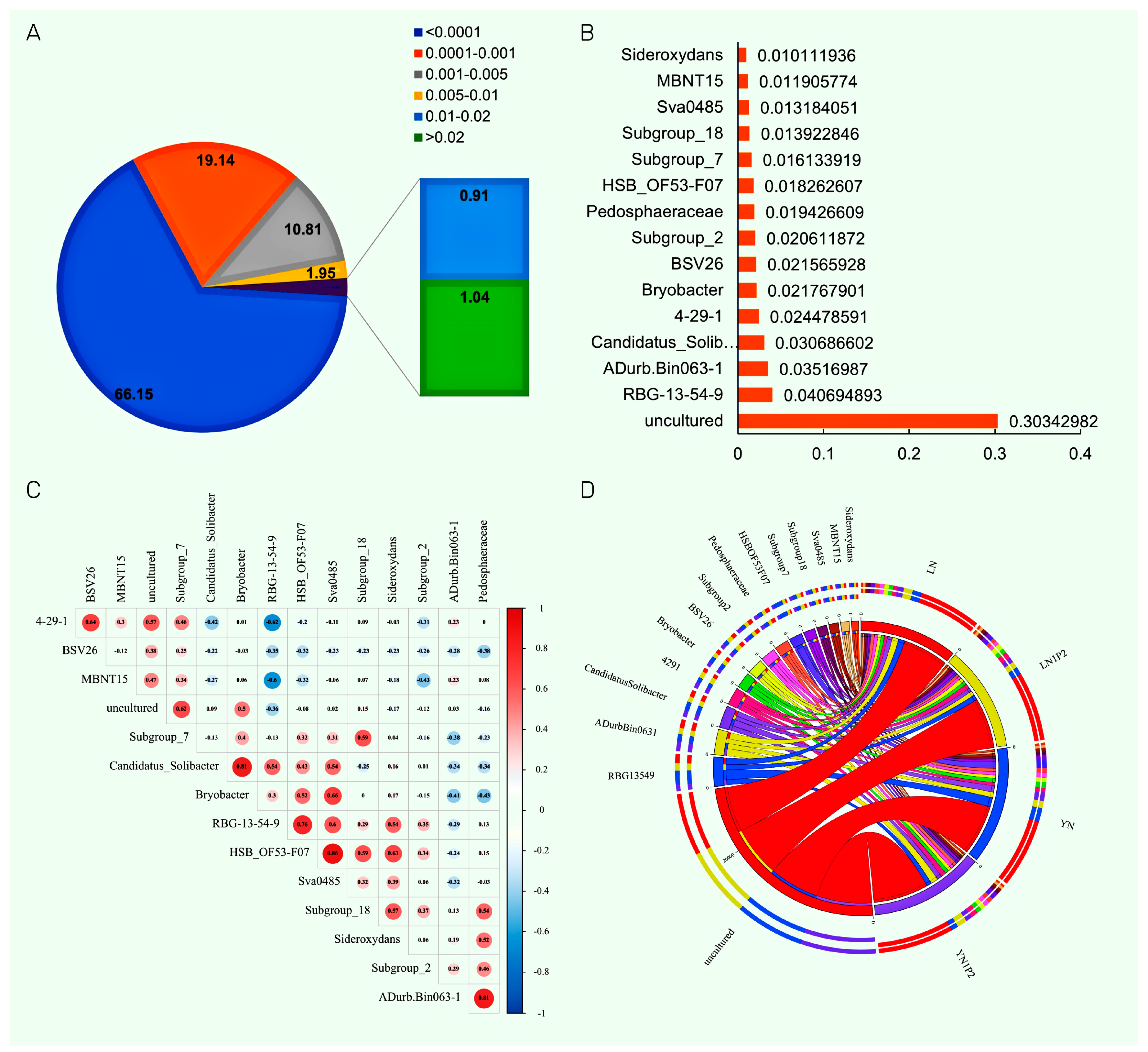
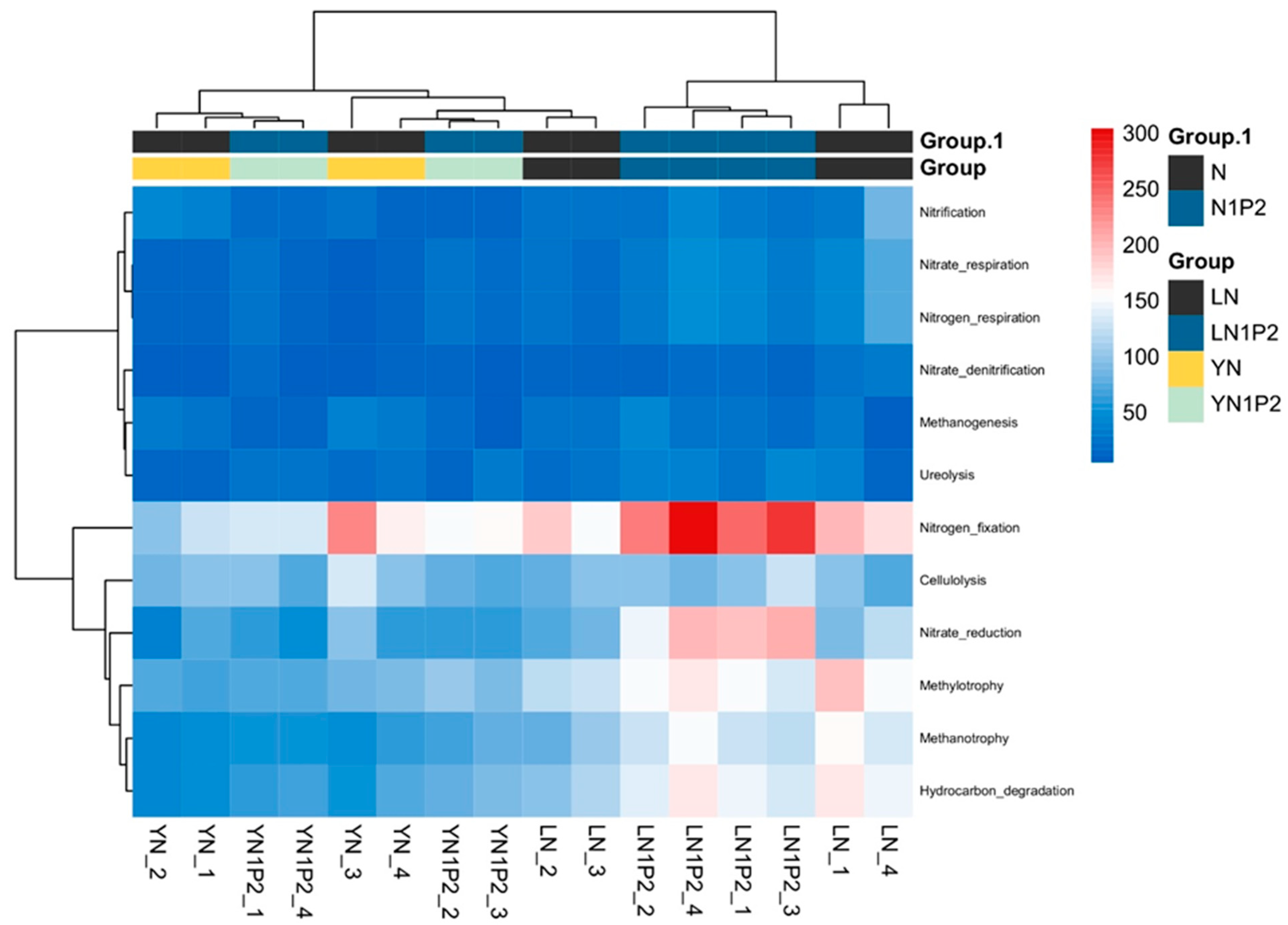

| Year | Varieties | Treatments | Indirect Emissions (kg CO2-eq·ha−1) | GWP (kg CO2-eq·ha−1) | GHGI (kg CO2-eq·ha−1) | CFA (kg CO2-eq·ha−1) | CFy (kg CO2-eq·ha−1) | CE (t·kg−1 CO2-eq) |
|---|---|---|---|---|---|---|---|---|
| 2022 | Luyou 1831 | N | 608.07 | 867.55 a | 166.37 a | 1475.63 a | 0.28 a | 3.57 d |
| N1P1 | 601.39 | 748.95 bc | 95.32 c | 1350.34 b | 0.17 c | 5.82 b | ||
| N1P2 | 623.40 | 558.38 d | 64.96 d | 1181.78 c | 0.14 c | 7.27 a | ||
| N1P3 | 644.59 | 689.87 c | 123.22 b | 1334.45 b | 0.24 b | 4.21 c | ||
| N2P1 | 572.70 | 808.93 ab | 173.52 a | 1381.63 ab | 0.3 a | 3.4 d | ||
| N2P2 | 594.71 | 778.77 abc | 120.02 b | 1373.48 ab | 0.21 b | 4.73 c | ||
| N2P3 | 615.90 | 678.9 c | 89.54 cd | 1294.8 b | 0.17 c | 5.86 b | ||
| Yongyou 1540 | N | 608.07 | 1253.38 b | 169.62 b | 1861.45 b | 0.25 b | 3.97 c | |
| N1P1 | 601.39 | 1029.41 c | 124.76 c | 1630.8 c | 0.2 c | 5.08 b | ||
| N1P2 | 623.40 | 713.24 d | 77.76 d | 1336.64 d | 0.15 d | 6.86 a | ||
| N1P3 | 644.59 | 1391.78 b | 182.5 b | 2036.37 b | 0.27 b | 3.8 c | ||
| N2P1 | 572.70 | 1317.74 b | 178.09 b | 1890.44 b | 0.26 b | 3.92 c | ||
| N2P2 | 594.71 | 2190.03 a | 290.97 a | 2784.74 a | 0.37 a | 2.73 d | ||
| N2P3 | 615.90 | 1388.6 b | 178.44 b | 2004.5 b | 0.26 b | 3.91 c | ||
| 2023 | Luyou 1831 | N | 608.07 | 1271.1 c | 282.59 b | 1879.17 c | 0.42 ab | 2.4 e |
| N1P1 | 601.39 | 1218.09 cd | 168.55 cd | 1819.49 cd | 0.25 cd | 4.1 c | ||
| N1P2 | 623.40 | 683.15 e | 80.97 e | 1306.55 e | 0.15 e | 6.48 a | ||
| N1P3 | 644.59 | 1035.17 d | 219.31 c | 1679.75 cd | 0.36 b | 2.89 de | ||
| N2P1 | 572.70 | 1853.6 a | 346.24 a | 2426.3 a | 0.45 a | 2.22 e | ||
| N2P2 | 594.71 | 1517.33 b | 199.47 c | 2112.04 b | 0.28 c | 3.61 cd | ||
| N2P3 | 615.90 | 995.34 d | 126.39 de | 1611.24 d | 0.2 de | 4.91 b | ||
| Yongyou 1540 | N | 608.07 | 2425.24 a | 371.38 a | 3033.32 a | 0.46 a | 2.16 e | |
| N1P1 | 601.39 | 1661.31 d | 193.03 d | 2262.7 d | 0.26 e | 3.81 b | ||
| N1P2 | 623.40 | 1026.26 e | 111.76 e | 1649.66 e | 0.18 f | 5.59 a | ||
| N1P3 | 644.59 | 1854.07 cd | 221.29 cd | 2498.66 c | 0.3 de | 3.39 bc | ||
| N2P1 | 572.70 | 1969.23 bc | 273.11 b | 2541.93 c | 0.35 c | 2.87 cd | ||
| N2P2 | 594.71 | 2482.32 a | 328.68 a | 3077.03 a | 0.41 b | 2.46 de | ||
| N2P3 | 615.90 | 2149.13 b | 263.4 bc | 2765.03 b | 0.34 cd | 2.96 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yao, F.; Sun, Y.; Yang, Z.; Li, R.; Bai, G.; Lin, W.; Chen, H. Appropriately Reduced Nitrogen and Increased Phosphorus in Ratooning Rice Increased the Yield and Reduced the Greenhouse Gas Emissions in Southeast China. Plants 2024, 13, 438. https://doi.org/10.3390/plants13030438
Yang Y, Yao F, Sun Y, Yang Z, Li R, Bai G, Lin W, Chen H. Appropriately Reduced Nitrogen and Increased Phosphorus in Ratooning Rice Increased the Yield and Reduced the Greenhouse Gas Emissions in Southeast China. Plants. 2024; 13(3):438. https://doi.org/10.3390/plants13030438
Chicago/Turabian StyleYang, Yuncheng, Feifei Yao, Yangbo Sun, Zhipeng Yang, Rong Li, Ge Bai, Wenxiong Lin, and Hongfei Chen. 2024. "Appropriately Reduced Nitrogen and Increased Phosphorus in Ratooning Rice Increased the Yield and Reduced the Greenhouse Gas Emissions in Southeast China" Plants 13, no. 3: 438. https://doi.org/10.3390/plants13030438
APA StyleYang, Y., Yao, F., Sun, Y., Yang, Z., Li, R., Bai, G., Lin, W., & Chen, H. (2024). Appropriately Reduced Nitrogen and Increased Phosphorus in Ratooning Rice Increased the Yield and Reduced the Greenhouse Gas Emissions in Southeast China. Plants, 13(3), 438. https://doi.org/10.3390/plants13030438





