Abstract
Gravitropism is the plant organ bending in response to gravity. Gravitropism, phototropism and sufficient mechanical strength define the optimal position of young shoots for photosynthesis. Etiolated wild-type Arabidopsis seedlings grown horizontally in the presence of sucrose had a lot more upright hypocotyls than seedlings grown without sucrose. We studied the mechanism of this effect at the level of cell wall biomechanics and biochemistry. Sucrose strengthened the bases of hypocotyls and decreased the content of mannans in their cell walls. As sucrose is known to increase the gravitropic bending of hypocotyls, and mannans have recently been shown to interfere with this process, we examined if the effect of sucrose on shoot gravitropism could be partially mediated by mannans. We compared cell wall biomechanics and metabolomics of hypocotyls at the early steps of gravitropic bending in Col-0 plants grown with sucrose and mannan-deficient mutant seedlings. Sucrose and mannans affected gravitropic bending via different mechanisms. Sucrose exerted its effect through cell wall-loosening proteins, while mannans changed the walls’ viscoelasticity. Our data highlight the complexity of shoot gravitropism control at the cell wall level.
1. Introduction
The aboveground position of young plant shoots is tightly regulated to ensure efficient photosynthesis. It is determined by their gravi- and phototropism, as well as requiring sufficient mechanical strength to maintain the shoots’ own weight in the gravity field. Gravitropism is defined as the plant organ bending in response to gravity [1]. Plant shoots demonstrate negative gravitropism by bending upward after their inclination from the vertical [2]. Gravitropism consists of three sequential steps: gravity perception, gravity signal transduction and gravity response [3]. In shoots, gravity perception takes place in the endodermis [4]. The most popular explanation of gravity perception is the starch–statolith hypothesis, which involves the sedimentation of amyloplasts (starch-filled plastids) that function as statoliths to a new lower side in gravisensing cells (statocytes). After a series of signal transduction events, this results in the gravity response in the form of plant organ bending [3]. According to the Cholodny–Went theory, growth curvature is due to an unequal distribution of auxin between the two sides of the bending organ. Recent studies have uncovered signal transduction steps that link amyloplast sedimentation with auxin redistribution in plant gravitropism. LZY proteins translocate from sedimenting amyloplasts to the nearby plasmalemma in statocytes [5]. After that, they recruit RLD proteins from the cytoplasm to the plasmalemma [6]. Finally, the LZY-RLD module induces the polar relocalization of PIN3 [6], or different factors of auxin transport machinery [5], to form the IAA gradient in the gravistimulated organ.
Plant shoot gravitropism is an interesting experimental model because it involves simultaneous and well-coordinated changes in different physical cell wall properties, including its extensibility on the opposite sides of a gravistimulated organ [7], compression resistance on the concave side [1] and possibly flexibility of different cell wall components [8]. When studied in specific cell wall mutants and/or under specific treatments, plant shoot gravitropism may help reveal novel functions of cell wall polymers that are difficult to discover while studying the linear growth of plant organs. For example, novel functions have been established for xyloglucans [9] and mannans [8] using this model.
Sucrose plays a pivotal role in sugar transport, metabolism and signaling in plants [10]. It stimulates gravitropism at the stage of gravity perception by elevating the starch load in amyloplasts, which increases their weight and hence the rate of sedimentation [11,12]. Additionally, sucrose increases the proportion of upright hypocotyls in Arabidopsis seedlings grown on horizontal Petri plates [12]. In the present work, we examined if this effect of sucrose on hypocotyl posture was supported by mechanical and biochemical changes in cell walls. Sucrose was found to decrease the content of mannans in hypocotyl cell walls. We have recently demonstrated that these minor hemicelluloses interfere with the gravitropic bending of Arabidopsis hypocotyls [8]. Thus, we speculated that the stimulation of shoot gravitropism in the presence of sucrose could be partially mediated by mannans. Our detailed biomechanical and metabolomic analyses did not support this hypothesis. We found that sucrose and mannans controlled gravitropic bending via different mechanisms. This highlighted the complexity of shoot gravitropism regulation at the cell wall level.
2. Results
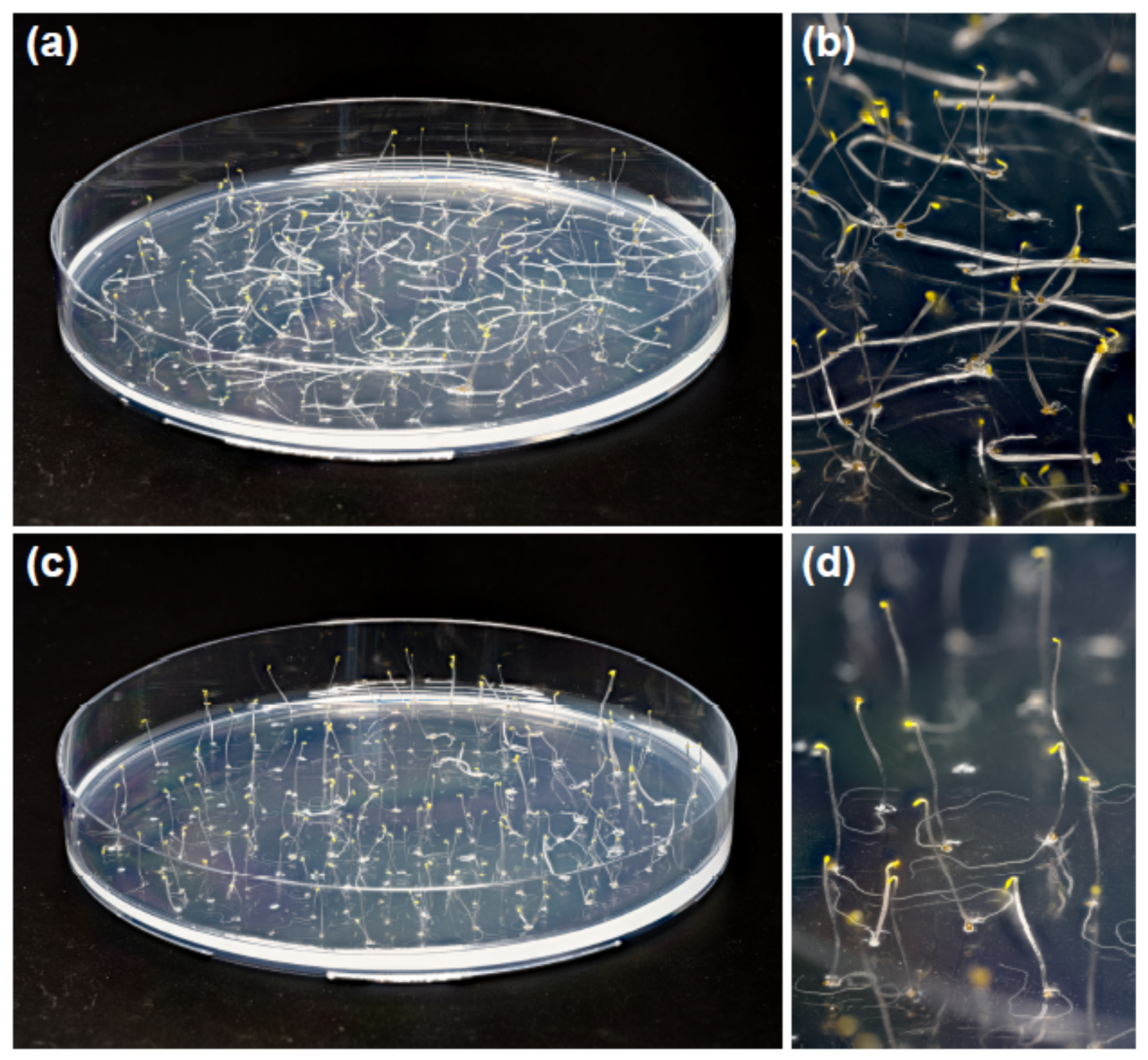
Five-day-old etiolated Col-0 Arabidopsis seedlings grown on horizontal Petri plates on one-half-strength MS medium demonstrated different posture of hypocotyls depending on the presence of sucrose in the medium. We observed that about 56 ± 10% (mean ± SD) of control seedlings grown without sucrose had upright hypocotyls, while the hypocotyls of the remaining 44% lay on the agar surface (Figure 1a,b). Sucrose (1% w/v) supplementing the medium increased the proportion of upright hypocotyls to 98 ± 2% (Figure 1c,d). This difference in hypocotyl posture was highly significant (p = 0.0035; n = 4; Student’s t-test) and persisted from the moment of seed germination. Additionally, sucrose inhibited the growth of hypocotyls. It decreased their length to 11.8 ± 0.9 mm compared with 15.4 ± 2.5 mm in the control (mean ± SD; n = 40; p < 0.0001; Student’s t-test).
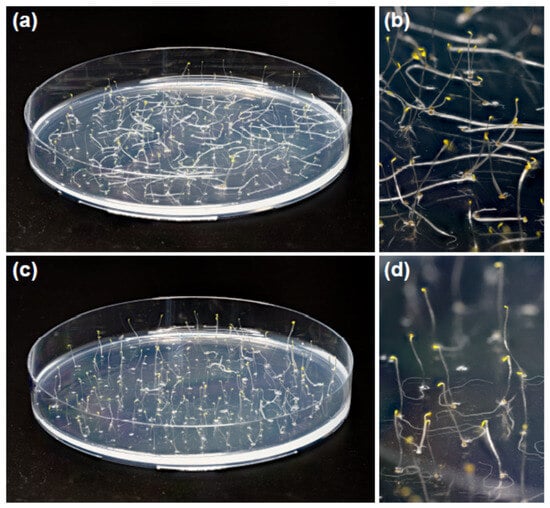
Figure 1.
Sucrose in the growth medium increased the proportion of upright hypocotyls in etiolated Col-0 Arabidopsis seedlings. Five-day-old plants grown without sucrose (a,b) or in the presence of 1% w/v sucrose (c,d) on horizontal Petri plates.
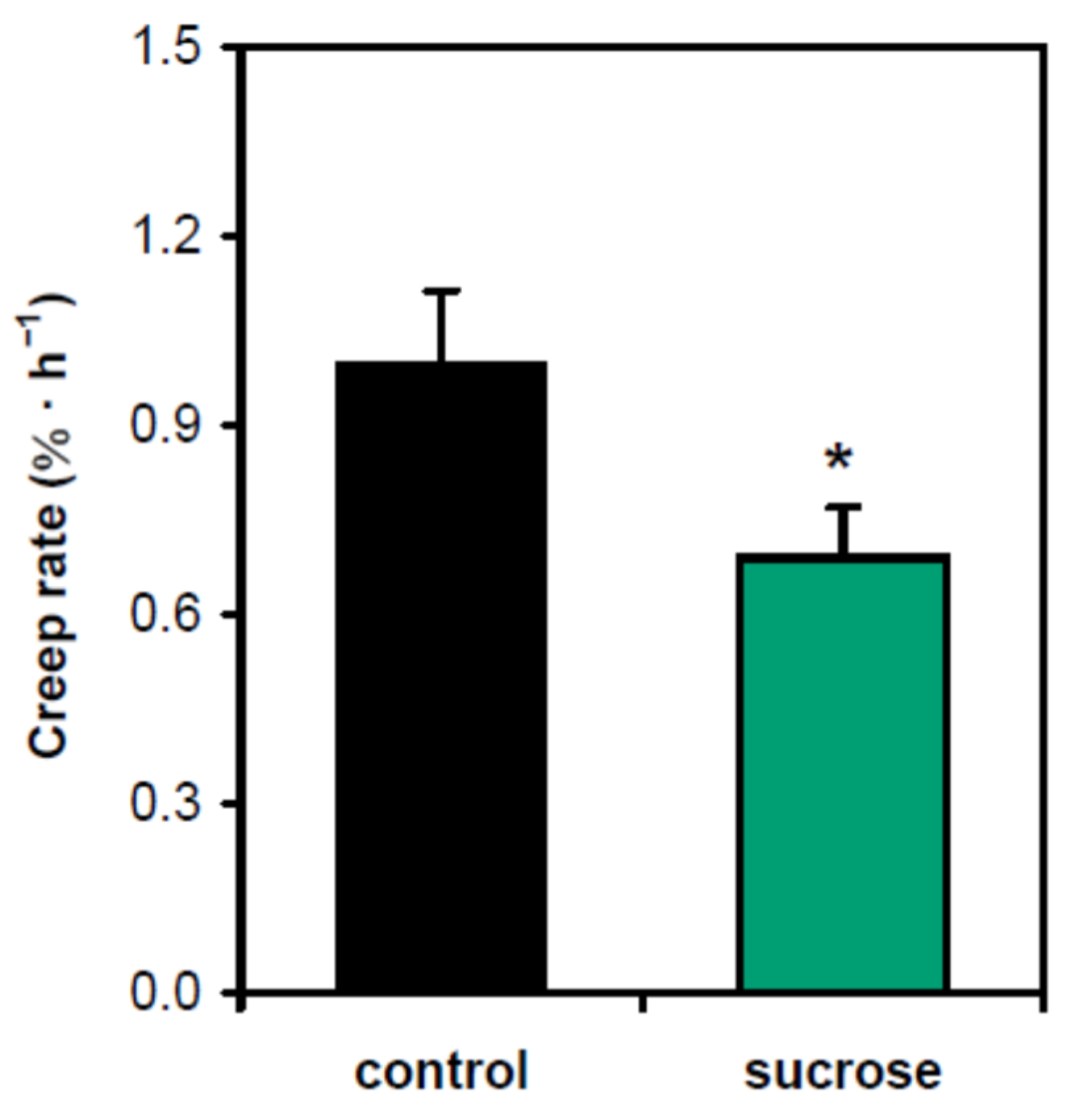
The effect of sucrose on gravitropism has traditionally been linked to its involvement at the stage of gravity perception, where it increases the load of starch in amyloplasts [11,12]. However, our previous findings suggested that sucrose could also affect the posture of shoots via cell wall mechanics [12]. To test this option directly, we studied the creep of cell walls (i.e., their time-dependent deformation under a constant load) at the base of hypocotyls, the zone that carries the main part of the organ weight. We used heat inactivation of cell walls to eliminate any contribution of cell wall-loosening proteins to the biomechanics [13]. Sucrose significantly decreased the wall creep rate, which is consistent with the strengthening of the lower portion of hypocotyls (Figure 2).

Figure 2.
Sucrose strengthened the lower part of hypocotyls in five-day-old Arabidopsis seedlings grown on horizontal Petri plates. Five-millimeter-long basal segments of frozen/thawed heat-inactivated hypocotyls were extended at pH 5.0 under a 600 mg load, and their creep rate was measured. ‘Control’—plants grown without sucrose; ‘sucrose’—plants grown in the presence of sucrose (1% w/v). Data are means ± SE (n = 10). An asterisk denotes significant difference (p < 0.05; Student’s t-test).
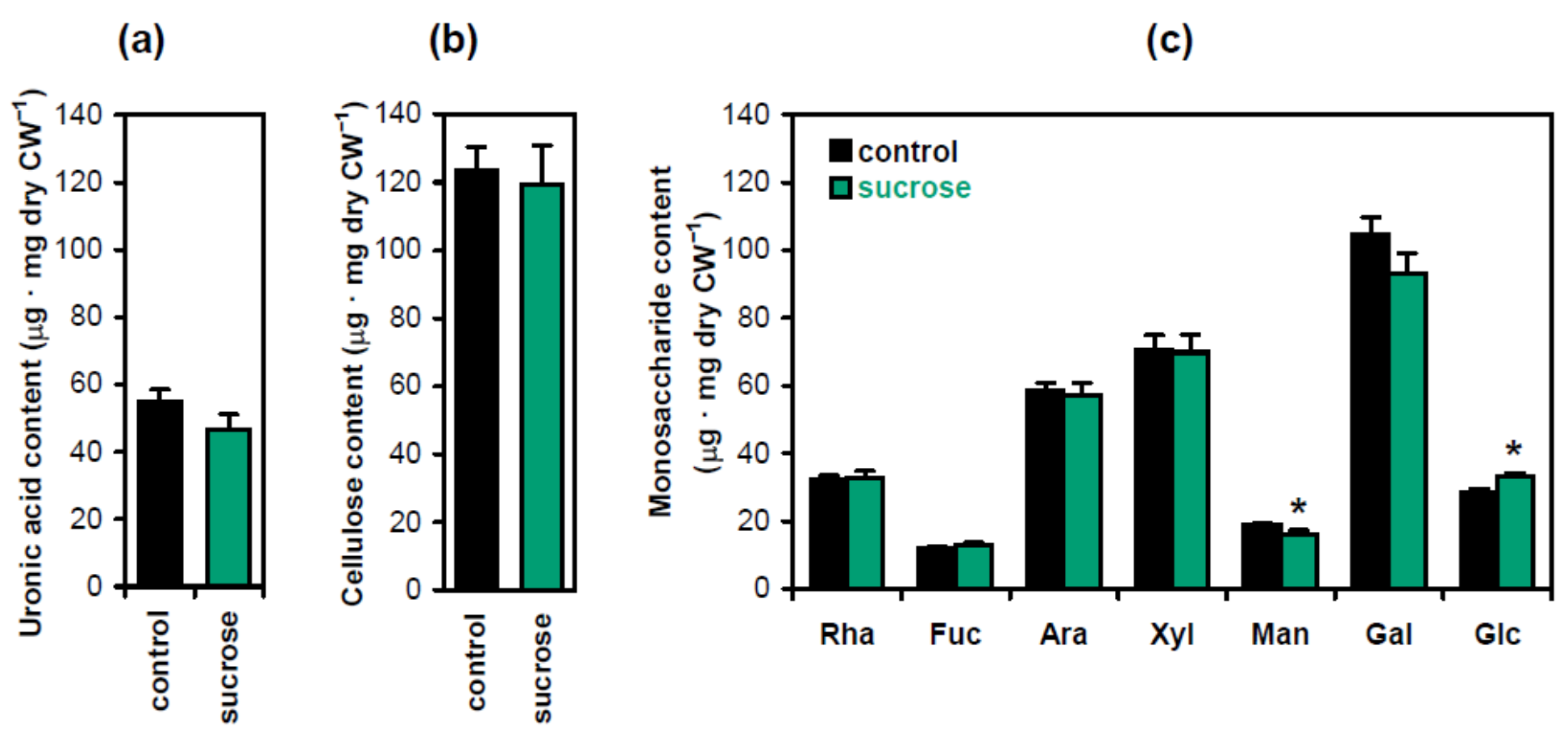
To investigate the biochemical basis of hypocotyl strengthening in the presence of sucrose, we carried out standard cell wall analyses measuring the levels of crystalline cellulose, uronic acids and monosaccharides derived from cell wall matrix polymers (Figure 3). Significant differences were only observed for monosaccharides: the content of glucose increased, which was accompanied by a decrease in mannose (Figure 3c). The increase in glucose was likely caused by starch. It accumulated in hypocotyls in the presence of sucrose [12] and could contaminate the wall residue, which was hydrolyzed with TFA (trifluoracetic acid) to release monosaccharides for subsequent analyses. The decrease in mannose suggested that the level of mannans was reduced in the presence of sucrose. These hemicelluloses have been known as the main source of mannose in Arabidopsis cell walls [14]. Their depletion is interesting in the context of shoot gravitropism. We have recently demonstrated that treatment with brassinazole, an inhibitor of brassinosteroid biosynthesis, and the triple mutation csla2csla3csla9, which both decreased mannans in Arabidopsis hypocotyl cell walls, stimulated gravitropic bending [8]. It might thus be hypothesized that sucrose, which also increased the gravitropic bending of Arabidopsis hypocotyls [12], could exert this effect, at least partially, at the expense of mannan depletion. We examined this hypothesis by studying the wall biomechanics and metabolomics of three-day-old Arabidopsis hypocotyls at the beginning of their gravitropic bending. Effects of sucrose in Col-0 plants were compared with those of the triple mutation csla2csla3csla9, which depleted mannans in Arabidopsis cell walls [14]. If the mechanism of sucrose action on gravitropic bending was partially mediated by mannans, we would observe similarities between the above two variants at the level of cell wall biomechanics and/or metabolomics.
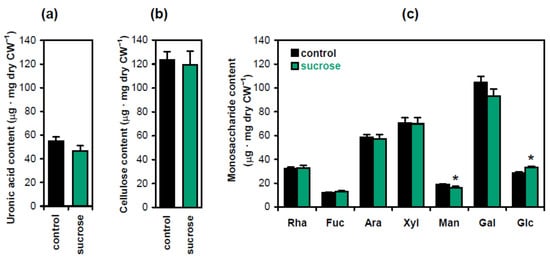
Figure 3.
Biochemical composition of cell walls in Arabidopsis seedlings as affected by sucrose. The contents of uronic acids (a), crystalline cellulose (b) and monosaccharides derived from cell wall matrix polymers (c) were determined in five-day-old etiolated Col-0 seedlings grown on horizontal Petri plates without sucrose (control) or in the presence of sucrose (1% w/v). Data are means ± SE (n = 9). Asterisks denote significant differences (p < 0.05; Student’s t-test).
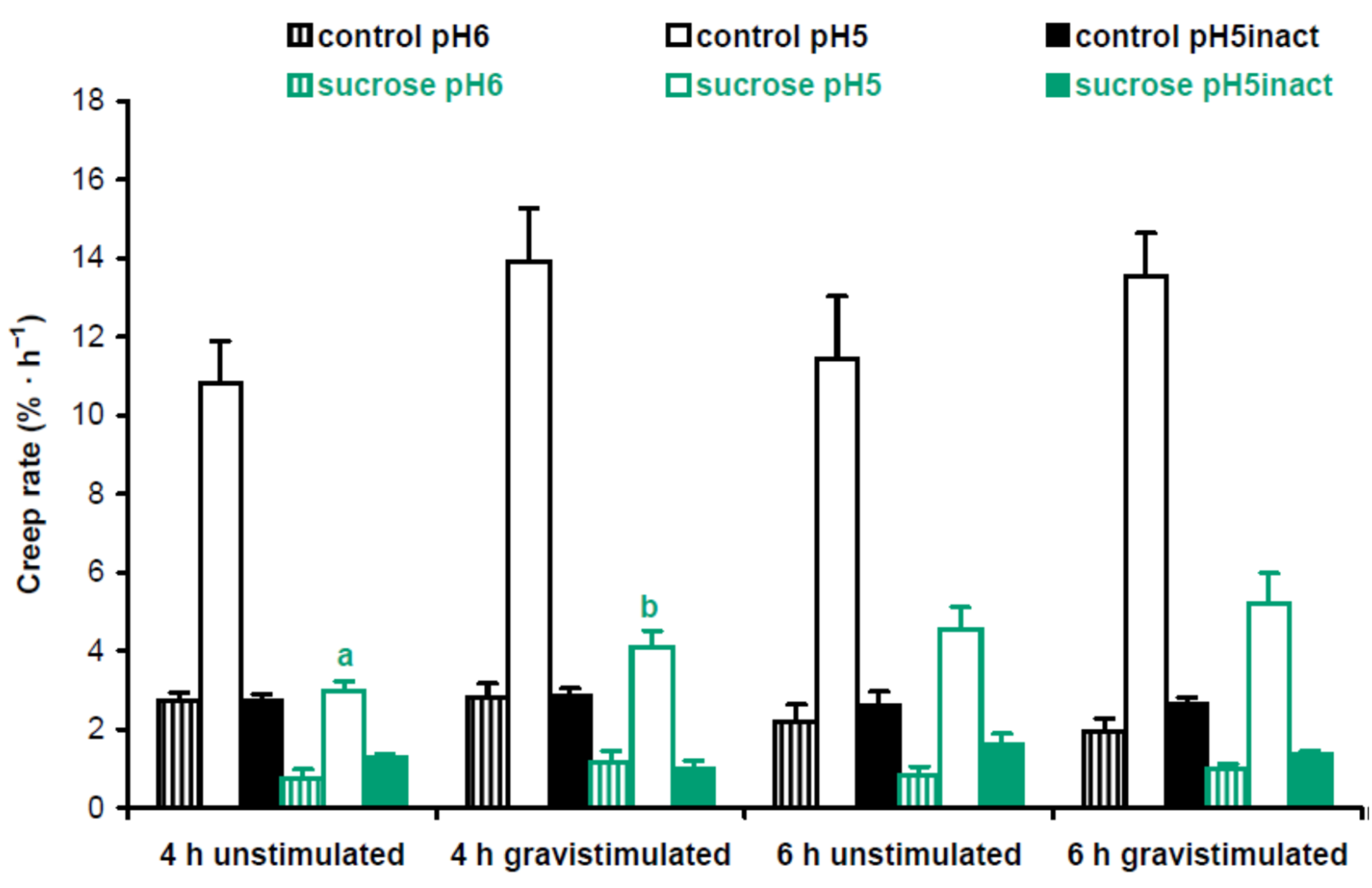
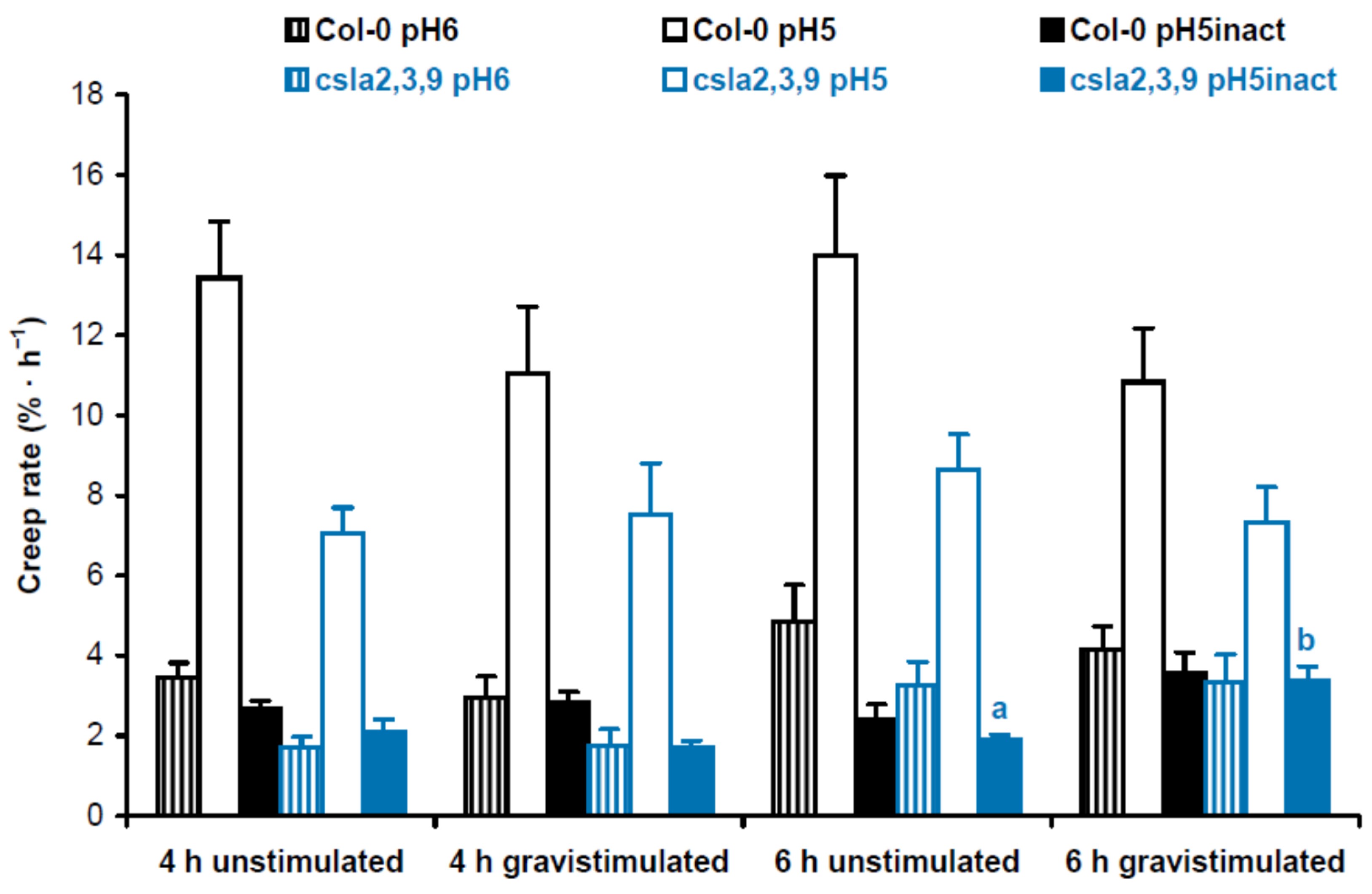
Cell wall mechanics were studied with the creep method using 3-mm-long subapical segments of hypocotyls from 3-day-old Arabidopsis seedlings grown on vertical Petri plates. We extended the zone of hypocotyls where their bending first appeared after gravistimulation. Cell wall creep was measured under three different conditions: (1) at pH 5, where expansin proteins, the mediators of ‘acid growth’, were active [15]; (2) at pH 6, where expansins were inactive, but different classes of cell wall-loosening proteins like XTHs (xyloglucan endotransglucosylase/hydrolases) could affect wall extensibility [16]; and (3) at pH 5 with heat inactivation, where activities of cell wall-loosening proteins were essentially eliminated, and in vitro extension of the wall was defined by its viscoelasticity [17]. Here ‘viscoelasticity’ denotes the range of material properties of cell walls that include elastic, viscous, plastic and retarded elastic deformations, but does not include enzyme-dependent, or chemorheological, flows [18]. To better understand the biomechanical mechanisms of gravitropic bending, we focused on comparisons between gravistimulated and nongravistimulated seedlings under each of the three conditions for creep measurements. In experiments with Col-0 plants grown with or without sucrose, we found only one significant difference in the creep rate between gravistimulated and nongravistimulated seedlings: at pH 5, 4 h after gravistimulation (Figure 4). This shows that increased gravitropic bending in the presence of sucrose [12] (Figure S1) could involve the accumulation of expansin proteins in the walls of hypocotyls.
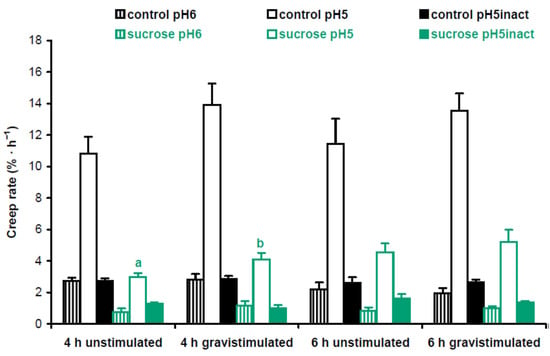
Figure 4.
Cell wall biomechanics of hypocotyls from gravistimulated and nongravistimulated Col-0 Arabidopsis seedlings grown without sucrose (control) or in the presence of sucrose (1% w/v). Etiolated seedlings grown on vertical Petri plates for 3 days were either gravistimulated via a 90° counterclockwise rotation of the plates or left unstimulated. Both groups of seedlings were frozen 4 h and 6 h after the moment of gravistimulation. Three-millimeter-long subapical segments of frozen/thawed hypocotyls were extended under a 600 mg load, and their creep rate was measured. Data are means ± SE (n = 8). Different letters denote the only significant difference between gravistimulated and nongravistimulated hypocotyls (p < 0.05; Student’s t-test).
Five-day-old mannan-deficient csla2csla3csla9 triple mutant and Col-0 wild-type seedlings grown on horizontal Petri plates did not differ in the percentage of upright hypocotyls (48.4 ± 5.8% vs. 47.7 ± 8.6%, respectively; mean ± SD; n = 7; p = 0.87; Student’s t-test). At the same time, the triple mutant demonstrated increased gravitropic bending of hypocotyls in three-day-old seedlings grown on vertical Petri plates (Figure S2) [8]. In biomechanical experiments where Col-0 wild-type plants were compared with csla2csla3csla9 triple mutants, we also found only one significant difference in the creep rate between gravistimulated and nongravistimulated seedlings: at pH 5 with heat inactivation 6 h after gravistimulation (Figure 5). Thus, the increased gravitropic bending in the mannan-deficient triple mutant seemingly resulted from changes in wall viscoelasticity.
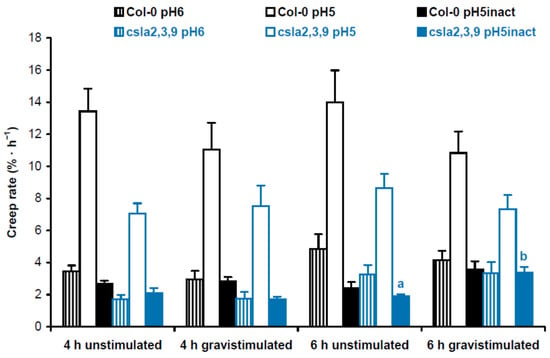
Figure 5.
Cell wall biomechanics of hypocotyls from gravistimulated and nongravistimulated wild-type Col-0 Arabidopsis seedlings or mannan-deficient csla2csla3csla9 triple mutants. Etiolated seedlings grown on vertical Petri plates for 3 days were either gravistimulated via a 90° counterclockwise rotation of the plates or left unstimulated. Both groups of seedlings were frozen 4 h and 6 h after the moment of gravistimulation. Three-millimeter-long subapical segments of frozen/thawed hypocotyls were extended under a 600 mg load, and their creep rate was measured. Data are means ± SE (n = 8). Different letters denote the only significant difference between gravistimulated and nongravistimulated hypocotyls (p < 0.05; Student’s t-test).
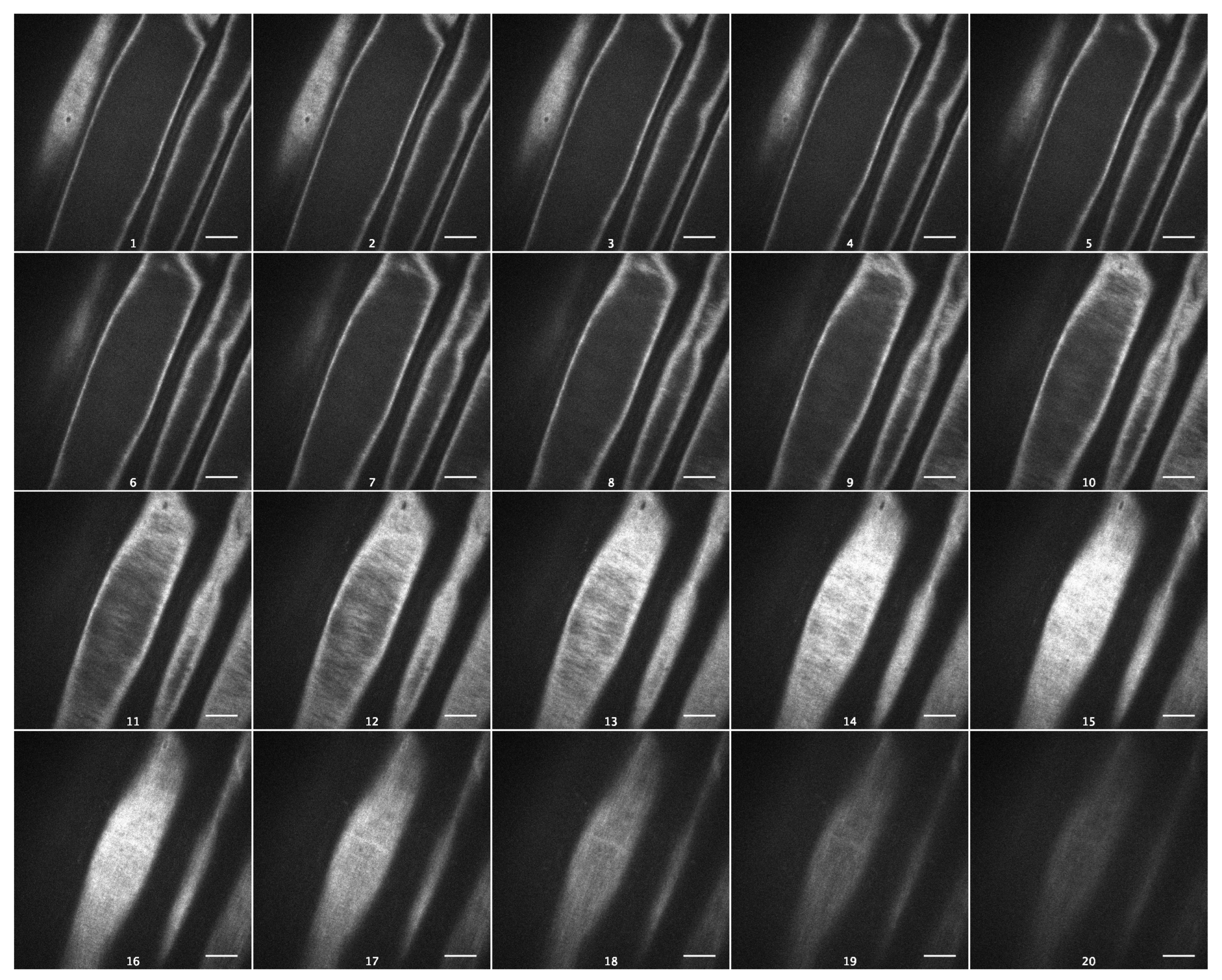
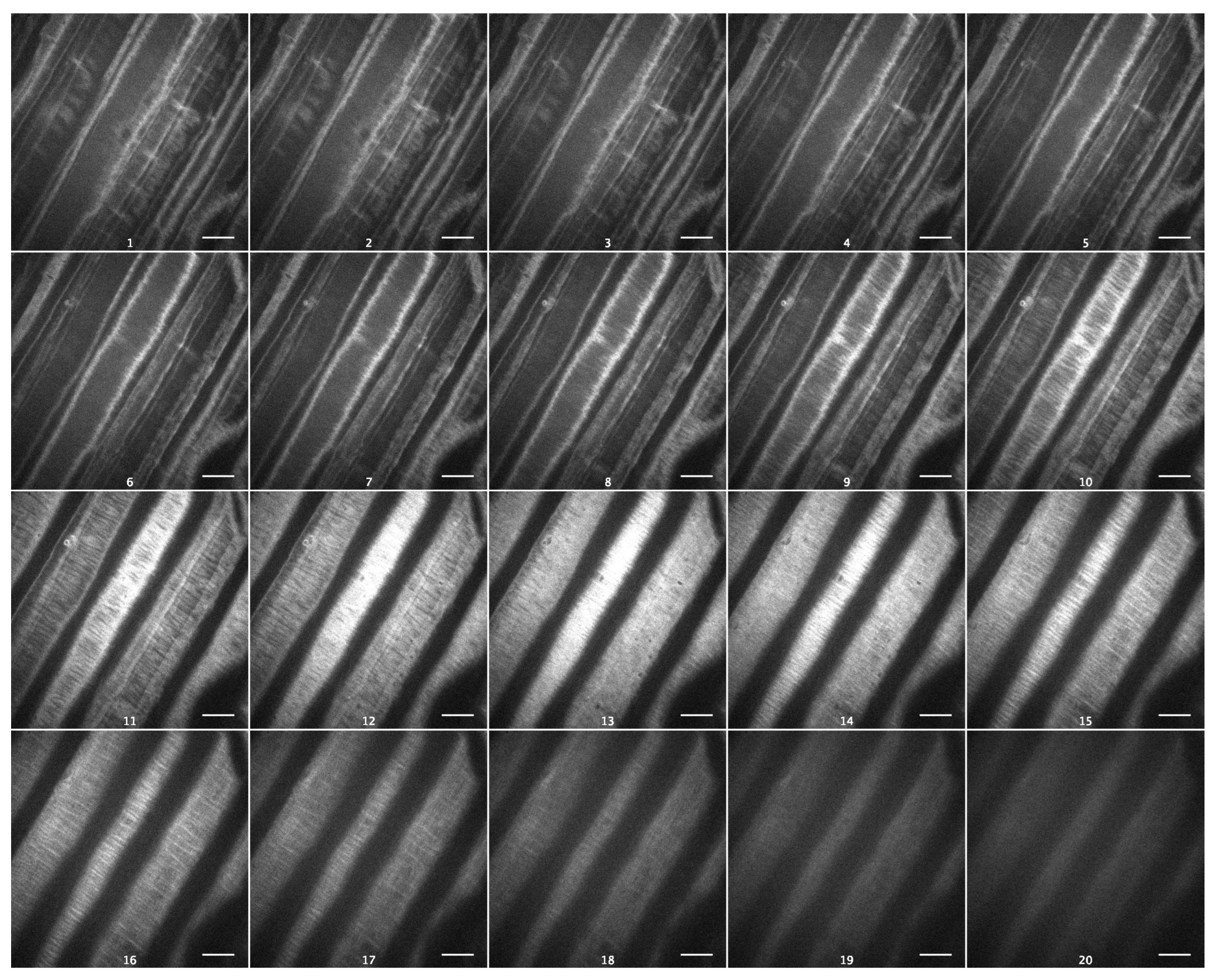
As cellulose is the strongest cell wall component, with a profound effect on its viscoelasticity [19], we visualized cellulose macrofibrils in outer epidermal cell walls of Arabidopsis hypocotyls using spinning-disc confocal microscopy and the specific fluorescent dye Pontamine Fast Scarlet 4B (S4B) [20]. Cellulose macrofibrils were thin and predominantly transverse in the inner wall layers and longitudinal in the outer layers of Col-0 epidermal cell walls (Figure 6, Video S1). In contrast to this, they were thicker and more uniformly transversely orientated in the inner wall layers of csla2csla3csla9 hypocotyls (Figure 7, Video S2). Thus, the depletion of mannans altered the arrangement of cellulose macrofibrils in the triple mutant, which could explain the effect on wall creep (Figure 5).
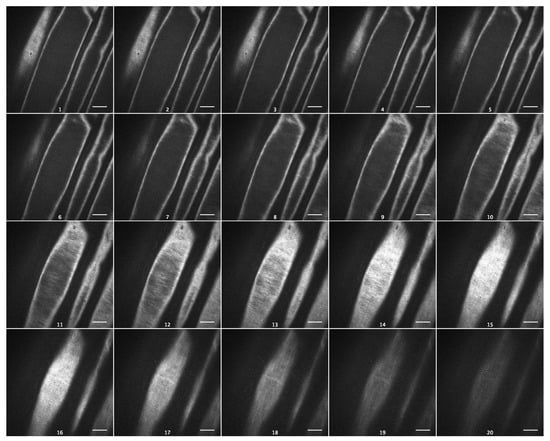
Figure 6.
Cellulose macrofibril arrangement in the outer epidermal cell wall from the subapical part of five-day-old Col-0 hypocotyls. Spinning-disc confocal microscopy on wall samples stained with Pontamine Fast Scarlet 4B. Subsequent optical sections of a representative Z-stack from the innermost to the outermost wall layer. Scale bars are 10 μm.

Figure 7.
Cellulose macrofibril arrangement in the outer epidermal cell wall from the subapical part of five-day-old csla2csla3csla9 hypocotyls. Spinning-disc confocal microscopy on wall samples stained with Pontamine Fast Scarlet 4B. Subsequent optical sections of a representative Z-stack from the innermost to the outermost wall layer. Scale bars are 10 μm.
Overall, our biomechanical data demonstrated that the stimulation of shoot gravitropism with sucrose (Figure 4) and in the triple mutant (Figure 5) occurred via different mechanisms. This does not support our hypothesis that the effect of sucrose on gravitropism is partially mediated by mannans in Arabidopsis hypocotyls.
In our metabolomic studies, we used Arabidopsis seedlings grown and gravistimulated under the same conditions and schedule as reported for biomechanical experiments (Figure 4 and Figure 5), but the plant material was harvested for analysis only at one timepoint: 4 h after gravistimulation. Only those metabolites that were present in all eight biological replicates (three independent experiments) were considered.
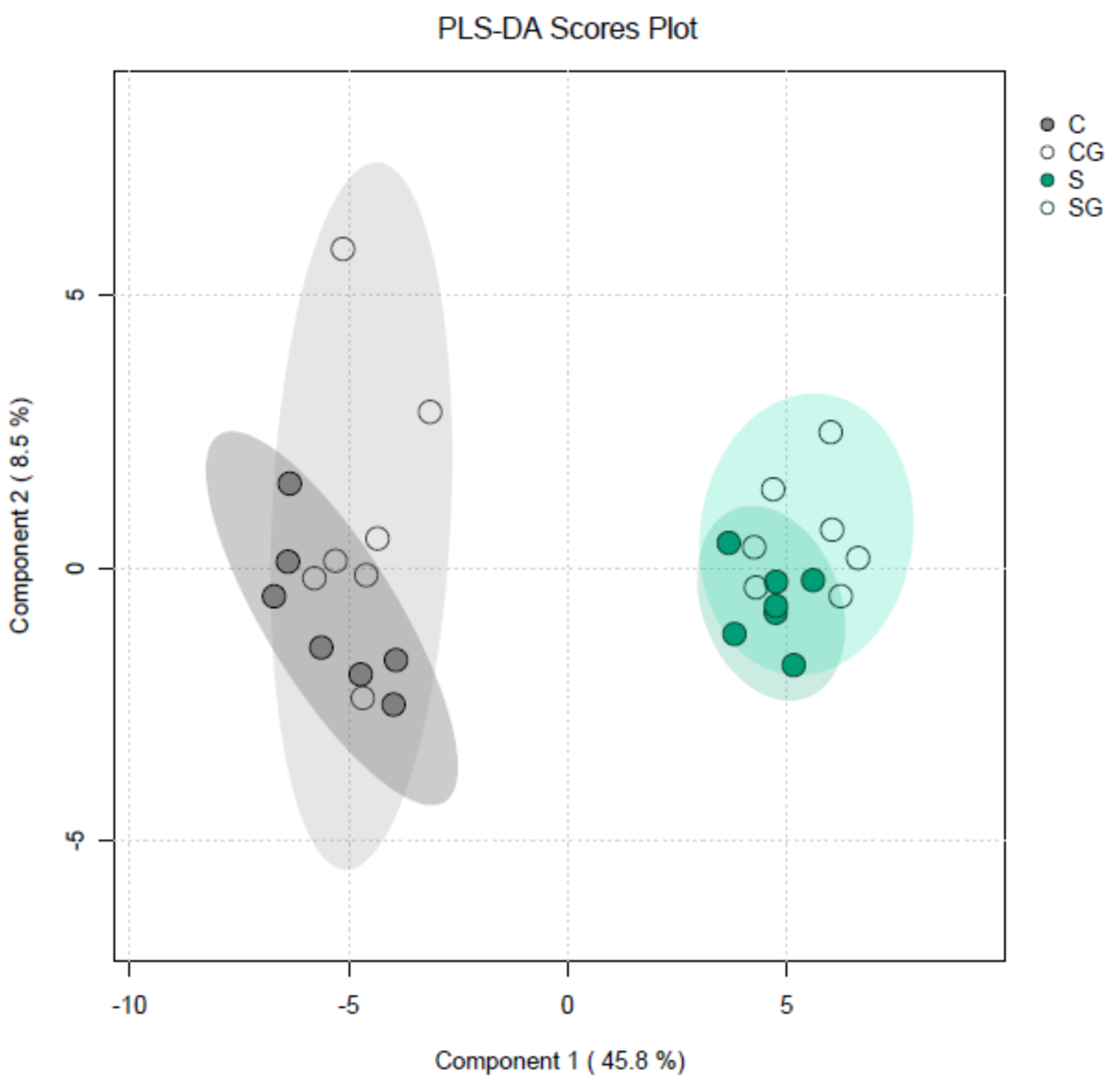
Sucrose induced profound metabolic changes in Arabidopsis hypocotyls. We found significant differences for 47 metabolites out of 60 detected in each experimental variant that were caused by the presence of sucrose in the growth medium (Table S1, comparisons of C vs. S and CG vs. SG). The effect of sucrose was very specific for particular metabolites: it increased and decreased the contents of 20 and 27 metabolites, respectively. The group of metabolites whose content decreased in the presence of sucrose included various amino acids. Apparently, this indicates that sucrose had a strong stimulatory effect on protein synthesis. At the same time, the group of metabolites whose content was higher in the presence of sucrose included a number of sugars. Interestingly, the effects of sucrose in the nongravistimulated variants (C vs. S) usually matched those in the gravistimulated variants (CG vs. SG). At the same time, we found only two metabolites: hydroxybutyric acid and ornithine, the content of which changed significantly as a result of gravistimulation in the presence of sucrose (S vs. SG). This means that sucrose’s effect on metabolism could outweigh and mask the majority of fine metabolic changes caused by gravistimulation. The plot of partial least squares discriminant analysis (PLS-DA) of metabolite profiles of Arabidopsis hypocotyls (Figure 8) illustrated this conclusion. The ellipses describing gravistimulated (SG) and nongravistimulated (S) plants grown with sucrose were not separated.

Figure 8.
Partial least squares discriminant analysis (PLS-DA) of metabolite profiles of Arabidopsis hypocotyls. Three-day-old etiolated Col-0 seedlings grown on vertical Petri plates without sucrose or in the presence of sucrose (1% w/v) were gravistimulated via a 90° counterclockwise rotation of the plates. PLS-DA scores are plotted along the X and Y axes, which are Component 1 and Component 2, respectively. Percentage in brackets indicates the explained variance of metabolite content. Abbreviations: C—nongravistimulated wild-type Col-0 seedlings, grown without sucrose; S—nongravistimulated wild-type Col-0 seedlings, grown in the presence of sucrose (1% w/v); CG—gravistimulated wild-type Col-0 seedlings, grown without sucrose; SG—gravistimulated wild-type Col-0 seedlings, grown in the presence of sucrose (1% w/v). Each circle shows a corresponding biological replicate. Large ellipses depict 95% confidence regions.
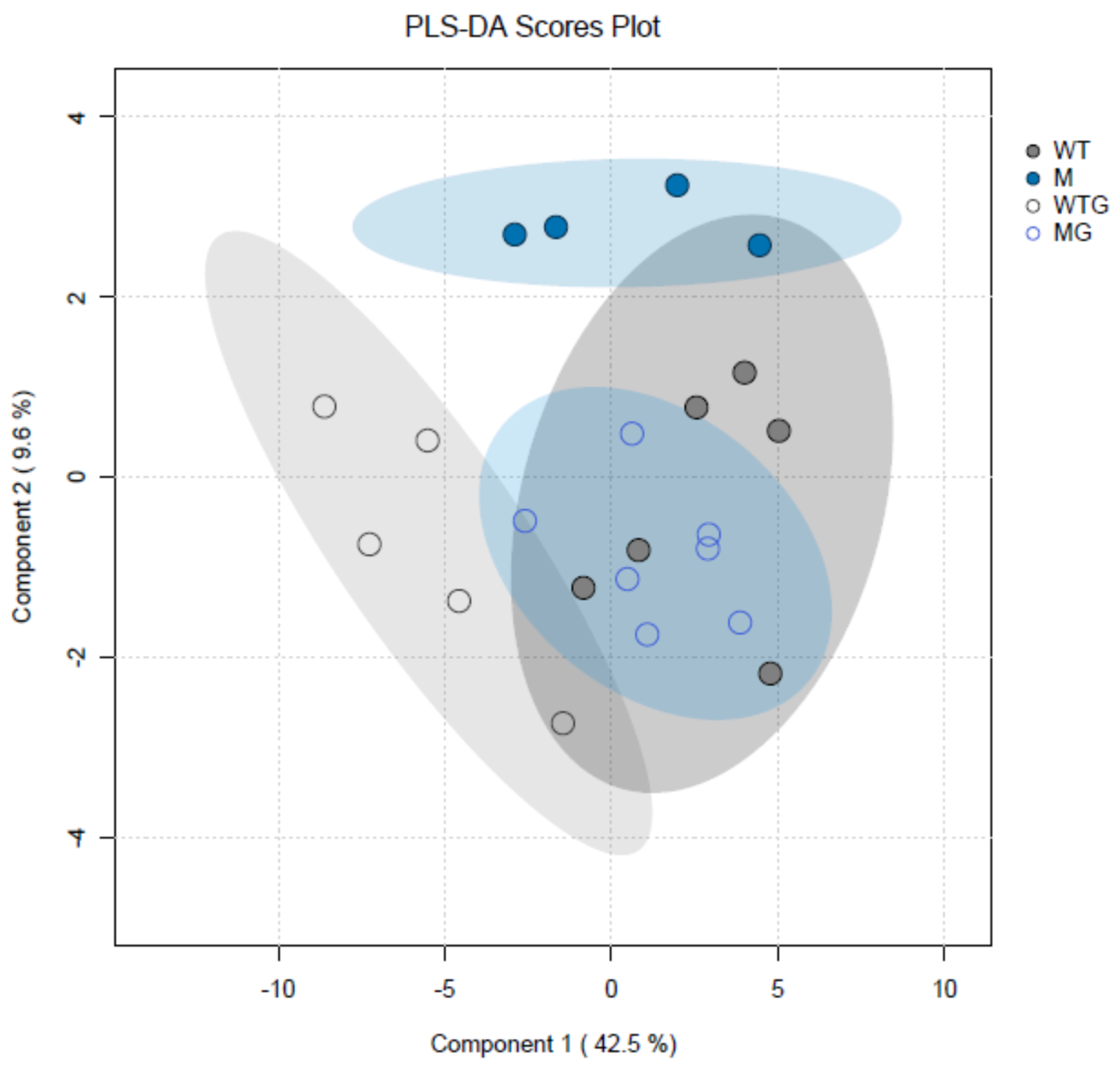
Metabolomic analyses of csla2csla3csla9 triple mutants vs. wild-type Col-0 plants gave very different results (Table S2) compared with the effects of sucrose (Table S1). Pairwise comparisons of the four experimental variants revealed only eight significant differences for the content of metabolites. Remarkably, seven of them were found when gravistimulated mutant and wild-type plants were compared (WTG vs. MG). Thus, gravistimulation caused stronger metabolic changes in the triple mutants compared with Col-0 plants (Figure 9). A decrease in 4-hydroxyproline and an increase in aspartic acid in gravistimulated csla2csla3csla9 seedlings could suggest that gravistimulation activated the metabolism of cell wall structural glycoproteins.
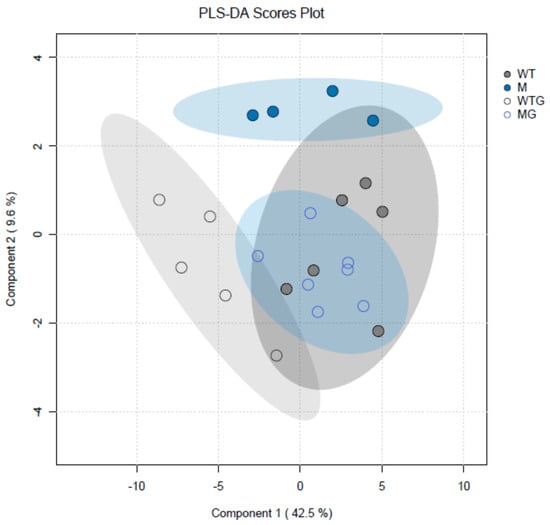
Figure 9.
Partial least squares discriminant analysis (PLS-DA) of metabolite profiles of wild-type Col-0 and csla2csla3csla9 Arabidopsis hypocotyls. Three-day-old etiolated wild-type Col-0 and csla2csla3csla9 seedlings grown on vertical Petri plates without sucrose were gravistimulated via a 90° counterclockwise rotation of the plates. PLS-DA scores are plotted along X and Y axes that are Component 1 and Component 2, respectively. Percentage in brackets indicate the explained variance for metabolite content. Abbreviations: WT—nongravistimulated wild-type Col-0 seedlings; M—nongravistimulated csla2csla3csla9 triple mutant seedlings; WTG—gravistimulated wild-type Col-0 seedlings; MG—gravistimulated csla2csla3csla9 triple mutant seedlings. Each circle shows a corresponding biological replicate. Large ellipses depict 95% confidence regions.
By analogy with the data on biomechanics (Figure 4 and Figure 5), the results of metabolomic analyses did not reveal any appreciable similarity in the effects of mannan deficiency and sucrose. Therefore, our hypothesis that the effect of sucrose on shoot gravitropism could be partially mediated by mannans was not supported.
3. Discussion
In the present work, we found that the effect of sucrose on hypocotyl posture (Figure 1c,d) was only partially due to improved gravity perception [11,12]. Sucrose also acted at the cell wall level: (1) it strengthened hypocotyls at the base (Figure 2), which aided them in supporting their own weight in the gravity field; (2) it accelerated gravitropic bending by an expansin-mediated mechanism (Figure 4).
Interestingly, the increase in hypocotyl strength was accompanied by minor changes in wall composition (Figure 3). Apparently, strengthening was mainly achieved through an increase in the amount of cell wall material, rather than through the accumulation of stronger wall material. Indeed, the rate of cell wall synthesis in Arabidopsis hypocotyls, estimated through the rate of cellulose synthase complex trafficking in the plasmalemma, was controlled by the availability of sugars from photosynthesis or the growth medium [13]. The increase in cellulose synthesis in the presence of sucrose [13] was seemingly accompanied by a comparable stimulation in the synthesis of all main cell wall constituents, with the exception of mannans, which was reflected in the limited sucrose-induced changes in the wall composition (Figure 3). In line with this assumption, we found that sucrose decreased the contents of arginine, asparagine, valine, glycine, isoleucine, leucine, proline, serine, threonine, tryptophan, phenylalanine and uronic acids (Table S1). A significant part of these amino acids might have been used in the synthesis of cell wall structural glycoproteins, while phenylalanine and uronic acids could have been consumed in the synthesis of cell wall phenylpropanoid compounds and pectins, respectively.
The in vivo strength of young plant organs is defined by turgor [21]. Both stronger cell walls and higher intracellular concentrations of osmotically active compounds increase turgor [22]. The higher proportion of upright hypocotyls in the presence of sucrose (Figure 1) could also result from elevated turgor due to the accumulation of intracellular osmotics. Our metabolomics data supported this additional mechanism of sucrose action on the hypocotyl posture: many abundant osmotically active compounds accumulated in hypocotyls in the presence of sucrose including arabinose, glucose, mannose, sucrose and others (Table S1).
Lastly, the inhibition of hypocotyl growth observed in the presence of sucrose, resulting from delayed germination of Arabidopsis seeds [23], could also contribute to the higher proportion of upright hypocotyls (Figure 1) because shortened hypocotyls would be more stable on a horizontal surface.
The only change in cell wall biochemical composition observed in the presence of sucrose was a decrease in mannans (Figure 3). Mannan deficiencies have been shown to disturb the arrangement of cellulose in Arabidopsis [24,25]. As cellulose is critical for wall strength [19], this change in mannans would hardly increase the percentage of upright hypocotyls. We found here that mannan depletion did not contribute to the control of hypocotyl posture, as the csla2csla3csla9 triple mutant and Col-0 wild-type seedlings had the same percentage of upright hypocotyls.
Gravitropic bending results from differential cell growth on the opposite sides of gravistimulated organs [26]. In elongating shoots placed horizontally, growth is always inhibited on the upper side [27]. In some plant species, this inhibition is accompanied by growth acceleration on the lower side of gravistimulated shoots [7,28,29]. The differential growth response is controlled by the corresponding decrease and increase in the wall extensibility, and these opposite changes were not necessarily equal in magnitude and synchronous in time on both sides of gravistimulated shoots [7,28]. The Arabidopsis hypocotyls used in the present study were very small and fragile. The longitudinal bisection of gravistimulated Arabidopsis hypocotyls into equal upper and lower halves to study their biomechanics separately is very difficult. Thus, we used nonbisected Arabidopsis hypocotyls to measure their wall properties during gravitropism. The reported wall creep rates (Figure 4 and Figure 5) were the net estimation of probable opposite changes in wall extensibility on upper and lower sides of gravistimulated hypocotyls. One limitation of our approach is that any significant differences between gravistimulated and nongravistimulated hypocotyls could be revealed only if the changes on both sides of the former were different in magnitude and/or timing.
Our previous data suggested that a decrease in mannans could stimulate gravitropic bending [8]. Therefore, it may be a part of the mechanism of the increased shoot gravitropism in the presence of sucrose [12] (Figure S1). However, this assumption was not supported by our biomechanical (Figure 4 and Figure 5) and metabolomic analyses (Figure 8 and Figure 9, Tables S1 and S2). We found that the increased gravitropic bending of hypocotyls in sucrose-grown plants (Figure S1) was accompanied by increased in vitro cell wall extension at pH 5 (Figure 4). This is consistent with the accumulation of expansin proteins, the mediators of the acid growth known as the early phase of auxin-induced plant organ extension [15,30,31]. Interestingly, the plasmalemma H+-ATPases were activated in the lower part of gravistimulated hypocotyls of Arabidopsis plants grown with sucrose [32]. They acidified the apoplast in this part of the hypocotyls, shifting its pH to optimal values for expansin activities [32]. These data suggest that the increased sucrose-induced gravitropic bending (Figure S1) was based on the enhanced auxin response, in which H+-ATPases and expansins worked in a concerted fashion. Apparently, heavier amyloplasts accumulated in the presence of sucrose [12] established a steeper auxin gradient in Arabidopsis hypocotyls after their gravistimulation.
The effect of mannans on gravitropic bending (Figure S2) [8] (p. 687) had a different mechanism compared with the effect of sucrose. It was accompanied by a significant increase in the creep rate of heat-inactivated cell walls (Figure 5). This shows that the wall viscoelasticity was changed as a result of mannan deficiency in the csla2csla3csla9 triple mutant. One mechanism for this change may involve cellulose macrofibrils that became thicker and more transversally orientated in mannan-deficient cell walls (Figure 6 and Figure 7; Videos S1 and S2) in the zone of hypocotyls where their gravitropic bending normally develops. Mannans were reported to interact with the surface of cellulose microfibrils [24,33] and may affect cellulose organization [33]. Our findings (Figure 6 and Figure 7) could be explained by the more abundant lateral interactions between the adjacent cellulose microfibrils in the absence of mannans, resulting in the formation of thicker bundles (Figure 7, Video S2). Additionally, mannan deficiency may expose more sites for the specific S4B binding on the surface of cellulose macrofibrils, such that they look brighter. A number of indirect data suggest that cellulose microfibrils considerably reoriented in the course of gravitropic bending [34,35,36,37]. One may hypothesize that mannan depletion eliminates steric barriers for these microfibril reorientations, which facilitates the gravity response.
The second mechanism for the effect of mannans on gravitropic bending could involve cell wall structural glycoproteins. We demonstrated that gravistimulation of the triple mutant resulted in a decrease in the content of 4-hydroxyproline and a simultaneous increase in the content of aspartic acid (Table S2). Hydroxyproline is a hallmark amino acid residue of cell wall structural glycoproteins, including extensins, that form a rigid network in the cell wall, decreasing its extensibility through the formation of isodityrosine cross-links [38,39]. Aspartic acid is an essential biosynthetic precursor for tyrosine, another abundant amino acid residue in extensins [40]. Thus, gravistimulation of the triple mutant could modulate the metabolism of extensin proteins in Arabidopsis hypocotyl cell walls. A possible link between mannans and extensins is illustrated in Figure S3. It is based on the fact that ascorbate and mannans have GDP-D-mannose as a common biosynthetic precursor [41], while ascorbate is an essential cofactor in the posttranslational synthesis of hydroxyproline from proline which is catalyzed by prolyl 4-hydroxylase enzymes [41,42]. Thus, ascorbate and mannan biosynthesis pathways could compete for the common biosynthetic precursor.
The third potential mechanism by which mannans could affect gravitropic bending is also mediated by ascorbate, a potent scavenger of reactive oxygen species (ROS) [41]. ROS are important in the early stages of shoot and root gravitropism, where they presumably have a role in signaling [43,44,45,46]. Thus, their scavenging by ascorbate could affect gravitropism, while the level of ascorbate, in turn, could be modulated by changes in mannan biosynthesis (Figure S3).
Further studies are needed to understand the mechanism underpinning the effect of mannans on shoot gravitropism.
To sum up, mannans did not mediate the stimulatory effect of sucrose on shoot gravitropism. Mannans and sucrose influenced the gravitropic bending of Arabidopsis hypocotyls through distinct cell wall-related mechanisms. Sucrose exerted its effect via the cell wall-loosening proteins expansins, while the effect of mannans was based on changes in wall viscoelasticity. This highlights the complexity of shoot gravitropism control at the cell wall level.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Arabidopsis thaliana (L. Heynh.) wild-type Columbia-0 and csla2csla3csla9 mutant plants were grown on half-strength Murashige and Skoog (MS) medium (pH 5.7) (Duchefa Biochemie, Haarlem, the Netherlands) containing 0.68% (w/v) microagar (Duchefa Biochemie) and, where indicated, 1% (w/v) sucrose (Sigma-Aldrich, St. Louis, MO, USA).
Surface-sterilized seeds were sown aseptically on the above-mentioned medium on 145 mm × 20 mm round Petri plates (Greiner Bio-One, Mosonmagyaróvár, Hungary) for experiments with plants grown on horizontal plates or 120 mm × 120 mm × 17 mm square Petri plates (Greiner Bio-One) for experiments with plants grown on vertical plates. The seeds were stratified for 2 days at 4 °C, and their synchronous germination was induced via exposure to white fluorescent light (150 μmol m−2 s−1) for 6 h at 21 °C. The moment of transfer to light was taken as zero age for experimental plants. After the light induction period, the Petri plates were wrapped in two layers of thick aluminum foil, placed horizontally or vertically in a growth chamber (ShSV-132 P, Termocon, St. Petersburg, Russia) at 21 °C and kept there for 3 or 5 days. Gravitropism in experimental vertical Petri plates with three-day-old seedlings was induced via a 90° counterclockwise rotation of the plates (gravistimulation), and the seedlings were harvested for analysis 4 h and 6 h after their gravistimulation. Control vertical Petri plates with three-day-old seedlings were not rotated, and these nongravistimulated plants were harvested at the same age (76 h and 78 h) as their gravistimulated counterparts.
4.2. Hypocotyl Length and Curvature Determination
Arabidopsis seedlings were photographed with a digital camera (Canon SX510 HS, Zhuhai, China). Hypocotyl length and gravitropic bending were determined on the photos obtained using the ‘Segmented line’ and ‘Angle’ tools in ImageJ 1.49b software (NIH, Bethesda, MD, USA), respectively.
4.3. Cell Wall Biochemical Analyses
Whole five-day-old etiolated Arabidopsis seedlings with their seeds removed were used in biochemical analysis of cell walls as detailed in [8].
4.4. Extensometry
Arabidopsis seedlings for creep tests were placed individually into 1.5-mL Eppendorf test tubes, frozen by immersing the closed tubes into liquid nitrogen, stored at −20 °C and used for biomechanical analyses within 2 weeks after freezing. In vitro extension of frozen/thawed hypocotyls was measured with a custom-built constant-load extensometer [47,48]. A 5-mm-long basal segment (located 1.5 mm above the root neck) of a five-day-old hypocotyl or a 3-mm-long subapical segment (located 1.5 mm below the apical hook) of a 76(78)-hour-old hypocotyl was secured between clamps of an extensometer and preincubated in a buffer (20 mM sodium acetate, pH 5.0, or 20 mM MES-KOH, pH 6.0) in a relaxed state for 2 min. Then, its time-dependent extension (creep) was measured in the same buffer under a 600 mg load for 15 min. The relative creep rate was calculated as described in [12]. Before some creep tests, hypocotyl cell walls were heat-inactivated at 90 °C for 3 min as described in [17].
4.5. Spinning-Disc Confocal Microscopy
Cellulose macrofibrils were visualized in cell walls with Pontamine Fast Scarlet 4B (S4B) dye [20]. To improve the dye penetration into the cell walls of Arabidopsis hypocotyls, plants were extracted under mild conditions via sequential washes with EtOH:100% acetic acid (7:1, v/v) for 1 h; 100% EtOH for 15 min; 50% EtOH in H2O for 15 min; and 1M KOH in H2O for 30 min. All washing steps were carried out on a rotator. Experimental samples were stored in 1M KOH at 4 °C before analysis or used immediately after the last alkaline wash for cellulose visualization. The samples were rinsed in H2O before staining to remove residual KOH, after which 0.2% (w/v) S4B was added for 30 min. Then they were rapidly rinsed with a large volume of H2O to remove excess dye and observed under a Nikon Ti-E inverted confocal microscope equipped with a CSU-X1 spinning disc head (Yokogawa, Musashino, Japan), a 100× CFI Apo oil immersion TIRF objective (NA 1.49, Nikon, Tokyo, Japan), an evolve charge-coupled device camera (Photometrics Technology, Tucson, AZ, USA), and a 31.2× lens between the spinning disc and camera. S4B was excited using a 561 nm laser (similar to [20]). Image acquisitions were performed using Metamorph software (Molecular Devices, San Jose, CA, USA) version 7.5. Corresponding Z-stacks were visualized in Fiji 2.0.0-rc-69 (ImageJ 1.52p) as a series of subsequent optical sections using the ‘Make Montage’ function or exported as movies at a frequency of 7 frames per second.
4.6. Metabolomics
Arabidopsis hypocotyls were harvested from Petri plates (from 50 to 120 plants per sample, plants from one Petri plate were combined into one biological replicate), dissected from their roots, put into screw-cap 2 mL test tubes (SSI, Lodi, CA, USA) and immediately frozen in liquid nitrogen, then stored at −80 °C. Frozen hypocotyls were then ground at the temperature of liquid nitrogen in pre-cooled holders in a Retsch MM 400 ball vibration mill (Haan, Germany) at 27 Hz for 3 min with one 2 mm steel ball per test tube. Polar metabolites were then extracted in methanol with ribitol as an internal standard and dried to a thin film in a Labconco Centrivap vacuum centrifuge (Kansas City, MO, USA) at +4 °C and stored at −80 °C. Metabolites were then chemically derivatized in two steps (methoxyamination and silylation) [49]. Chromatography analysis was performed on a Leco Pegasus 4D GC × GC TOFMS equipped with a Gerstel autosampler on a Zebron ZB-5MS column (5% phenylmethylsiloxane, 30 m length, 250 μm internal diameter, 0.25 μm film thickness; Phenomenex, Torrance, CA, USA). Metabolite identification and their quantification was performed in MS-DIAL v. 4.8 software [50] using the Golm Metabolome database [51] and Fiehn BinBase [52]. Final matrices containing information on relative metabolite content were analyzed in Metaboanalyst [53].
4.7. Statistical Analysis
All data reported were based on two to three independent experiments, and the total number of biological replicates was indicated in the respective figures and tables. One biological replicate in different assays was as follows: about 120 seedlings grown on one Petri plate for hypocotyl posture determination; individual Arabidopsis seedlings grown on at least four different Petri plates for hypocotyl length, gravitropic bending and creep rate measurements; about 300 seedlings from one Petri plate with their seeds removed for cell wall biochemical analyses; and hypocotyls from 50–120 seedlings grown on one Petri plate for metabolomics. Means and SD(SE) values were calculated on the basis of the above biological replicates. Statistical differences between the means were revealed using Student’s t-tests, and the difference was considered as significant at p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13020209/s1, Figure S1: Sucrose increases the gravitropic bending of hypocotyls in Arabidopsis plants; Figure S2: Mannan-deficient csla2csla3csla9 triple mutants of Arabidopsis plants demonstrate increased gravitropic bending of hypocotyls; Figure S3: Biosynthesis pathways explain why the cell wall levels of mannans and extensins could be interdependent; Table S1: Relative content of metabolites in hypocotyls of gravistimulated and nongravistimulated wild-type Col-0 Arabidopsis seedlings, grown without sucrose (control) or in the presence of sucrose (1% w/v) in the growth medium; Table S2: Relative content of metabolites in hypocotyls of gravistimulated and nongravistimulated wild-type Col-0 and csla2csla3csla9 Arabidopsis seedlings; Video S1: Cellulose macrofibril arrangement from the innermost to the outermost layer of the outer epidermal cell wall from the subapical part of five-day-old Col-0 hypocotyls. Video S2: Cellulose macrofibril arrangement from the innermost to the outermost layer of the outer epidermal cell wall from the subapical part of five-day-old csla2csla3csla9 hypocotyls.
Author Contributions
Conceptualization, D.S.; methodology, G.P. and D.S.; validation, D.S. and G.P.; formal analysis, G.P. and D.S.; investigation, D.S. and G.P.; resources, D.S.; writing—original draft preparation, D.S.; writing—review and editing, G.P. and D.S.; visualization, D.S. and G.P.; supervision, D.S.; project administration, D.S.; funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-24-00379, Available online: https://rscf.ru/project/23-24-00379/ (accessed on 29 November 2023).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
D.S. and G.P. acknowledge the Research Park of Saint Petersburg State University: Center for Molecular and Cell Technologies, and Chromas Core Facility. We are very grateful to Staffan Persson (Copenhagen Plant Science Center, Department of Plant & Environmental Sciences, University of Copenhagen) for the supervision of cell wall biochemical analyses, spinning-disc confocal microscopy and the critical reading of the manuscript, and Paul Dupree (University of Cambridge) for the kind gift of csla2csla3csla9 seeds.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tsugawa, S.; Miyake, Y.; Okamoto, K.; Toyota, M.; Yagi, H.; Morita, M.T.; Hara-Nishimura, I.; Demura, T.; Ueda, H. Shoot gravitropism and organ straightening cooperate to arrive at a mechanically favorable shape in Arabidopsis. Sci. Rep. 2023, 13, 11165. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.T. Directional gravity sensing in gravitropism. Annu. Rev. Plant Biol. 2010, 61, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.; Blancaflor, E.B. Plant Gravitropism: From Mechanistic Insights into Plant Function on Earth to Plants Colonizing Other Worlds. Methods Mol. Biol. 2022, 2368, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z. Mechanisms of the early phases of plant gravitropism. Crit. Rev. Plant Sci. 2000, 19, 551–573. [Google Scholar] [CrossRef]
- Nishimura, T.; Mori, S.; Shikata, H.; Nakamura, M.; Hashiguchi, Y.; Abe, Y.; Hagihara, T.; Yoshikawa, H.Y.; Toyota, M.; Higaki, T.; et al. Cell polarity linked to gravity sensing is generated by LZY translocation from statoliths to the plasma membrane. Science 2023, 381, 1006–1010. [Google Scholar] [CrossRef]
- Furutani, M.; Hirano, Y.; Nishimura, T.; Nakamura, M.; Taniguchi, M.; Suzuki, K.; Oshida, R.; Kondo, C.; Sun, S.; Kato, K.; et al. Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun. 2020, 11, 76. [Google Scholar] [CrossRef]
- Ikushima, T.; Soga, K.; Hoson, T.; Shimmen, T. Role of xyloglucan in gravitropic bending of azuki bean epicotyl. Physiol. Plant. 2008, 132, 552–565. [Google Scholar] [CrossRef]
- Somssich, M.; Vandenbussche, F.; Ivakov, A.; Funke, N.; Ruprecht, C.; Vissenberg, K.; Van Der Straeten, D.; Persson, S.; Suslov, D. Brassinosteroids Influence Arabidopsis Hypocotyl Graviresponses through Changes in Mannans and Cellulose. Plant Cell Physiol. 2021, 62, 678–692. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Guo, X.; Gallemi, M.; Aryal, B.; Venhuizen, P.; Barbez, E.; Dünser, K.A.; Darino, M.; Pĕnčík, A.; Novák, O.; et al. Xyloglucan Remodeling Defines Auxin-Dependent Differential Tissue Expansion in Plants. Int. J. Mol. Sci. 2021, 22, 9222. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Guisinger, M.M.; Miller, A.J.; Stackhouse, K.S. Reduced Gravitropism in Hypocotyls of Starch-Deficient Mutants of Arabidopsis. Plant Cell Physiol. 1997, 38, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Suslov, D.; De Grauwe, L.; Leroux, O.; Vissenberg, K.; Van der Straeten, D. The role of brassinosteroids in shoot gravitropism. Plant Physiol. 2011, 156, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Ivakov, A.; Flis, A.; Apelt, F.; Fünfgeld, M.; Scherer, U.; Stitt, M.; Kragler, F.; Vissenberg, K.; Persson, S.; Suslov, D. Cellulose synthesis and cell expansion are regulated by different mechanisms in growing Arabidopsis hypocotyls. Plant Cell 2017, 29, 1305–1315. [Google Scholar] [CrossRef]
- Goubet, F.; Barton, C.J.; Mortimer, J.C.; Yu, X.; Zhang, Z.; Miles, G.P.; Richens, J.; Liepman, A.H.; Seffen, K.; Dupree, P. Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J. 2009, 60, 527–538. [Google Scholar] [CrossRef] [PubMed]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar] [CrossRef]
- Maris, A.; Kaewthai, N.; Eklöf, J.M.; Miller, J.G.; Brumer, H.; Fry, S.C.; Verbelen, J.P.; Vissenberg, K. Differences in enzymic properties of five recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 261–271. [Google Scholar] [CrossRef]
- Boron, A.K.; Van Loock, B.; Suslov, D.; Markakis, M.N.; Verbelen, J.P.; Vissenberg, K. Over-expression of AtEXLA2 alters etiolated arabidopsis hypocotyl growth. Ann. Bot. 2015, 115, 67–80. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Wang, X.; Durachko, D.M.; Zhang, S.; Cosgrove, D.J. Molecular insights into the complex mechanics of plant epidermal cell walls. Science 2021, 372, 706–711. [Google Scholar] [CrossRef]
- Anderson, C.T.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef]
- Shah, D.U.; Reynolds, T.P.S.; Ramage, M.H. The strength of plants: Theory and experimental methods to measure the mechanical properties of stems. J. Exp. Bot. 2017, 68, 4497–4516. [Google Scholar] [CrossRef]
- Ray, P.M.; Green, P.B.; Cleland, R. Role of turgor in plant cell growth. Nature 1972, 239, 163–164. [Google Scholar] [CrossRef]
- Stewart, J.L.; Maloof, J.N.; Nemhauser, J.L. PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS ONE 2011, 6, e19894. [Google Scholar] [CrossRef]
- Yu, L.; Yoshimi, Y.; Cresswell, R.; Wightman, R.; Lyczakowski, J.J.; Wilson, L.F.L.; Ishida, K.; Stott, K.; Yu, X.; Charalambous, S.; et al. Eudicot primary cell wall glucomannan is related in synthesis, structure, and function to xyloglucan. Plant Cell 2022, 34, 4600–4622. [Google Scholar] [CrossRef]
- Yu, L.; Shi, D.; Li, J.; Kong, Y.; Yu, Y.; Chai, G.; Hu, R.; Wang, J.; Hahn, M.G.; Zhou, G. CELLULOSE SYNTHASE-LIKE A2, a glucomannan synthase, is involved in maintaining adherent mucilage structure in Arabidopsis seed. Plant Physiol. 2014, 164, 1842–1856. [Google Scholar] [CrossRef]
- Jonsson, K.; Ma, Y.; Routier-Kierzkowska, A.L.; Bhalerao, R.P. Multiple mechanisms behind plant bending. Nat. Plants 2023, 9, 13–21. [Google Scholar] [CrossRef]
- Edelmann, H.G. Gravistimulated asymmetries in the outer epidermal cell walls of graviresponding coleoptiles. Planta 1997, 203 (Suppl. S1), S123–S129. [Google Scholar] [CrossRef]
- Bagshaw, S.L.; Cleland, R.E. Wall extensibility and gravitropic curvature of sunflower hypocotyls: Correlation between timing of curvature and changes in extensibility. Plant Cell Environ. 1990, 13, 85–89. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Rapid, bilateral changes in growth rate and curvature during gravitropism of cucumber hypocotyls: Implications for mechanism of growth control. Plant Cell Environ. 1990, 13, 227–234. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Arsuffi, G.; Braybrook, S.A. Acid growth: An ongoing trip. J. Exp. Bot. 2018, 69, 137–146. [Google Scholar] [CrossRef]
- Fendrych, M.; Leung, J.; Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. Elife 2016, 5, e19048. [Google Scholar] [CrossRef]
- Yu, L.; Lyczakowski, J.J.; Pereira, C.S.; Kotake, T.; Yu, X.; Li, A.; Mogelsvang, S.; Skaf, M.S.; Dupree, P. The Patterned Structure of Galactoglucomannan Suggests It May Bind to Cellulose in Seed Mucilage. Plant Physiol. 2018, 178, 1011–1026. [Google Scholar] [CrossRef]
- Blancaflor, E.B.; Hasenstein, K.H. Time course and auxin sensitivity of cortical microtubule reorientation in maize roots. Protoplasma 1995, 185, 72–82. [Google Scholar] [CrossRef]
- Himmelspach, R.; Wymer, C.L.; Lloyd, C.W.; Nick, P. Gravity-induced reorientation of cortical microtubules observed in vivo. Plant J. 1999, 18, 449–453. [Google Scholar] [CrossRef]
- Zhang, Z.; Friedman, H.; Meir, S.; Rosenberger, I.; Halevy, A.H.; Philosoph-Hadas, S. Microtubule reorientation in shoots precedes bending during the gravitropic response of cut snapdragon spikes. J. Plant Physiol. 2008, 165, 289–296. [Google Scholar] [CrossRef]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef]
- Liu, X.; Wolfe, R.; Welch, L.R.; Domozych, D.S.; Popper, Z.A.; Showalter, A.M. Bioinformatic Identification and Analysis of Extensins in the Plant Kingdom. PLoS ONE 2016, 11, e0150177. [Google Scholar] [CrossRef]
- Fry, S.C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem. J. 1982, 204, 449–455. [Google Scholar] [CrossRef]
- De la Torre, F.; Cañas, R.A.; Pascual, M.B.; Avila, C.; Cánovas, F.M. Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J. Exp. Bot. 2014, 65, 5527–5534. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Ricardi, M.M.; Poulsen, C.P.; Oikawa, A.; Dilokpimol, A.; Halim, A.; Mangano, S.; Denita Juarez, S.P.; Marzol, E.; Salgado Salter, J.D.; et al. Complex regulation of prolyl-4-hydroxylases impacts root hair expansion. Mol. Plant. 2015, 8, 734–746. [Google Scholar] [CrossRef]
- Clore, A.M.; Doore, S.M.; Tinnirello, S.M. Increased levels of reactive oxygen species and expression of a cytoplasmic aconitase/iron regulatory protein 1 homolog during the early response of maize pulvini to gravistimulation. Plant Cell Environ. 2008, 31, 144–158. [Google Scholar] [CrossRef]
- Krieger, G.; Shkolnik, D.; Miller, G.; Fromm, H. Reactive Oxygen Species Tune Root Tropic Responses. Plant Physiol. 2016, 172, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.L.; Mukherjee, A.; Kar, R.K. Early axis growth during seed germination is gravitropic and mediated by ROS and calcium. J. Plant Physiol. 2017, 216, 181–187. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, H.; Yang, T.; Lian, Y.; Sun, Y.; Bian, Z.; Wang, C. Exogenous hydrogen peroxide inhibits primary root gravitropism by regulating auxin distribution during Arabidopsis seed germination. Plant Physiol. Biochem. 2018, 128, 126–133. [Google Scholar] [CrossRef]
- Suslov, D.; Verbelen, J.P. Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls. J. Exp. Bot. 2006, 57, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Suslov, D.; Vissenberg, K. Cell wall expansion as viewed by the creep method. In Plant Biomechanics: From Structure to Function at Multiple Scales; Geitmann, A., Gril, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 305–320. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef]
- Hummel, J.; Strehmel, N.; Bölling, C.; Schmidt, S.; Walther, D.; Kopka, J. Mass Spectral Search and Analysis Using the Golm Metabolome Database. In The Handbook of Plant Metabolomics; Weckwerth, W., Kahl, G., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 321–343. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).