Expanding the Distribution of Prosthechea jauana (Orchidaceae) in the Pantepui and Highlighting the Urgent Need for Conservation Strategies in the Region in Face of Climate Change
Abstract
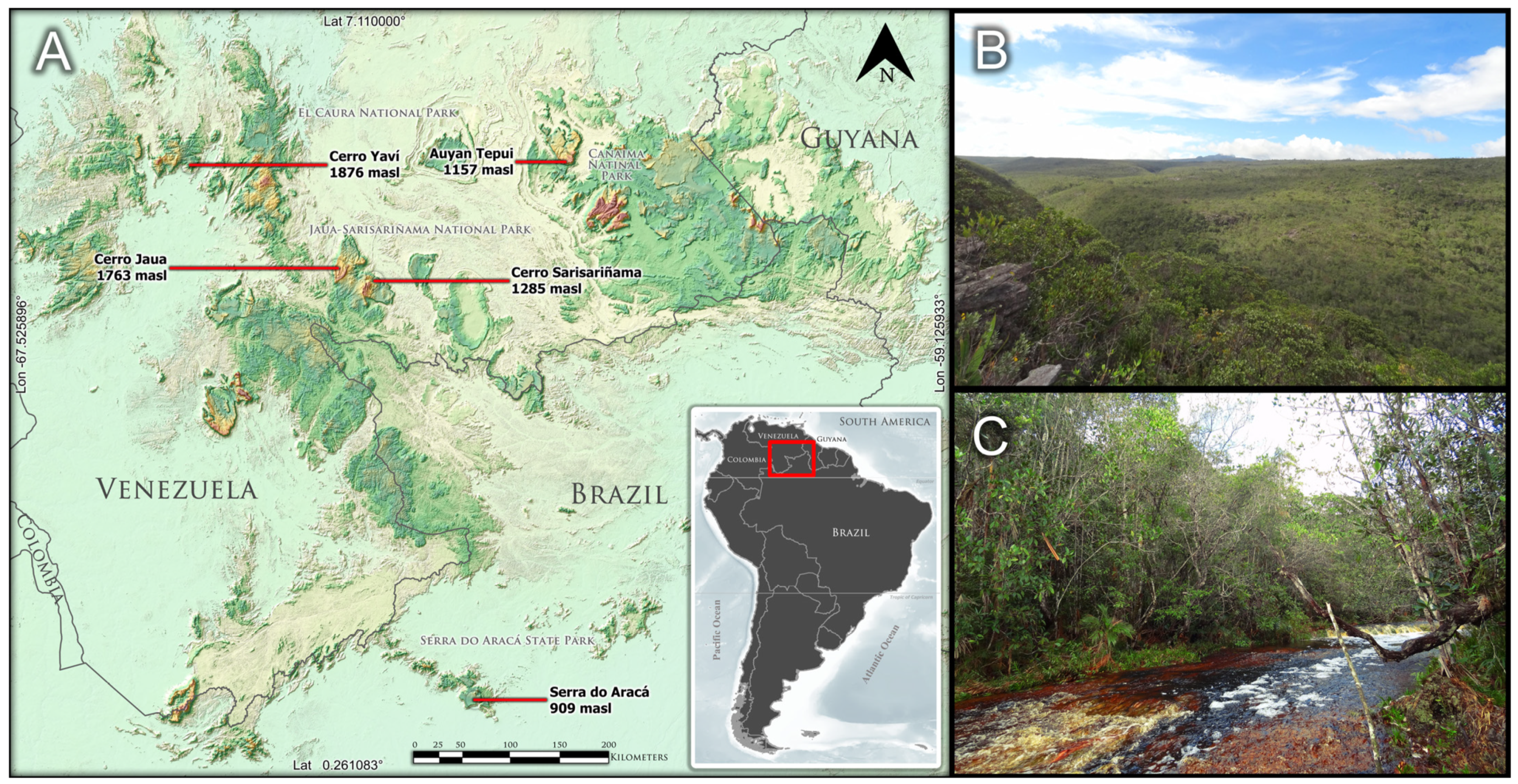
1. Introduction
2. Results
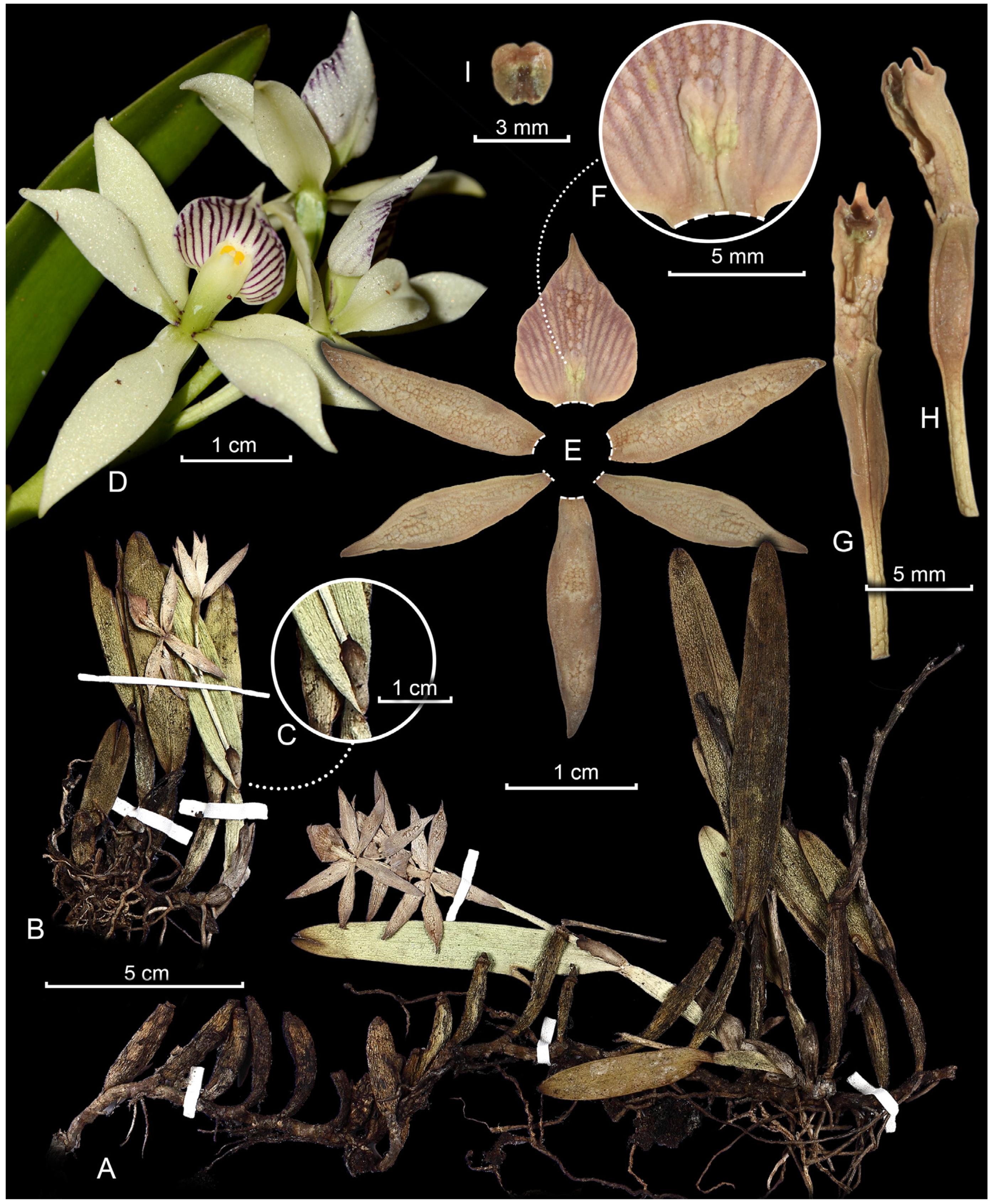
2.1. Taxonomy
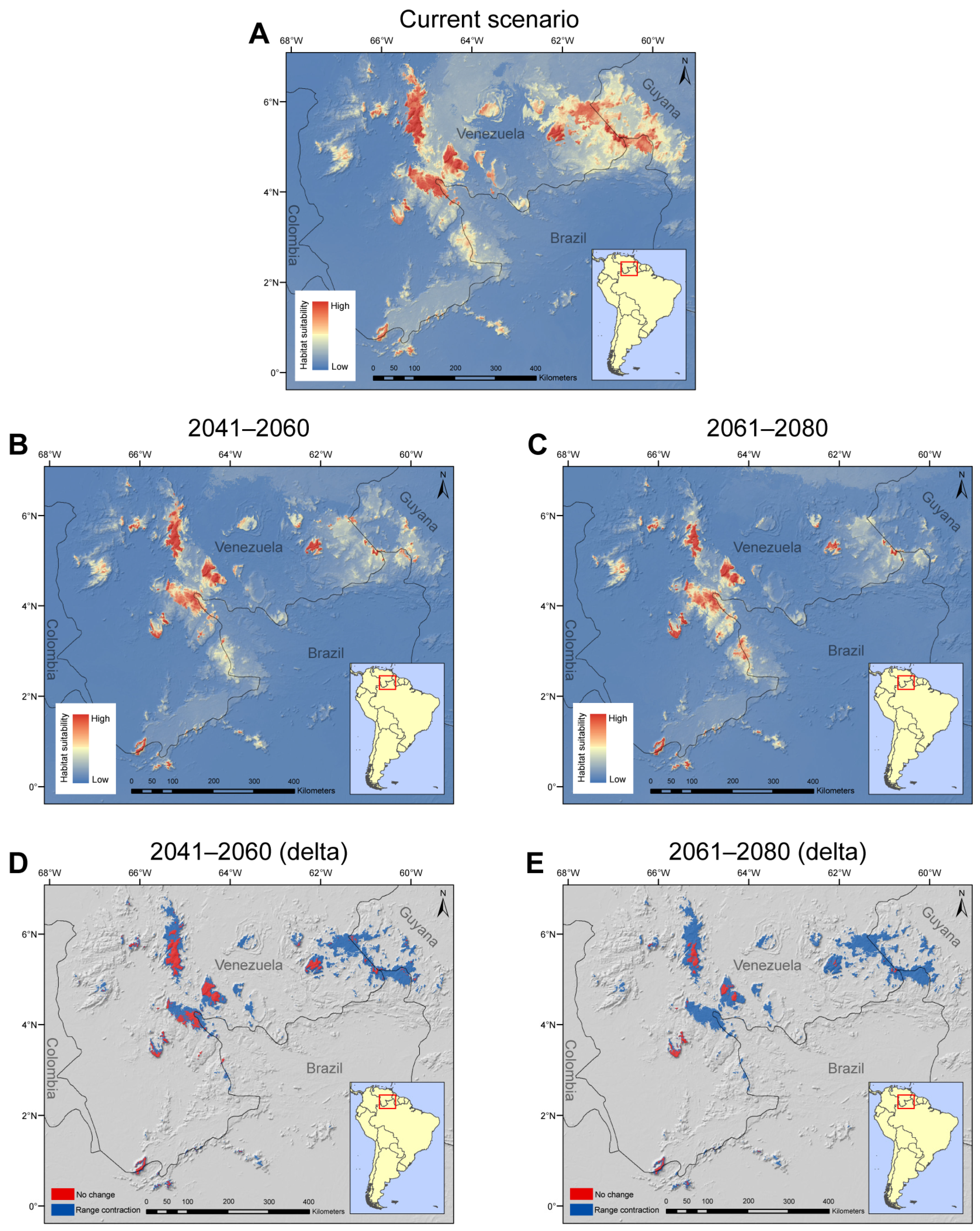
2.2. Species Distribution Modeling
3. Discussion
3.1. A Species Widely Distributed throughout a Sky Island Archipelago
3.2. The Relevance of Taxon-Focused Studies
3.3. Implications of Climate Change over a Pantepui Endemic Orchid
4. Materials and Methods
4.1. Study of Herbarium Materials
4.2. Species Distribution Modeling
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Bioclimatic Variable | Rate of Contribution (%) |
|---|---|
| Mean annual temperature (Bio 1) | 75.9 |
| Temperature seasonality (Bio 4) | 3.7 |
| Annual range of temperature (Bio 7) | 2.2 |
| Annual precipitation (Bio 12) | 0.4 |
| Precipitation of coldest quarter (Bio 19) | 17.8 |
References
- Carnevali, G.; Ramírez, I.M.; Romero, G.A. Orchidaceae Dunstervillorum VIII: New Encyclia species and combinations from Venezuela. Lindleyana 1994, 9, 59–70. [Google Scholar]
- Rull, V.; Huber, O.; Vegas-Vilarrúbia, T.; Señaris, C. (Eds.) Definition and characterization of the Pantepui biogeographical province. In Biodiversity of Pantepui; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–32. [Google Scholar] [CrossRef]
- Riina, R.; Berry, P.E.; Huber, O.; Michelangeli, F.A. Vascular plants and bryophytes. In Biodiversity of Pantepui; Rull, V., Vegas-Vilarrúbia, T., Huber, O., Señaris, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–147. [Google Scholar] [CrossRef]
- Dressler, R.L. El complejo Encyclia fragrans en los países andinos. Orquideología 1971, 6, 195–203. [Google Scholar]
- Carnevali, G.; Ramírez-Morillo, I. Prosthechea. In Flora of the Venezuelan Guayana; Steyermark, J., Berry, P.E., Yatskievych, K., Holst, B.K., Eds.; Missouri Botanical Garden Press: St. Louis, MI, USA, 2003; Volume 7—Myrtaceae-Plumbaginaceae, pp. 536–540. [Google Scholar]
- Vieira, T.L.; van den Berg, C. Prosthechea. In Flora e Funga do Brasil; Jardim Botânico do Rio de Janeiro, Brazil, 2023. Available online: https://floradobrasil.jbrj.gov.br/FB12106 (accessed on 4 July 2023).
- Barbosa-Silva, R.G.; Bueno, M.L.; Labiak, P.H.; Coelho, M.A.N.; Martinelli, G.; Forzza, R.C. The Pantepui in the Brazilian Amazon: Vascular flora of Serra Do Aracá, a cradle of diversity, richness and endemism. Bot. Rev. 2020, 86, 359–375. [Google Scholar] [CrossRef]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef]
- Kress, W.J.; Krupnick, G.A. Lords of the biosphere: Plant winners and losers in the Anthropocene. Plants People Planet 2022, 4, 350–366. [Google Scholar] [CrossRef]
- Steffen, W.; Grinevald, J.; Crutzen, P.; McNeill, J. The Anthropocene: Conceptual and historical perspectives. Philos. Trans. R. Soc. A 2011, 369, 842–867. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.C. The herbarium of the future. Trends Ecol. Evol. 2022, 38, 412–423. [Google Scholar] [CrossRef]
- Lughadha, E.N.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Morueta-Holme, N.; Engemann, K.; Sandoval-Acuña, P.; Jonas, J.D.; Segnitz, R.M.; Svenning, J.C. Strong upslope shifts in Chimborazo’s vegetation over two centuries since Humboldt. Proc. Natl. Acad. Sci. USA 2015, 112, 12741–12745. [Google Scholar] [CrossRef]
- Nogué, S.; Rull, V.; Vegas-Vilarrúbia, T. Modeling biodiversity loss by global warming on Pantepui, northern South America: Projected upward migration and potential habitat loss. Clim. Chang. 2009, 94, 77–85. [Google Scholar] [CrossRef]
- Rull, V.; Nogué, S.; Safont, E.; Vegas-Vilarrúbia, T. Pantepui and global warming. In Biodiversity of Pantepui; Rull, V., Vegas-Vilarrúbia, T., Huber, O., Señaris, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–417. [Google Scholar] [CrossRef]
- Safont, E.; Vegas-Vilarrúbia, T.; Rull, V. Use of Environmental Impact Assessment (EIA) tools to set priorities and optimize strategies in biodiversity conservation. Biol. Conserv. 2012, 149, 113–121. [Google Scholar] [CrossRef]
- Safont, E.; Rull, V.; Vegas-Vilarrúbia, T.; Holst, B.K.; Huber, O.; Nozawa, S.; Vivas, Y.; Silva, A. Establishing a baseline of plant diversity and endemism on a neotropical mountain summit for future comparative studies assessing upward migration: An approach from biogeography and nature conservation. Syst. Biodivers. 2014, 12, 292–314. [Google Scholar] [CrossRef]
- Vegas-Vilarrúbia, T.; Nogué, S.; Rull, V. Global warming, habitat shifts and potential refugia for biodiversity conservation in the neotropical Guayana Highlands. Biol. Conserv. 2012, 152, 159–168. [Google Scholar] [CrossRef]
- Lozada, J.R.; Hernández, L.; Carrero, Y.A. Amenazas en el Parque Nacional Canaima y áreas protegidas por la minería indígena illegal en Venezuela. Rev. Geogr. Venez 2020, 61, 380–395. Available online: http://www.saber.ula.ve/handle/123456789/47146 (accessed on 2 November 2023).
- Pinto-Hidalgo, J.J.; Silva-Centeno, J.A. Geospatial Intelligence and Artificial Intelligence for Detecting Potential Coca Paste Production Infrastructure in the Border Region of Venezuela and Colombia. J. Appl. Secur. Res. 2023, 18, 1000–1050. [Google Scholar] [CrossRef]
- Ackerman, J.D. Orchid Flora of the Greater Antilles; The New York Botanical Garden Press: New York, NY, USA, 2014; 625p. [Google Scholar]
- Steyermark, J.A. Flora del Ayuan-Tepui. Acta Bot. Venez. 1960, 2, 166–199. Available online: https://www.jstor.org/stable/41740412#metadata_info_tab_contents (accessed on 2 November 2023).
- Dunsterville, G.C.K.; Dunsterville, E. Jaua, Sarisarinama and holes in the ground. Orchid Rev. 1974, 82, 205–213. [Google Scholar]
- Berry, P.E.; Holst, B.K.; Yatskievych, K. Introduction. In Flora of the Venezuelan Guayana; Steyermark, J., Berry, P.E., Yatskievych, K., Holst, B.K., Eds.; Missouri Botanical Garden Press: St. Louis, MI, USA, 1995; Volume 1, pp. 1–320. [Google Scholar]
- Araújo, A.M.; Farroñay, F.; Perdiz, R.O.; Pessoa, E.; Giacomin, L. The discovery of Scaphyglottis punctulata (Laeliinae) in the highlands of Brazilian Amazonia with a key to the species of the region. Lankesteriana 2022, 22, 123–131. [Google Scholar] [CrossRef]
- Barbosa-Silva, R.G.; Labiak, P.H.; Gil, A.S.B.; Goldenberg, R.; Michelangeli, F.A.; Martinelli, G.; Coelho, M.A.N.; Zappi, D.C.; Forzza, R.C. Over the hills and far away: New plant records for the Guayana Shield in Brazil. Brittonia 2016, 68, 397–408. [Google Scholar] [CrossRef]
- Hágsater, E.; Wrazidlo, M. Epidendrum katarun-yariku (Orchidaceae), a new species of the Schistochilum group from the tepuis of the Guiana Highlands in South America. Phytotaxa 2020, 472, 33–40. [Google Scholar] [CrossRef]
- Liu, B.; Su, J.; Chen, J.; Cui, G.; Ma, J. Anthropogenic Halo Disturbances Alter Landscape and Plant Richness: A Ripple Effect. PLoS ONE 2013, 8, e56109. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, M.A.; Beaudrot, L.; Meléndez-Ackerman, E.J.; Tremblay, R.L. Local extinction risk under climate change in a neotropical asymmetrically dispersed epiphyte. J. Ecol. 2020, 108, 1553–1564. [Google Scholar] [CrossRef]
- Larrea, M.L.; Werner, F.A. Response of vascular epiphyte diversity to different land-use intensities in a neotropical montane wet forest. For. Ecol. Manag. 2010, 260, 1950–1955. [Google Scholar] [CrossRef]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkis, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 3–24. [Google Scholar] [CrossRef]
- Seaton, P.T.; Hu, H.; Perner, H.; Pritchard, H.W. Ex Situ Conservation of Orchids in a Warming World. Bot. Rev. 2010, 76, 193–203. [Google Scholar] [CrossRef]
- Campos, P.V.; Schaefer, C.E.G.R.; Pontara, V.; Senra, E.O.; Viana, P.L.; Oliveira, F.S.; Candido, H.G.; Villa, P.M. Exploring the relationship between soil and plant evolutionary diversity in the Roraima table mountain OCBIL, Guayana Highlands. Biol. J. Linn. Soc. 2021, 133, 587–603. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 1012, 9, 671–675. [Google Scholar] [CrossRef]
- Radford, A.E.; Dickison, W.C.; Massey, J.R.; Bell, C.R. Vascular Plant Systematics; Harper and Row: New York, NY, USA, 1974; 891p. [Google Scholar]
- Dressler, R.L. Phylogeny and Classification of the Orchid Family; Timber Press: Portland, OR, USA, 1993; 330p. [Google Scholar]
- Beentje, H. The Kew Plant Glossary, an Illustrated Dictionary of Plant Terms, 2nd ed.; Kew Publishing: Kew, UK, 2016; 164p. [Google Scholar]
- ESRI. ArcGIS Desktop (Release 10.5), Environmental Systems Research Institute: Redlands, CA, USA, 2011.
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN: Gland, Switzerland, 2012; 32p. [Google Scholar]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria: Version 15.1; Prepared by the Standards and Petitions Committee; 2022; 114p, Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 2 November 2023).
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scot, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Gotelli, N.J.; Ellison, A.M. MaxEnt versus MaxLike: Empirical comparisons with ant species distributions. Ecosphere 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Lopes, A.; Demarchi, L.O.; Piedade, M.T.F.; Schöngart, J.; Wittmann, F.; Munhoz, C.B.R.; Ferreira, C.S.; Franco, A.C. Predicting the range expansion of invasive alien grasses under climate change in the Neotropics. Perspect. Ecol. Conserv. 2023, 21, 128–135. [Google Scholar] [CrossRef]
- Colli-Silva, M.; Pirani, J.R.; Zizka, A. Ecological niche models and point distribution data reveal a differential coverage of the cacao relatives (Malvaceae) in South American protected areas. Ecol. Inform. 2022, 69, 101668. [Google Scholar] [CrossRef]
- Bueno, M.L.; Pennington, R.T.; Dexter, K.G.; Kamino, L.H.Y.; Pontara, V.; Neves, D.M.; Ratter, J.A.; Oliveira-Filho, A.T. Effects of Quaternary climatic fluctuations on the distribution of Neotropical savanna tree species. Ecography 2016, 40, 403–414. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Serna-Chavez, H.M.; Villalobos-Arambula, A.R.; Pérez de la Rosa, J.A.; Raes, N. Similar but not equivalent: Ecological niche comparison across closely–related Mexican white pines. Divers. Distrib. 2015, 21, 245–257. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). 2023. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 19 October 2023).
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Zhu, Z.; Tao, Y.; Xiang, J. Predicting the potential suitable distribution area of Emeia pseudosauteri in Zhejiang Province based on the MaxEnt model. Sci. Rep. 2023, 13, 1806. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Luoto, M.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog. Phys. Geogr. 2006, 30, 751–777. [Google Scholar] [CrossRef]
- Shiogama, H.; Abe, M.; Tatebe, H. MIROC MIROC6 Model Output Prepared for CMIP6 ScenarioMIP ssp585; Earth System Grid Federation, 2019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, T.L.; Barbosa-Silva, R.G.; Acosta, A.L.; van den Berg, C. Expanding the Distribution of Prosthechea jauana (Orchidaceae) in the Pantepui and Highlighting the Urgent Need for Conservation Strategies in the Region in Face of Climate Change. Plants 2024, 13, 222. https://doi.org/10.3390/plants13020222
Vieira TL, Barbosa-Silva RG, Acosta AL, van den Berg C. Expanding the Distribution of Prosthechea jauana (Orchidaceae) in the Pantepui and Highlighting the Urgent Need for Conservation Strategies in the Region in Face of Climate Change. Plants. 2024; 13(2):222. https://doi.org/10.3390/plants13020222
Chicago/Turabian StyleVieira, Tiago L., Rafael G. Barbosa-Silva, André L. Acosta, and Cássio van den Berg. 2024. "Expanding the Distribution of Prosthechea jauana (Orchidaceae) in the Pantepui and Highlighting the Urgent Need for Conservation Strategies in the Region in Face of Climate Change" Plants 13, no. 2: 222. https://doi.org/10.3390/plants13020222
APA StyleVieira, T. L., Barbosa-Silva, R. G., Acosta, A. L., & van den Berg, C. (2024). Expanding the Distribution of Prosthechea jauana (Orchidaceae) in the Pantepui and Highlighting the Urgent Need for Conservation Strategies in the Region in Face of Climate Change. Plants, 13(2), 222. https://doi.org/10.3390/plants13020222








