Abstract
The effects of different types of biostimulants on crops include improving the visual quality of the final products, stimulating the immune systems of plants, inducing the biosynthesis of plant defensive biomolecules, removing heavy metals from contaminated soil, improving crop performance, reducing leaching, improving root development and seed germination, inducing tolerance to abiotic and biotic stressors, promoting crop establishment and increasing nutrient-use efficiency. Protein hydrolysates are mixtures of polypeptides and free amino acids resulting from enzymatic and chemical hydrolysis of agro-industrial protein by-products obtained from animal or plant origins, and they are able to alleviate environmental stress effects, improve growth, and promote crop productivity. Amino acids involve various advantages such as increased yield and yield components, increased nutrient assimilation and stress tolerance, and improved yield components and quality characteristics. They are generally achieved through chemical or enzymatic protein hydrolysis, with significant capabilities to influence the synthesis and activity of some enzymes, gene expression, and redox-homeostasis. Increased yield, yield components, and crop quality; improved and regulated oxidation-reduction process, photosynthesis, and physiological activities; decreased negative effects of toxic components; and improved anti-fungal activities of plants are just some of the more important benefits of the application of phenols and phenolic biostimulants. The aim of this manuscript is to survey the impacts of amino acids, different types of protein hydrolysates, phenols, and phenolic biostimulants on different plants by presenting case studies and successful paradigms in several horticultural and agricultural crops.
1. Introduction
Biostimulants are considered bioactive substances that are either inorganic or organic microorganisms that can increase crop performance when utilized in small quantities [1] as they can enhance both performance and growth as well as improve nutrient- and water-use efficiencies of different crops [2,3,4,5,6,7,8]. Amino acids have a dual function as building blocks for proteins and as providers of organic nitrogen, which can alleviate the negative impacts of drought and salt stress [9], and promote cell growth. They are vital in metabolite synthesis, growth, and development, and appropriate in plants because of their structure as protein units [10,11,12,13,14]. The positive effects of the foliar application of amino acids and biostimulants based on amino acids on both the qualitative and quantitative characteristics of Foeniculum vulgare Mill, Coriandrum sativum L., Achillea millefolium L., Nigella sativa L., Ocimum basilicum L., Urtica pilulifera L., Mentha piperita, Calendula officinalis L., and Satureja hortensis L. plants have been reported [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
Amino acids used for the production of biostimulants are obtained from the chemical synthesis of plant proteins, such as algae, soybean, and corn, as well as from animal proteins by both chemical and enzymatic hydrolysis. Amino acids that have been used for foliar usage are the result of enzymatic hydrolysis from both animal and plant protein hydrolysates, and as it is very energy-consuming, foliar application is a common process in the agricultural industry. Protein hydrolysate is related to the product of the hydrolytic action of protease(s) on a pure protein sample, or a complicated proteinaceous sample [26,27], which is necessarily a mixture of peptides, free amino acids, and probably partially degraded proteins [28,29]. Protein hydrolysates and amino acids, which are also known as protein-based biostimulants, are usually readily available because of the abundance of raw materials and their affordable cost [30,31,32]. Protein-based biostimulants can usually be obtained from the hydrolysis of protein-rich agro-wastes, which includes chemical, thermal, and enzymatic processes, or a combination of them [33,34,35,36]. They are usually considered as a crude peptide mixture, and they are usually used as the initial raw material for bioactivity testing [37,38,39]. Fish protein hydrolysates are famous in different parts of the world for pharmaceutical, cosmetic, and nutritional usage [40,41,42].
Several studies have reported that biostimulants promote plant resilience, especially by improving antioxidant activity within the plant under negative environmental conditions [43,44]. It could behave directly on the plant through an adjustment of the nitrogen and carbon metabolisms and the plant hormonal profile, or indirectly through the microbiome [45]. Food-derived bioactive proteins have physiological impacts on major body systems, such as opioid agonists and opioid antagonists on the nervous system; anti-hyperlipidemic, anti-thrombotic, anti-oxidative, and anti-hypertensive effects on the cardiovascular system; cytomodulatory, immunomodulatory, and anti-microbial effects on the immune system; and mineral binding, anti-appetizing, and anti-microbial impacts on the gastrointestinal system [46,47,48,49,50]. Phenols have notable roles in plant development and growth [51,52,53], as they are products of secondary metabolic procedures and are generally converted from sugars via the pentose phosphate pathway, the manganiferous acid pathway, the glycolytic pathway, or the benzene-propane pathway [54,55,56]. Phenolic acids include a carboxylic acid group in addition to the basic phenolic structure and are categorized into hydroxybenzoic and hydroxycinnamic acids. Phenolic acids can be utilized to grow crops by soil and foliar application as well as seed treatment, but foliar utilization of phenolic acids is usually suggested. This research examines the scientific literature on biostimulants from 1990 to October 2022 by conducting a bibliometric analysis of the literature published on the Web of Science database, including more than one thousand articles. The goal of this review article is to survey the effects of different biostimulants, such as amino acids, protein hydrolysates, and phenols, by presenting case studies and successful paradigms in different agricultural and horticultural crops. The information provided is obtained from randomized control experiments, review articles, and analytical observations and studies that have been gathered from various literature sources such as PubMed, Science Direct, Scopus, and Google Scholar. The keywords used were the Latin and common names of different agricultural and horticultural species, amino acids, protein hydrolysates, phenols, phenolic biostimulants, and medicinal plants.
2. Amino Acids
Amino acids for the production of biostimulants are derived by chemical synthesis from plant proteins such as soybean, corn, algae, corn, etc., as well as from animal proteins by enzymatic and chemical hydrolysis [57,58,59,60,61,62]. Amino acids act as vital molecules with various physiological roles [63] and play an important function in seed germination [64,65], and under salinity stress, they can behave as osmolytes, which can promote stomatal opening control, transport regulation, enzyme activation, heavy metals detoxification, redox homeostasis maintenance, and gene expression [66,67,68,69,70]. Supplementing plants with environmentally friendly amino acid biostimulants can decrease the application of inorganic fertilizers [71,72].
Amino acids are also important in the agriculture industry as chelates of metal ions and microelements chelated with amino acids from very small, electrically neutral molecules increase their transport and absorption within the plant [73,74,75]. Some of the most important products in the market which contain amino acids are Delfan Plus (Tradecorp, Madrid, Spain), Natural Crop SL (Natural Crop Poland Sp. Z o.o., Warsaw, Poland), Bosfoliar Activ (COMPO EXPERT, Munster, Germany), Amino Quelant Ca (Bioiberica, Barcelona, Spain), Tecamin Max, Tecamin Brix, Tecnokel Amino Mix, Terra-Sorb Foliar (Agritecno Fertilizants, Valencia, Spain), Agrocean B (Agrimer, Plouguerneau, France), Metalosate Calcium and Metalosate Fe (Albion Minerals, Layton, UT, USA) [76,77,78,79]. The usage of amino acids can increase co-enzyme formation and the photosynthesis procedure [80], and supports different plant organisms that may face environmental stresses [81]. It has been also reported that the exogenous utilization of amino acids can enhance nitrogen status, and the contents of mineral elements in plant tissues [82,83]. Depending on environmental conditions and plant species, plants reduce inorganic nitrogen to amino acids in roots, nodules, and leaves [84,85,86]. Many studies have reported the important and notable effects of the foliar application of concentrations with phenylalanine and tyrosine solutions on essential oil, the total amount of phenols, and their compositions in Ocimum basilicum L., Melissa officinalis L., and Coleus blumei L. plants [87,88,89]. Phenylalanine is an amino acid [90,91], and its foliar application can help mustard (Brassica campestris L.) plants overcome drought stress and increase total chlorophyll contents, shoot length, and biological yield [92]. Roman et al. [93] reported that foliar application of methyl jasmonate and phenylalanine can increase the content of volatile compounds in grapes, and Portu et al. [94] introduced it as an important management tool for boosting grape quality. The impacts of different amino acids on several experimental plants are shown in Table 1. The roles of different amino acids as biostimulants are shown in Table 2. The main mechanisms of amino acids biostimulants are shown in Figure 1.

Table 1.
The effects of amino acids on different plants.

Table 2.
The roles of different amino acids as biostimulants.
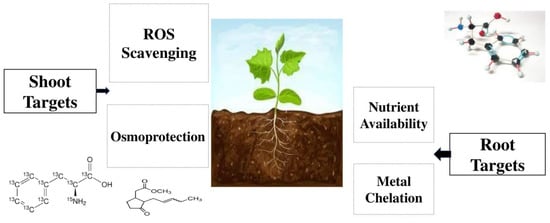
Figure 1.
The most important mechanisms of amino acids biostimulants.
3. Protein Hydrolysates
Protein hydrolysates, especially those that contain antioxidant peptides, are obtained from natural components, and many researchers and scholars consider them biostimulants because of their minimum side effects, easy absorption, low cost, high activity, and lower molecular weight [123,124,125,126,127,128,129,130]. Protein hydrolysates and peptides can be used as notable ingredients in the formulation of functional foods [131,132,133,134,135,136,137,138,139,140,141]. They can be used as foliar sprays or through drip irrigation systems, and the amino acids can be absorbed through both leaves and roots [142,143,144]. Their utilization can significantly affect nitrogen metabolism in plants, and boost productivity, particularly when applied as a seed pre-treatment [144]. For separating the amino acids in protein hydrolysates, a liquid chromatography process can be used [145,146]. Numerous methods have been considered to produce hydrolysates from fish and fish by-products such as thermal hydrolysis, autolysis, chemical hydrolysis, and enzymatic hydrolysis [146,147]. The basic procedures utilized following hydrolysis of protein are heat inactivation, which has a function in the inactivation of proteolytic enzymes; ultrafiltration, which is important in the removal of high molecular weight peptides and proteins; use of specific enzymes, which can reduce the content of specific amino acids; hydrolysis by exoproteases, which is active in hydrolysis and the reduction of bitterness; carob activation, which has a notable role in the reduction of bitterness; and absorption chromatography, which can decrease the content of aromatic amino acids. Microbial-based biostimulants such as Environoc 401®, Bioyield®, Rootshield Plus+ WP ®, Spectrum + Myco®, Select®, and Endomaxx® inconsistently increased the quality of bell pepper (Capsicum annuum L.) in a greenhouse experiment [148]. Ghorbel-Bellaaj et al. [149] reported that five proteolytic enzymes, namely Alcalase®, trypsin, a crude enzyme extract from sardinelle (Sardinella aurita) viscera, and an enzyme preparation from Aspergillus clavatus ESA and Bacillus licheniformis NH1, which are protein hydrolysates, were obtained from shrimp via by-products processing, and they have revealed notable degrees of antioxidant activities, such as β-carotene bleaching, reducing power, and 1,1-diphenyl-2-picrylhydrazyl (DPPH)-scavenging activity assays, which can be a promising and helpful alternative for accessible commercial nitrogen sources from other origins. It can be a good source for microbial growth and protease production by Saccharomyces cerevisiae, Escherichia coli, Bacillus subtilis A26, and Bacillus mojavensis A21.
Some of the available plant biostimulants, their composition, and application strategies are C Fish, which contain peptides and amino acids that are used on vegetables and fruits to increase the plant’s resistance to insect pressure, disease and drought or heat stress which originates from white fish/mixed fish composition autolysates and hydrolysates in fruits and vegetables; Radifarm, which contains peptides, amino acids, betaines, saponins, vitamins, polysaccharides, and microelements, has been used to promote the formation of an extensive root system by speeding up the elongation of adventitious and lateral roots of vegetables and fruits; Megafol, which contains betaines, amino acids, auxin, vitamins, proteins, cytokine, and gibberellin, can improve the balance between vegetative productivity and development as well as plant resistance to stressors such as hail, weeding, root asphyxia, and frost; Biozyme, which includes plant hormones, algae extract, and chelated micronutrients, can boost nutrient uptake, photosynthesis, and the activity of chlorophyll of legumes, vegetables and fruits; BioRoot, which contains humates, plant and mineral-derived organic acids, enhances rooting ability, protein content, and chlorophyll of fruits and vegetables; Grow-plex SP, which contain humic acids, can increase soil bacteria, shoot and root growth, and zinc and iron uptake of vegetables and fruits; Ergonfil, which has cysteine, animal protein hydrolysates, keratin derivatives, and folic acid, can promote chlorophyll synthesis and indole acetic acid, increase chelation, and improve translocation in fruits and vegetables; Benefit, which contains nucleotides, amino acids, vitamins, free enzymatic proteins, can improve cell division and increase the number of cells per fruit [150,151,152,153]. Animal-derived gelatin, which has peptides and amino acids, can improve shoot dry weight and promote root nitrogen assimilation in broccoli, arugula, tomato, pepper, cucumber, and field corn [154]. There are notable reports and evidence that the application of non-structural and structural amino acids, such as histidine, proline, taurine, and glutamate, can provide protection to the plant from environmental stresses or play an important function in metabolic signaling by regulating nitrogen acquisition by the roots [155,156]. Amino acids can act as osmoprotectants, which stabilize membranes, enzymes, and proteins against denaturing caused by high salt components and non-physiological temperatures [157]; moreover, arginine has been proven to have an important function in nitrogen transport and storage in plants during biotic and abiotic stress conditions [158]. Amino acids can also reduce plant toxicity by heavy metals by acting as metal chelators [159,160]. Rouphael et al. [161] reported that the application of vegetal-protein hydrolysates based microgranules can increase carotenoids and total chlorophyll content. Protein hydrolysate has a positive influence on total root area and on root length, which can increase mineral-nutrient and water-use efficiency as well as promote plant productivity and resistance to harmful conditions [162,163,164]. It can also positively influence the leaf area and yield of horticultural plants and fruit trees [165,166]. The exogenous utilization of protein hydrolysate and isolated amino acids can promote plant antioxidant performance by improving the non-enzymatic and enzymatic antioxidant defense machinery of the cell [167]. The most important effects of different kinds of protein hydrolysates have been shown in Table 3.

Table 3.
The impacts of different protein hydrolysates on various plants.
4. Phenols and Phenolic Biostimulants
Phenols are a major type of antioxidant phytochemical, which have significant importance because of their free radical scavenging and biological characteristics [233,234,235,236]. Phenolic compounds are the most abundant secondary metabolites in many plants which are usually discovered in the cell walls of subepidermal and in the vacuoles of epidermal cells [237,238]. Endogenous phenolic components in plants have different functions, which can be used by plants to defend themselves against pathogens, herbivores, and weeds. They are implicated in seed germination and dormancy, appropriate as screens against damaging UV radiation, and act as pigments to attract seed dispersal agents and pollinators [239,240,241]. The function of phenolic acids as signaling molecules in plant-microbe symbioses has been reported in previous research [242]. Some of the most important phenolic compounds with bioprotectant activities are ferulic acid, curcumin, ellagic acid, catechol, gallic acid, coumarin, caffeic acid, catechin, quercetin, sinapic acid, rutin, resveratrol, salicylic acid, and syringic acid [243,244]. The accumulation of phenolic compounds and the subsequent production of quinones in turnip (Brassica rapa L.) may happen when plants are susceptible to Boron deficiency [245]. Phenolic compound concentration can be important in the biochemical pathway of toxigenic fungal species because of the induction of stress via sub-lethal contents and depletion of the phenolic compounds [246]. Phenolics have meaningful functions in plant development, especially in pigment and lignin biosynthesis as well as considerable roles in plant protection against stress. It has been reported the correlation between antifungal activity and total phenolics of plants [247] and the accumulation of amino acids and phenolics may boost tolerance to both copper and cobalt stress in barley [248]. Silva et al. [249] reported that tyrosol, which is a phenolic compound from olive oil and several endophytic fungi such as Phomopsis sp., can be used as an important biostimulant in soybean seed treatment, which can alter soybean plant metabolism without meaningful impacts on crop yield. Masondo et al. [250] reported that two phenolic biostimulants, namely eckol and phloroglucinol, isolated from brown algae Ecklonia maxima can have a significant effect on the phytochemical and growth of Eucomis autumnalis. While the phenolic acid metabolism in Kandelia obovata may decrease the negative impacts of cadmium and zinc [251], it has been reported that the phenolic compounds of leave extracts of Calligonum arich L. are effectual against pathogenic bacteria [252], and the phenolic compounds of apricot branches have shown antifungal activity against Monilinia laxa growth [253,254,255]. One of the notable impacts of phenolics is to improve the resistance of Nicotiana langsdorffii to Cr(VI) [256,257,258,259].
5. Conclusions and Future Prospects
The innovative agronomic tools of agriculture are biostimulants, which are composed of inorganic and organic substances that consist of several microorganisms and substances. Biostimulants can do various agronomic functions such as increasing the growth and development of plants during their entire life cycle; promoting the resistance of plants to abiotic stresses such as cold, heat, and lack of water; improving soil fertility, especially increasing the development of soil microorganisms; promoting the use efficiency of nutrients by plants; and finally, increasing yield and crop quality. They can also be used as the best alternative for chemical fertilizers and are the best strategy for promoting organic agriculture. Amino acids are appropriate candidates to boost stress tolerance through metal chelation, nutrient availability, osmo-protection, and reactive oxygen species (ROS), which can notably affect the synthesis and stimulation of gene expression and some enzymes. Amino acids are organic components, which contain amine and carboxyl C(=O)OH) functional groups together with a side chain (R group). They can promote and stimulate the process of protein synthesis and photosynthesis; promote nutrient assimilation, translocation, and utilization; and strengthen plant growth and yield formation. Protein hydrolysates are manufactured from plant-derived protein sources using partial thermal hydrolysis, chemical hydrolysis, and enzymatic hydrolysis. Different sources of protein hydrolysates on the basis of protein sources are animal origin, leather by-products, blood meal, fish by-products, chicken feathers, casein, plant origin, legume seeds, alfalfa hay, and vegetable by-products. The positive impacts of the utilization of amino acids have been discovered; however, there is not enough knowledge about the effects of each amino acid on both the physiological and metabolic processes of plants. A better understanding of biostimulants, such as amino acids, protein hydrolysates, phenols, and phenolic biostimulants, while considering their various effects on different functions of crops, namely crop yield and yield components, growth promotion, and nutrient availability, may help agricultural scientists and farmers to better understanding and utilization of them.
Author Contributions
W.S., writing-original draft preparation; M.H.S., writing-original draft preparation and editing; Y.K., writing-original draft preparation; N.W., writing-original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China (Grant No. 2019YFA0904700). This research was also funded by the Natural Science Foundation of Beijing, China (Grant No. M21026).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- La Bella, S.; Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Sabatino, L. Impact of Ecklonia maxima seaweed extract and Mo foliar treatments on biofrotification, spinach yield, quality and NUE. Plants 2021, 10, 1139. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Ngcobo, B.L.; Manyevere, A.; Bertling, I.; Fawole, O.A. Assessing the usefulness of Moringa oleifera leaf extract as a biostimulant to supplement synthetic fertilizers: A review. Plants 2022, 11, 2214. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Lin, M. Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation 2022, 8, 688. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants-natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing sustainable agriculture systems in medicinal and aromatic plant production by using chitosan and chitin-based biostimulants. Plants 2023, 12, 2469. [Google Scholar] [CrossRef]
- Rodriguez-Morgado, B.; Gomez, I.; Parrado, J.; Garcia-Martinez, A.M.; Aragon, C.; Tejada, M. Obtaining edaphic biostimulants/biofertilizers from different sewage sludges. Effects on soil biological properties. Environ. Technol. 2015, 36, 2217–2226. [Google Scholar] [CrossRef]
- Orts, A.; Tejada, M.; Parrado, J.; Paneque, P.; Garcia, C.; Hernandez, T.; Gomez-Parrales, I. Production of biostimulants from okara through enzymatic hydrolysis and fermentation with Bacillus licheniformis: Comparative effect on soil biological properties. Environ. Technol. 2019, 40, 2073–2084. [Google Scholar] [CrossRef]
- Abdelkader, M.M.; Gaplaev, M.S.; Terekbaev, A.A.; Puchkov, M.Y. The influence of biostimulants on tomato plants cultivated under hydroponic systems. J. Hortic. Res. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Various techniques for molecular and rapid detection of infectious and epidemic diseases. Lett. Org. Chem. 2023, 20, 779–801. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Survey on multi-omics, and multi-omics data analysis, integration and application. Curr. Pharm. Anal. 2023, 19, 267–281. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the influences of microbial biostimulants on horticultural crops: Case studies and successful paradigms. Horticulturae 2023, 9, 193. [Google Scholar] [CrossRef]
- Aghaye Noroozlo, Y.; Souri, M.K.; Delshad, M. Stimulation effects of foliar application of glycine and glutamine amino acids on growth and quality of sweet basil. Adv. Hortic. Sci. 2019, 33, 495–501. [Google Scholar] [CrossRef]
- Ayyat, A.M.; Kenawy, A.G.M.; Aboel-Ainin, M.A.; Abdel-Mola, M.A.M. Improving growth, productivity and oil yield of Nigella sativa L. Plants by foliar spraying with some stimulants. J. Plant Prod. 2021, 12, 339–344. [Google Scholar] [CrossRef]
- Elsayed, A.A.A.; El-Gohary, A.E.; Khalid, K.A.; Ahmed, A.M.A. Changes in bitter fennel essential oils exposed to foliar spray with L-phenylalanine. Egypt. J. Bot. 2022, 62, 241–253. [Google Scholar] [CrossRef]
- Rafiee, H.; Mehrafarin, A.; Qaderi, A.; Jari, S.K.; Badi, H.N. Phytochemical, agronomical and morphological response of pot marigold (Calendula officinalis L.) to foliar application of biostimulators (Bioactive amino acid compounds). J. Med. Plants 2013, 12, 48–61. [Google Scholar]
- Mehrabi, S.; Mehrafarin, A.; Badi, H.N. Clarifying the role of methanol and amino acids application on savory (Satureja hortensis L. ). Ann. Biol. Res. 2013, 4, 190–195. [Google Scholar]
- Hendawy, S.F.; Hussein, M.S.; El-Gohary, A.E.; Ibrahim, M.E. Effect of foliar organic fertilization on the growth, yield and oil content of Mentha piperita var. citrata. Asian J. Agric. Res. 2015, 9, 237–248. [Google Scholar] [CrossRef]
- Shafie, F.; Bayat, H.; Aminifard, M.H.; Saeid Daghighi, S. Biostimulant effects of seaweed extract and amino acids on growth, antioxidants, and nutrient content of Yarrow (Achillea millefolium L.) in the field and greenhouse conditions. Commun. Soil Sci. Plant Anal. 2021, 52, 964–975. [Google Scholar] [CrossRef]
- Wafaa, H.A.A.A.; Rania, M.R.K.; El-Shafay, R.M.M. Effect of spraying with extracts of plants and amino acids on growth and productivity on Coriandrum sativum plants under Shalateen condition. Plant Arch. 2021, 21, 300–307. [Google Scholar] [CrossRef]
- Wahba, H.E.; Motawe, H.M.; Ibrahim, A.Y. Growth and chemical of Urtica pilulifera L. plant as influenced by foliar application of some amino acids. J. Mater. Environ. Sci. 2015, 6, 499–506. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Shen, H.; Cheng, Q. Chinese herbal medicine for SARS and SARS-CoV-2 treatment and prevention, encouraging using herbal medicine for COVID-19 outbreak. Acta Agric. Scand. Sec. B. Soil Plant Sci. 2020, 70, 437–443. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Traditional herbal medicine for the prevention and treatment of cold and flu in the autumn of 2020, overlapped with COVID-19. Nat. Prod. Commun. 2020, 15, 1934578X20951431. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the degree of hydrolysis on functional properties and antioxidant activity of enzymatic soybean protein hydrolysates. Molecules 2022, 27, 6110. [Google Scholar] [CrossRef] [PubMed]
- Peslerbes, M.; Fellenberg, A.; Jardin, J.; Deglaire, A.; Ibanez, R.A. Manufacture of whey protein hydrolysates using plant enzymes: Effect of processing conditions and simulated gastrointestinal digestion on angiotensin-I-converting enzyme (ACE) inhibitory activity. Foods 2022, 11, 2429. [Google Scholar] [CrossRef]
- Cosovanu, D.; Acosta, A.M.; Lopez, P.C.; Gernaey, K.V.; Li, Q.; Lametsch, R.; Canela-Garayoa, R.; Eras, J.; Villorbina, G. Rendered-protein hydrolysates as a low-cost nitrogen source for the fungal biotransformation of 5-hydroxymethylfurfural. Catalysts 2022, 12, 839. [Google Scholar] [CrossRef]
- Aluko, R.E. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier Inc.: Cambridge, UK, 2015; pp. 105–140. [Google Scholar]
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with seaweed extract and microbial-based commercial biostimulants influences seed germination of five Abelmoschus esculentus genotypes. Plants 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arriogoni, G.; Masi, A. Quantitative proteomics of maize roots treated with a protein hydrolysate: A comparative study with transcriptomics highlights the molecular mechanisms responsive to biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- Baqer, H.A.A.-R.; Zeboon, N.; Al-Behadili, A. The tole and importance of amino acids within plants: A review. Plant Arch. 2019, 19, 1402–1410. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.-T.; Ee, K.-Y.; Kumar, D.T.; Manan, F.A.; Wong, F.-C. Plant bioactive peptides: Current status and prospects towards use on human health. Protein Pept. Lett. 2021, 28, 623–642. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Ryu, B.; Shin, K.-H.; Kim, S.-K. Muscle protein hydrolysates and amino acid composition in fish. Mar. Drugs 2021, 19, 377. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Sierra Lopera, L.M.; Sepulveda Rincon, C.T.; Vasquez Mazo, P.; Figueroa Moreno, O.A.; Zapata Montoya, J.E. Byproducts of aquaculture processes: Development and prospective uses. Review. Vitae 2018, 25, 128–140. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Gonzalez-Morales, S.; Solis-Gaona, S.; Valdes-Caballero, M.V.; Juarez-Maldonado, A.; Loredo-Trevino, A.; Benavides-Mendoza, A. Transcriptomics of biostimulation of plants under abiotic stress. Front. Genet. 2021, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Bazsefidar, N.; Yazdi, A.P.G.; Karimi, A.; Yahyavi, M.; Amini, M.; Gavlighi, H.A.; Simal-Gandara, J. Brewers spent grain protein hydrolysate as a functional ingredient for muffins: Antioxidant, antidiabetic, and sensory evaluation. Food Chem. 2024, 435, 137565. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.L.; O’Callaghan, Y.C.; O’Brien, N.M. Protein hydrolysates from agricultural crops-bioactivity and potential for functional food development. Agriculture 2013, 3, 112–130. [Google Scholar] [CrossRef]
- Kong, Y.; Toh, N.P.; Wu, Y.; Huang, D. Trypsin-treated chickpea protein hydrolysate enhances the cytoaffinity of microbeads for cultures meat application. Food Res. Int. 2023, 173, 113299. [Google Scholar] [CrossRef]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Thimmanagari, M.; Dutta, A. Exploration of corn distillers solubles from selective milling technology as a novel source of plant-based ACE inhibitory protein hydrolysates. Food Chem. 2022, 388, 133036. [Google Scholar] [CrossRef]
- Wang, C.; Rao, J.; Li, X.; He, D.; Zhang, T.; Xu, J.; Chen, X.; Wang, L.; Yuan, Y.; Zhu, X. Chickpea protein hydrolysate as a novel plant-based cryoprotectant in frozen surimi: Insights into protein structure integrity and gelling behaviors. Food Res. Int. 2023, 169, 112871. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, H.; Xie, M.; Shi, T.; Shi, P.; Yu, M. Effects of different cooking methods on phenol content and antioxidant activity in sprouted peanut. Molecules 2023, 28, 4684. [Google Scholar] [CrossRef]
- Zakraoui, M.; Hannachi, H.; Paskovic, I.; Vidovic, N.; Paskovic, M.P.; Palcic, I.; Major, N.; Ban, S.G.; Hamrouni, L. Effect of geographical location on the phenolic and mineral composition of Chetoui olive leaves. Foods 2023, 12, 2565. [Google Scholar] [CrossRef]
- Horvat, D.; Simic, G.; Drezner, G.; Lalic, A.; Ledencan, T.; Tucak, M.; Pavsic, H.; Andric, L.; Zdunic, Z. Phenolic acid profiles and antioxidant activity of major cereal crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Trepiana, J.; Leniz, A.; Gomez-Garcia, I.; Carr-Ugarte, H.; Gonzalez, M.; Portillo, M.P. Variability in beneficinal effects of phenolic compounds: A review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A comparative investigation on phenolic composition, characterization and antioxidant potentials of five different Australian grown pear varieties. Antioxidants 2021, 10, 151. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in phenolic compounds and antioxidant activity during development of Qiangcuili and Cuihongli fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Using bacteria and fungi as plant biostimulants for sustainable agricultural production systems. Rec. Pat. Biotechnol. 2022, 17, 206–244. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Foliar application of nutrients on medicinal and aromatic plants, the sustainable approaches for higher and better production. Beni-Suef Uni. J. Basic Appl. Sci. 2022, 11, 26. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Mechanism of action of collagen and epidermal growth factor: A review on theory and research methods. Mini Rev. Med. Chem. 2023, 23, 453–477. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Five important seeds in traditional medicine, and pharmacological benefits. Seeds 2023, 2, 290–308. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. The golden spice for life: Turmeric with the pharmacological benefits of curcuminoids components, including curcumin, bisdemethoxycurcumin, and demethoxycurcumin. Curr. Org. Synth. 2023, 20. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Kuang, Y.; Cui, H.; Fu, L.; Sun, W. Metabolic changes of active components of important medicinal plants on the basis of traditional Chinese medicine under different envrionmental stresses. Curr. Org. Chem. 2023, 27, 782–806. [Google Scholar] [CrossRef]
- Sierras, N.; Botta, A.; Staasing, L.; Martinez, M.J.; Bru, R. Understanding the effect of amino acids based biostimulant by an enantiomeric analysis of their active principles and a proteomic profiling approach. Acta Hortic. 2016, 1148, 93–100. [Google Scholar] [CrossRef]
- Atilio, J.B.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Soleymani, A.; Cheng, Q. Traditional herbal medicines to overcome stress, anxiety and improve mental health in outbreaks of human coronaviruses. Phytother. Res. 2020, 2020, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Different methods for molecular and rapid detection of human novel coronavirus. Curr. Pharm. Des. 2021, 27, 2893–2903. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Sustainable approaches to boost yield and chemical constituents of aromatic and medicinal plants by application of biostimulants. Recent Pat. Food Nutr. Agric. 2022, 13, 72–92. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Cheng, Q.; Sun, W. The effects of amino acids, phenols and protein hydrolysates as biostimulants on sustainable crop production and alleviated stress. Rec. Pat. Biotechnol. 2022, 16, 319–328. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, Q.; Zhang, W.-H. Glutamate receptors are involved in mitigating effects of amino acids on seed germination of Arabidopsis thaliana under salt stress. Environ. Exp. Bot. 2016, 130, 68–78. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The synthesis and the role of B-alanine in plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of basil (Ocimum bacilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Paleckiene, R.; Sviklas, A.; Slinksiene, R. Physicochemical properties of a microelement fertilizer with amino acids. Russ. J. Appl. Chem. 2007, 80, 352–357. [Google Scholar] [CrossRef]
- Johansson, A. Conversations on chelation and mineral nutrition. Aust. J. Grape Wine Res. 2008, 583, 53–56. [Google Scholar]
- Seadh, S.R.; El-Abady, M.I.; Farouk, S.; El-Saidy Amal, E.A. Effect of foliar nutrition with humic and amino acids under N-levels on wheat productivity and quality of grains and seeds. Egypt. J. Appl. Sci. 2008, 23, 543–558. [Google Scholar]
- Toscano, S.; Gomez-Bellot, M.J.; Romano, D.; Sanchez-Blanco, M.J. Physiological and biochemical changes in response to Moringa oleifera biostimulant in petunia plants under water deficit. Sci. Hortic. 2023, 319, 112187. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Gorecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Amin, A.A.; Gharib, F.A.; El-Awadi, M.; Rashad, E.-S.M. Physiological response of onion plants to foliar application of putrescine and glutamine. Sci. Hortic. 2011, 129, 353–360. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef]
- Das, C.; Sengupta, T.; Chattopadhyay, S.; Setua, M.; Das, N.K.; Saratchandra, B. Involvement of kinetin and spermidine in controlling salinity stress in mulberry (Morus alba L. cv. S1). Acta Physiol. Plant. 2002, 24, 53–57. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Musbah, H.M.; Ibrahim, K.M. Effects of feeding tyrosine or phenylalanine on the accumulation of polyphenols in Coleus blumei in vivo and in vitro. J. Biotechnol. Res. Cent. 2019, 13, 35–43. [Google Scholar] [CrossRef]
- Reham, M.S.; Khattab, M.E.; Ahmed, S.S.; Kandil, M.A.M. Influence of foliar spray with phenylalanine and nickel on growth, yield quality, and chemical composition of genoveser basil plant. Afr. J. Agric. Res. 2016, 14, 934–941. [Google Scholar] [CrossRef]
- Baharlou, M.J.; Pirbalouti, A.G.; Malekpoor, F. Effect of different concentrations of L-phenylalanine on chemical compositions and yield of essential oil of lemon balm (Melissa officinalis). J. Herb. Drugs 2019, 10, 175–183. [Google Scholar]
- Noviyanti, R.; Sari, R.L.K.; Kristanti, A.N.; Yachya, A.; Manuhara, Y.S.W. Biomass and flavonoid production of gynura procumbens adventitious roots induced by sucrose, phenylalanine, and tyrosine. Biosci. Res. 2017, 14, 934–941. [Google Scholar]
- Portu, J.; Santamaria, P.; Lopez, R.; Garde-Cerdan, T. Phenolic composition of Tempranillo grapes following foliar applications of phenylalanine and urea: A two-year study. Sci. Hortic. 2017, 219, 191–199. [Google Scholar] [CrossRef]
- Feng, Z.; Xie, X.; Wu, P.; Chen, M.; Qin, Y.; Zhou, Y.; Zhu, H.; Yao, Q. Phenylalanine-mediated changes in the soil bacterial community promote nitrogen cycling and plant growth. Microbiol. Res. 2023, 275, 127447. [Google Scholar] [CrossRef]
- Ramzan, T.; Shahbaz, M.; Maqsood, M.F.; Zulfiqar, U.; Saman, R.U.; Lili, N.; Irshad, M.; Maqsood, S.; Haider, A.; Shahzad, B.; et al. Phenylalanine supply alleviates the drought stress in mustard (Brassica campestris) by modulating plant growth, photosynthesis, and antioxidant defense system. Plant Physiol. Biochem. 2023, 201, 107828. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.M.-S.; Garde-Cerdan, T.; Baroja, E.; Rubio-Breton, P.; Perez-Alvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515. [Google Scholar] [CrossRef]
- Portu, J.; Lopez-Alfaro, I.; Gomez-Alonso, S.; Lopez, R.; Garde-Cerdan, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, A.; Mirkalaeia, S.A.M.; Sanavy, S.A.M.M.; Eftekhari, A.; Moghadam, M.N. Effect of foliar application of amino acids on grain yield and physiological traits of chickpea under drought stress. Gesunde Pflanz. 2023, 75, 1705–1718. [Google Scholar] [CrossRef]
- Wang, D.; Deng, X.; Wang, B.; Zhang, N.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Effects of foliar application of amino acid liquid fertilizers, with or without Bacillus amyloliquefaciens SQR9, on cowpea yield and leaf microbiota. PLoS ONE 2019, 14, e0222048. [Google Scholar] [CrossRef]
- Mian, G.; Belfiore, N.; Musetti, R.; Tomasi, D.; Cantone, P.; Lovat, L.; Lupinelli, S.; Iacumin, L.; Celotti, E.; Golinelli, F. Effects of a triacontanol-rich biostimulant on the ripening dynamic and wine must technological parameters in Vitis vinifera cv. Ribolla Gialla. Plant Physiol. Biochem. 2022, 188, 60–69. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Othman, Y. Effect of foliar application of amino acid biostimulants on growth, macronutrient, total phenol contents and antioxidant activity of soilless grown lettuce cultivars. S. Afr. J. Bot. 2023, 154, 225–231. [Google Scholar] [CrossRef]
- Mpai, S.; Mokganya, L.M.; Raphoko, L.; Masoko, P.; Ndhlala, A.R. Untargeted metabolites and chemometric approach to elucidate the response of growth and yield attributes on different concentrations of an amino acid based biostimulant in two lettuce cultivars. Sci. Hortic. 2022, 306, 111478. [Google Scholar] [CrossRef]
- Hidalgo-Santiago, L.; Navarro-Leon, E.; Lopez-Moreno, F.J.; Arjo, G.; Gonzalez, L.M.; Ruiz, J.M.; Blasco, B. The application of the silicon-based biostimulant Codasil® offset water deficit of lettuce plants. Sci. Hortic. 2021, 285, 110177. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Baratova, L.; Shelepova, O.; Zargar, M.; Puchkov, M.; Lktionova, E.; Amantayev, B.; Kipshakbaeva, A.; Arinov, B. Biostimulants-based amino acids augment physio-biochemical responses and promote salinity tolerance of lettuce plants (Lactuca sativa L.). Horticulturae 2023, 9, 807. [Google Scholar] [CrossRef]
- Navarro-Leon, E.; Lopez-Moreno, F.J.; Borda, E.; Marin, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Effect of L-amino acid-based biostimulants on nitrogen use efficiency (NUE) in lettuce plants. J. Sci. Food Agric. 2022, 102, 7098–7106. [Google Scholar] [CrossRef] [PubMed]
- Velicka, A.; Taraseviciene, Z.; Hallmann, E.; Kieltyka-Dadasiewicz, A. Impact of foliar application of amino acids on essential oil content, odor profile, and flavonoids content of different mint varieties in field conditions. Plants 2022, 11, 2938. [Google Scholar] [CrossRef]
- Seyedi, A.; Fathi, S.; Movlodzadeh, R. The effect of biostimulants based on free amino acids on some growth and physiological parameters of Dracocephalum moldavica L. under salinity stress. J. Med. Plants By-Prod. 2023; in press. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Neshev, N.; Balabanova, D.; Yanev, M.; Mitkov, A. Is the plant biostimulant application ameliorative for herbicide-damaged sunflower hybrids? Ind. Crops Prod. 2022, 182, 114926. [Google Scholar] [CrossRef]
- Klokic, I.; Koleska, I.; Hasanagic, D.; Murtic, S.; Bosancic, B.; Todorovic, V. Biostimulants, influence on tomato fruit characteristics at conventional and low-input NPK regime. Acta Agric. Scand. Sec. B Soil Plant Sci. 2020, 70, 233–240. [Google Scholar] [CrossRef]
- Niu, C.; Wang, G.; Sui, J.; Liu, G.; Ma, F.; Bao, Z. Biostimulants alleviate temperature stress in tomato seedlings. Sci. Hortic. 2022, 293, 110712. [Google Scholar] [CrossRef]
- Alfosea-Simon, M.; Zavala-Gonzalez, E.A.; Camara-Zapata, J.M.; Martinez-Nicolas, J.J.; Simon, I.; Simon-Grao, S.; Garcia-Sanchez, F. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 2020, 272, 109509. [Google Scholar] [CrossRef]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A biostimulant based on protein hydrolysates promotes the growth of young olive trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Nargesi, M.M.; Sedaghathoor, S.; Hashemabadi, D. Effect of foliar application of amino acid, humic acid, and fulvic acid on the soil content and quality of olive. Saudi J. Biol. Sci. 2022, 29, 3473–3481. [Google Scholar] [CrossRef]
- Sadak, M.S.; Bakry, B.A.; Abdel-Razik, T.M.; Hanafy, R.S. Amino acids foliar application for maximizing growth, productivity and quality of peanut grown under sandy soil. Braz. J. Biol. 2023, 6, e256338. [Google Scholar] [CrossRef]
- Barrajon-Catalan, E.; Alvarez-Martinez, F.J.; Borras, F.; Perez, D.; Herrero, N.; Ruiz, J.J.; Micol, V. Metabolomic analysis of the effects of a commercial complez biostimulant on pepper crops. Food Chem. 2020, 310, 125818. [Google Scholar] [CrossRef] [PubMed]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Biostimulative effect of amino acids on the enzymatic and metabolic response of two Capsicum annuum L. cultivars grown under salt stress. Sci. Hortic. 2023, 309, 111713. [Google Scholar] [CrossRef]
- Raza, S.; Zia-ur-Rehman, M.; Alghamdi, S.A.; Alghanem, S.M.S.; Usman, M.; Ahmed, R.; Waris, A.A.; Rizwan, M.; Abeed, A.H.A.; Al-Haithloul, H.A.S. Effects of zinc-enriched amino acids on rice plants (Oryza sativa L.) for adaptation in saline-sodic soil conditions: Growth, nutrient uptake and biofortification of zinc. S. Afr. J. Bot. 2023, 162, 370–380. [Google Scholar] [CrossRef]
- Mirtaleb, S.H.; Niknejad, Y.; Fallah, H. Foliar spray of amino acids and potassic fertilizer improves the nutritional quality of rice. J. Plant Nutr. 2021, 44, 2029–2041. [Google Scholar] [CrossRef]
- Kocira, S. Effect of amino acid biostimulant on the yield and nutraceutical potential of soybean. Chil. J. Agric. Res. 2019, 79, 17–25. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Soares, J.N.; Reichardt, K.; Neto, D.D. Seed and foliar application of amino acids improve variables of nitrogen metabolism and productivity in soybean crop. Front. Plant Sci. 2018, 9, 396. [Google Scholar] [CrossRef]
- Kausar, A.; Zahra, N.; Tahir, H.; Hafeez, M.B.; Abbas, W.; Raza, A. Modulation of growth and biochemical responses in spinach (Spinacia oleracea L.) through foliar application of some amino acids under drought conditions. S. Afr. J. Bot. 2023, 158, 243–253. [Google Scholar] [CrossRef]
- Hosseini, S.; Shabani, L.; Sabzalian, M.R.; Gharibi, S. Foliar spray of commercial seaweed and amino-acid derived biostimulants promoted phytoremediation potential and salinity stress tolerance in halophytic grass, Puccinellia distans. Int. J. Phytoremed. 2023, 25, 415–429. [Google Scholar] [CrossRef]
- Popko, M.; Wilk, R.; Gorecki, H. New amino acid biostimulators based on protein hydrolysate of keratin. Przem. Chem. 2014, 93, 1012–1015. [Google Scholar]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Annals Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Lin, H.-C.; Alashi, A.M.; Aluko, R.E.; Pan, B.S.; Chang, Y.-W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2017, 61, 1391666. [Google Scholar] [CrossRef]
- Czelej, M.; Czernecki, T.; Garbacz, K.; Wawrzykowski, J.; Jamiol, M.; Michalak, K.; Walczak, N.; Wilk, A.; Wasko, A. Egg yolk as a new source of peptides with antioxidant and antimicrobial properties. Foods 2023, 12, 3394. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.S.; Ben-Hamadou, R.; Hamdi, H.; Saadaoui, I.; Ahmed, T. Application of cyanobacteria (Roholtiella sp.) liquid extract for the alleviation of salt stress in bell pepper (Capsiucum annuum L.) plants grown in a soilless system. Plants 2022, 11, 104. [Google Scholar] [CrossRef]
- Chan, Y.-J.; Lu, W.-C.; Lin, H.-Y.; Wu, Z.-R.; Liou, C.-W.; Li, P.-H. Effect of rice protein hydrolysates as an egg replacement on the physicochemical properties of flaky egg rolls. Foods 2020, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhu, Z.; Wu, S.; Chen, M.; Huang, R.; Wang, J.; Wu, Q.; Ding, Y. Preparation of antioxidant protein hydrolysates from Pleurotus geesterans and their protective effects on H2O2 oxidative damaged PC12 cells. Molecules 2020, 25, 5408. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Soetemans, L.; Caligiani, A.; Sforza, S.; Bastiaens, L. Degree of hydrolysis affects the techno-functional properties of lesser mealworm protein hydrolysates. Foods 2020, 9, 381. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Kanbargi, K.D.; Sonawane, S.K.; Arya, S.S. Encapsulation characteristics of protein hydrolyaste extracted from Ziziphus jujube seed. Int. J Food Prop. 2017, 20, 3215–3224. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Organic waste utilization and urban food waste composting strategies in China—A review. Not. Sci. Biol. 2021, 13, 10881. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Archaea, bacteria and termite, nitrogen fixation and sustainable plants production. Not. Bot. Horti Agrobot. 2021, 49, 1–32. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Barberry (Berberis vulgaris), a medicinal fruit and food with traditional and modern pharmaceutical uses. Isr. J. Plant Sci. 2021, 68, 61–71. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek cultivation with emphasis on historical aspects and its uses in traditional medicine and modern pharmaceutical science. Mini Rev. Med. Chem. 2021, 21, 724–730. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Natural dietary and medicinal plants with anti-obesity therapeutics activities for treatment and prevention of obesity during lock down and in post-COVID-19 era. Appl. Sci. 2021, 11, 7889. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The effectiveness of Rhizobium bacteria on soil fertility and sustainable crop production under cover and catch crops management and green manuring. Not. Bot. Horti Agrobot. 2022, 50, 12560. [Google Scholar] [CrossRef]
- Tchorbanov, B.; Iliev, I.; Litchev, V. Enzymic protein hydrolysates. Biotechnol. Biotechnol. Equip. 1991, 5, 32–36. [Google Scholar] [CrossRef]
- Povolo, C.; Avolio, R.; Doria, E.; Marra, A. Development and validation of an analytical method to ensure quality requirements of hydrolysed proteins intended for agricultural use as biostimulants. Talanta Open 2022, 5, 100082. [Google Scholar] [CrossRef]
- Jensen, C.; Dale, H.F.; Hausken, T.; Hatlebakk, J.G.; Bronstad, I.; Lied, G.A.; Hoff, D.A.L. Supplementation with low doses of a cod protein hydrolysate on glucose regulation and lipid metabolism in adults with metabolic syndrome: A randomized, double-blind study. Nutrients 2020, 12, 1991. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M. Spray-drying encapsulation of protein hydrolysates and bioactive peptides: Opportunities and challenges. Dry. Technol. 2020, 38, 577–595. [Google Scholar] [CrossRef]
- Balan, D.; Luta, G.; Stanca, M.; Jerca, O.; Niculescu, M.; Gaidau, C.; Jurcoane, S.; Mihalcea, A. Effect of protein gel treatments on biometric and biochemical attributes of tomato seedlings in greenhouse condition. Agriculture 2023, 13, 54. [Google Scholar] [CrossRef]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Ferrari, E.; Schiavon, M.; Nardi, S. Spectroscopic-chemical fingerprint and biostimulant activity of a protein-based product in solid form. Molecules 2018, 23, 1031. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Amino acid profiling and antimicrobial activity of Cucurbita moschata and Lagenaria siceraria seed protein hydrolysates. Nat. Prod. Res. 2018, 32, 2050–2053. [Google Scholar] [CrossRef]
- Schmidt, G.J.; Olson, D.C.; Slavin, W. Amino acid profiling of protein hydrolysates using liquid chromatography and fluorescence detection. J. Liquid Chromatogr. 1979, 2, 1031–1045. [Google Scholar] [CrossRef]
- Phetchthumrongchai, T.; Tachapuripunya, V.; Chintong, S.; Roytrakul, S.; E-kobon, T.; Klaypradit, W. Properties of protein hydrolysates and bioinformatics prediction of peptides derived from thermal and enzymatic process of Skipjack tuna (Katsuwonus pelamis) roe. Fishes 2022, 7, 255. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Dinesh Kumar, B. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef]
- Bilenky, M.; Nair, A. Biostimulants combined with water soluble fertilizer had little effect on transplant growth and pepper yield grown under greenhouse conditions. Int. J. Veg. Sci. 2023, 29, 25–39. [Google Scholar] [CrossRef]
- Ghorbel-Bellaaj, O.; Jellouli, K.; Maalej, H. Shrimp processing by-products protein hydrolysates: Evaluation of antioxiant activity and application in biomass and proteases production. Biocatal. Biotransform. 2017, 35, 287–297. [Google Scholar] [CrossRef]
- Li, H.; Aluko, R.E. Structural modulation of calmodulin and calmodulin-dependent protein kinase II by pea protein hydrolysates. Int. J. Food Sci. Nutr. 2006, 57, 178–189. [Google Scholar] [CrossRef]
- Paradikovic, N.; Vinkovic, T.; Vinkovic Vrcek, I.; Zuntar, I.; Bojic, M.; Medic-Saric, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Paradikovic, N.; Teklic, T.; Zelikovic, S.; Lisjak, M.; Spoljarevic, M. Biostimulants research in some horticultural plant species- A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Anthoxidants Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Formanek, P. Non-protein amino acids: Plant, soil, and ecosystem interactions. Plant Soil 2011, 342, 31–48. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Flores, T.; Todd, C.D.; Tovar-Mendez, A.; Dhanoa, P.K.; Correa-Aragunde, N.; Hoyos, M.E.; Brownfield, D.M.; Mullen, R.T.; Lamattina, L.; Polacco, J.C. Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol. 2008, 147, 1936–1946. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzalka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Hoagland, L.; Giordano, M.; El-Nakhel, C.; Cardarelli, M. Vegetal-protein hydrolysates based microgranule enhances growth, mineral content, and quality traits of vegetable transplants. Sci. Hortic. 2021, 290, 110554. [Google Scholar] [CrossRef]
- Colantoni, A.; Recchia, L.; Bernaberi, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the environmental impact of chemically-produced protein hydrolysate from leather waste vs. enzymatically-produced protein hydrolysate from legume grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Porterfield, D.M. Environmental sensing and directional growth of plant roots. In Plant Roots: The Hidden Half, 4th ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-sequencing analysis reveals transcriptional changes in root of maize seedings treated with two increasing concentrations of a new biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Kisvarga, S.; Farkas, D.; Boronkay, G.; Nemenyi, A.; Orloci, L. Effects of biostimulants in horticulture, with emphasis on ornamental plant production. Agronomy 2022, 12, 1043. [Google Scholar] [CrossRef]
- Tanou, G.; Ziogas, V.; Molassiotis, A. Foliar nutrition, biostimulants and prime-like dynamics in fruit tree physiology: New insights on an old topic. Front. Plant Sci. 2017, 8, 75. [Google Scholar] [CrossRef]
- Malecange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef] [PubMed]
- Soppela, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Gurav, R.; Jadhav, J. A novel source of biofertilizer from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Consentino, B.B.; Vultaggio, L.; Sabatino, L.; Ntatsi, G.; Rouphael, Y.; Bondi, C.; Pasquale, C.D.; Guarino, V.; Iacuzzi, N.; Capodici, G.; et al. Combined effects of biostimulants, N level and drought stress on yield, quality and physiology of greenhouse-grown basil. Plant Stress 2023, 10, 100268. [Google Scholar] [CrossRef]
- Rosiane, L.S.L.; Liv, S.S.; Ligia, R.S.; Sofiatti, V.; Gomes, J.A.; Beltrao, N.E.M. Blends of castor meal and castor husks for optimized use as organic fertilizer. Ind. Crops Prod. 2011, 33, 364–368. [Google Scholar] [CrossRef]
- Consentino, B.B.; Virga, G.; Placa, G.G.L.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; Bella, S.L.; Mauro, R.P.; Anna, F.D.; et al. Celery (Apium graveolens L.) performances as subjected to different sources of protein hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef]
- Paul, T.; Halder, S.K.; Das, A.; Bera, S.; Maity, C.; Mandal, A.; Das, P.S.; Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocatal. Agric. Biotechnol. 2013, 2, 50–57. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Mohammadipour, N.; Souri, M.K. Effects of different levels of glycine in the nutrient solution on the growth, nutrient composition, and antioxidant activity of coriander (Coriandrum sativum L.). Acta Agrobot. 2019, 72, 13–16. [Google Scholar] [CrossRef]
- Carillo, P.; Pannico, A.; Cirillo, C.; Ciriello, M.; Colla, G.; Cardarelli, M.; Pascale, S.D.; Rouphael, Y. Protein hydrolysates from animal or vegetal sources affect morpho-physiological traits, ornamental quality, mineral composition, and shelf-life of Chrysanthemum in a distinctive manner. Plants 2022, 11, 2321. [Google Scholar] [CrossRef]
- Garcia-Santiago, J.C.; Cavazos, C.J.; Gonzalez-Fuentes, J.; Zermeno-Gonzalez, A.; Alvarado, E.R.; Duarte, A.R.; Preciado-Rangel, P.; Troyo-dieguez, E.; Ramos, F.M.P.; Valdez-Aguilar, L.A.; et al. Effect of fish-derived protein hydrolysate, animal-based organic fertilisers and irrigation method on the growth and quality of grape tomatoes. Biol. Agric. Hortic. 2021, 37, 107–124. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Squeri, C.; Zamboni, M.; Frioni, T. Protein hydrolysates modulate leaf proteome and metabolome in water-stressed grapevines. Sci. Hortic. 2020, 270, 109413. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-based protein hydrolysate improves salinity tolerance in hemp: Agronomical and physiological aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Wise, K.; Selby-Pham, J.; Chai, X.; Simovich, T.; Gupta, S.; Gill, H. Fertiliser supplementation with a biostimulant complex of fish hydrolysate, Aloe vera extract, and kelp alters cannabis root architecture to enhance nutrient uptake. Sci. Hortic. 2023, 323, 112483. [Google Scholar] [CrossRef]
- Massa, D.; Lenzi, A.; Montoneri, E.; Ginepro, M.; Prisa, D.; Burchi, G. Plant response to biowaste soluble hydrolysates in hibiscus grown under limiting nutrient availability. J. Plant Nutr. 2018, 41, 396–409. [Google Scholar] [CrossRef]
- Qurartieri, M.; Lucchi, A.; Cavani, L. Effects of the rate of protein hydrolysis and spray concentration on growth of potted kiwifruit (Actinidia deliciosa) plants. Acta Hortic. 2022, 594, 341–347. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. Hortechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Naroozlo, Y.A.; Souri, K.M.; Mojtaba, D. Stimulation effects of foliar applied glycine and glutamine amino acids on lettuce growth. Open Agric. 2019, 4, 164. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D Anna, F.; La Bella, S. Protein hydrolysates and Mo-biofortification interactively modulate plant performance and quality of Canasta lettuce grown in a protected environment. Agronomy 2021, 11, 1023. [Google Scholar] [CrossRef]
- Dass, S.M.; Chai, T.-T.; Cao, H.; Ooi, A.L.; Wong, F.-C. Application of enzyme-digested soy protein hydrolysate on hydroponic-planted lettuce: Effects on phytochemical contents, biochemical profiles and physical properties. Food Chem. 2021, 12, 100132. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cristofano, F.; Colla, G.; Pii, Y.; Lucini, L.; Rouphael, Y. A Graminaceae-derived protein hydrolysate and its fractions provide differential growth and modulate qualitative traits of lettuce grown under non-saline and mild salinity conditions. Sci. Hortic. 2023, 319, 112130. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous pig blood-derived protein hydrolysates as a promising method for alleviation of salt stress in tomato (Solanum lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- Liu, N.; Qu, P.; Huang, H.; Wei, Z. Soybean protein hydrolysate-formaldehyde-urea block copolymer for controlled release fertilizer. Environ. Pollut. Bioavailab. 2019, 31, 94–102. [Google Scholar] [CrossRef]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Tsopmo, A.; Thimmanagari, M.; Dutta, A. Corn distillers solubles by two-step proteolytic hydrolysis as a new source of plant-based protein hydrolysates with ACE and DPP4 inhibition activities. Food Chem. 2023, 401, 134120. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Simova-Stoilova, L.; Kostadinova, A.; Yuperlieva-Mateeva, B.; Karakicheva, T.; Vassileva, V. Heat-stress-mitigating effects of a protein-hydrolysate-based biostimulant are linked to changes in Protease, DHN, and HSP gene expression in maize. Agronomy 2022, 12, 1127. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Ambrosini, S.; Prinsi, B.; Zamboni, A.; Espen, L.; Zanzoni, S.; Santi, C.; Varanini, Z.; Pandolfini, T. Chemical characterization of a collagen-derived protein hydrolysate and biostimulant activity assessment of its peptidic components. J. Agric. Food Chem. 2020, 70, 11201–11211. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhang, J.; Jia, C.; Feng, J.; Liang, L.; Chen, Q.Q.; Li, T.; Hao, J. Foliar application of fish protein peptide improved the quality of deep-netted melon. J. Plant Nutr. 2023, 46, 3683–3696. [Google Scholar] [CrossRef]
- Eguchi, Y.; Milazzo, M.C.; Ueno, K.; Shetty, K. Partial improvement of vitrification and acclimation of oregano (Origanum vulgare L.) tissue culture by fish protein hydrolysates. J. Herb Spice Med. Plants 2000, 6, 29–38. [Google Scholar] [CrossRef]
- Morales-Payan, P.; Stall, W. Passion fruit (Passiflora Edulis) transplant production is affected by selected biostimulants. In Proceedings of the Florida State Horticultural Society, Fort Lauderdale, FL, USA, 6–8 June 2004; Volume 117, pp. 224–227. [Google Scholar]
- Osman, A.; Merwad, A.-R.M.; Mohamed, A.H.; Sitohy, M. Foliar spray with pepsin-and papain-whey protein hydrolysates promotes the productivity of pea plants cultivated in clay loam soil. Molecules 2021, 26, 2805. [Google Scholar] [CrossRef] [PubMed]
- Aktsoglou, D.-C.; Kasampalis, D.S.; Sarrou, E.; Tsouvaltzis, P.; Chatzopoulou, P.; Martens, S.; Siomos, A.S. Protein hydrolysates supplement in the nutrient solution of soilless grown fresh peppermint and spearmint as a tool for improving product quality. Agronomy 2021, 11, 317. [Google Scholar] [CrossRef]
- Visconti, F.; de Paz, J.M.; Bonet, L.; Jorda, M.; Quinones, A.; Intrigliolo, D.S. Effects of a commercial calcium protein hydrolysate on the salt tolerance of Diospyros kaki L. cv. Rojo Brillante grafted on Diospyros lotus L. Sci. Hortic. 2015, 185, 129–138. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Wang, Y.; Cheng, Q.; Mou, H. Production of a water-soluble fertilizer containing amino acids by solid-state fermentation of soybean meal and evaluation of its efficacy on the rapeseed growth. J. Biotechnol. 2014, 187, 34–42. [Google Scholar] [CrossRef]
- Luo, Y.; Niu, L.; Li, D.; Xiao, J. Synergistic effects of plant protein hydrolysates and xanthan gum on the short- and long-term retrogradation of rice starch. Int. J. Biol. Macromol. 2020, 144, 967–977. [Google Scholar] [CrossRef]
- Calderon-chiu, C.; Calderon-santoyo, M.; Damasceno-gomes, S.; Ragazzo-Sanchez, J.A. Use of jackfruit leaf (Artocarpus heterophyllus L.) protein hydrolysates as a stabilizer of the nanoemulsions loaded with extract-rich pentacyclic triterpenes obtained from Coccoloba uvifera L. leaf. Food Chem. 2021, 12, 100138. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Dickinson, N. Nutrient interactions influence the efficacy of biostimulants. J. Plant Nutr. 2023, 46, 1616–1626. [Google Scholar] [CrossRef]
- Lachhab, N.; Sanzani, S.M.; Adrian, M.; Chiltz, A.; Balacey, S.; Boselli, M.; Ippolito, A.; Pointssot, B. Soybean and casein hydrolysates induce grapevine immune responses and resistance against Plasmopara viticola. Front. Plant Sci. 2014, 5, 716. [Google Scholar] [CrossRef] [PubMed]
- Liatile, P.C.; Potgieter, G.; Moloi, M.J. A natural biostimulant consisting of a mixture of fish protein hydrolysates and kelp extract enhances the physiological, biochemical, and growth responses of Spinach under different water levels. Plants 2022, 11, 3374. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Colla, G.; Fusco, G.M.; Aversana, E.D.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal-versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Jolayemi, O.L.; Malik, A.H.; Vetukuri, R.R.; Saripella, G.V.; Kalyandurg, P.B.; Ekblad, T.; Yong, J.W.H.; Olsson, M.E.; Johansson, E. Metabolic processes and biological macromolecules defined the positive effects of protein-rich biostimulants on sugar beet plant development. Int. J. Mol. Sci. 2023, 24, 9720. [Google Scholar] [CrossRef]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-based biostimulants to enhance plant growth-state of the art and future direction with sugar beet as an example. Agronomy 2022, 12, 3211. [Google Scholar] [CrossRef]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-based biostimulants as sustainable alternative to synthetic growth regulators in two sweet cherry cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef]
- Testani, E.; Campanelli, G.; Leteo, F.; Ciaccia, C.; Canali, S.; Tittarelli, F. Sweet pepper (Capsicum annuum L.) organic seedling production: The role of compost, cultivar, and protein hydrolyzate. Compost Sci. Util. 2017, 25, 112–119. [Google Scholar] [CrossRef]
- Elwaziri, E.; Ismail, H.; El-Khairi, E.-S.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; El-Gawad, H.G.A.; Omar, W.A.; Abdelaal, K.; Osman, A. Biostimulant application of whey protein hydrolysates and potassium fertilization enhances the productivity and tuber quality of sweet potato. Not. Bot. Horti Agrobot. 2023, 51, 13122. [Google Scholar] [CrossRef]
- Raguraj, S.; Kasim, S.; Md Jaafar, N.; Nazli, M.H. Growth of tea nursery plants as influenced by different rates of protein hydrolysate derived from chicken feathers. Agronomy 2022, 12, 299. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A novel protein hydrolysate-based biostimulant improves tomato performances under drought stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef]
- Barrada, A.; Delisle-Houde, M.; Nguyen, T.T.A.; Tweddell, R.J.; Dorais, M. Drench application of soy protein hydrolysates increases tomato plant fitness, fruit yield, and resistance to a Hemibiotrophic pathogen. Agronomy 2022, 12, 1761. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Tallarita, A.V.; Vecchietti, L.; Golubkina, N.A.; Sekara, A.; Cozzolino, E.; Mirabella, M.; Cuciniello, A.; Maiello, R.; Cenvinzo, V.; Lombardi, P.; et al. Effects of plant biostimulation time span and soil electrical conductivity on greenhouse tomato Miniplum yield and quality in diverse crop seasons. Plants 2023, 12, 1423. [Google Scholar] [CrossRef]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.-J. Effects of plant-derived protein hydrolysates on yield, quality and nitrogen use efficiency of greenhouse grown lettuce and tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Francesca, S.; Najai, S.; Zhou, R.; Decros, G.; Cassan, C.; Delmas, F.; Ottosen, C.-O.; Barone, A.; Rigano, M.M. Phenotyping to disset the biostimulant action of a protein hydrolysate in tomato plants under combined abiotic stress. Plant Physiol. Biochem. 2022, 179, 32–43. [Google Scholar] [CrossRef]
- Munaro, D.; Mazo, C.H.; Bauer, C.M.; Gomes, L.D.S.; Teodoro, E.B.; Mazzarino, L.; Veleirinho, M.B.D.R.; Silva, S.M.; Maraschin, M. A novel biostimulant from chitosan nanoparticles and microalgae-based protein hydrolysate: Improving crop performance in tomato. Sci. Hortic. 2024, 323, 112491. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Lv, H.; Wang, Q.; Liang, B.; Li, J. Foliar application of pig blood-derived protein hydrolysates improves antioxidant activities in lettuce by regulating phenolic biosynthesis without compromising yield production. Sci. Hortic. 2022, 291, 110602. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, W.; Lv, H.; Liang, B.; Jin, S.; Li, J.; Zhou, W. Animal-derived plant biostimulant alleviates drought stress by regulating photosynthesis, osmotic adjustment, and antioxidant systems in tomato plants. Sci. Hortic. 2022, 305, 111365. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; Pascale, S.D. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Casadesus, A.; Perez-Llorca, M.; Munne-Bosch, S.; Polo, J. An enzymatically hydrolyzed animal protein-based biostimulant (Pepton) increases salicylic acid and promotes growth of tomato roots under temperature and nutrient stress. Front. Plant Sci. 2020, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. yield and quality as influenced by cropping season, protein hydrolysates and Trichoderma applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef]
- Mironenko, G.A.; Zagorskii, I.A.; Bystrova, N.A.; Kochetkov, K.A. The effect of a biostimulant based on a protein hydrolysate of a rainbow trout (Oncorhynchus mykiss) on the growth and yield of wheat (Triticum aestivum L.). Molecules 2022, 27, 6663. [Google Scholar] [CrossRef]
- Trakselyte-Rupsiene, K.; Juodeikiene, G.; Cernauskas, D.; Bartkiene, E.; Klupsaite, D.; Zadeike, D.; Bendoraitiene, J.; Damasius, J.; Ignatavicius, J.; Sikorskaite-Gudziuniene, S. Integration of ultrasound into the development of plant-based protein hydrolysate and its bio-stimulatory effect for growth of wheat grain seedlings in vivo. Plants 2021, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.A.I.; El-Yazied, A.A.; El-Gawad, H.G.A.; Azab, E.; Gobouri, A.A.; Sitohy, M.; Osman, A. Enhancing grain yield and nitrogen accumulation in wheat plants grown under a Mediterranean arid environment by foliar spray with papin-released whey peptides. Agronomy 2021, 11, 1913. [Google Scholar] [CrossRef]
- Chwil, S.; Matraszek, R.; Kozlowska-Strawska, J.; Chwil, M.; Zapalski, P. Effects of protein hydrolysate on soil fertility and heavy-metal accumulation in Sinapis alba L. Commun. Soil Sci. Plant Anal. 2016, 47, 298–304. [Google Scholar] [CrossRef]
- Bajpai, M.; Pande, A.; Tewari, S.K.; Prakash, D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutr. 2005, 56, 287–291. [Google Scholar] [CrossRef]
- Owen-Going, T.N.; Beninger, C.W.; Sutton, J.C.; Hall, J.C. Accumulation of phenolic compounds in plants and nutrient solution of hydroponically grown peppers inoculated with Pythium aphanidermatum. Can. J. Plant Pathol. 2008, 30, 214–225. [Google Scholar] [CrossRef]
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28. [Google Scholar] [CrossRef]
- Wong, J.Y.; Matanjun, P.; Ooi, Y.B.H.; Chia, K.F. Characterization of phenolic compounds, carotenoids, vitamins and antioxidant activities of selected Malaysian wild edible plants. Int. J. Food Sci. Nutr. 2013, 5, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communications during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; Volume 661, pp. 23–67. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 3242. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar]
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B Biol. 2019, 193, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Hajiboland, R.; Farhanghi, F. Remobilization of boron, photosynthesis, phenolic metabolism and anti-oxidant defense capacity in boron-deficient turnip (Brassica rapa L.) plants. Soil Sci. Plant Nutr. 2010, 56, 427–437. [Google Scholar] [CrossRef]
- Schoneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contamin. 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.M.; Saleh, A.M.; Abdel-Farid, I.B.; El-Naggar, S.A. Growth, hydrolases and ultrastructure of Fusarium oxysporum as affected by phenolic rich extracts from several xerophytic plants. Pestic. Biochem. Physiol. 2017, 141, 57–64. [Google Scholar] [CrossRef]
- Lwalaba, J.L.W.; Zvobgo, G.; Mwamba, T.M.; Louis, L.T.; Fu, L.; Kirika, B.A.; Tshibangu, A.K.; Adil, M.F.; Sehar, S.; Mukobo, R.P. High accumulation of phenolics and amino acids confers tolerance to the combined stress of cobalt and copper in barley (Hordeum vulgare). Plant Physiol. Biochem. 2020, 155, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.H.; Souza, J.A.R.D.; Macedo, W.R.; Pinto, F.G. Tyrosol, a phenolic compound from Phomopsis sp., is a potential biostimulant in soybean seed treatment. Phytochem. Lett. 2021, 43, 40–44. [Google Scholar] [CrossRef]
- Masondo, N.A.; Aremu, A.O.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Subrtova, M.; Dolezal, K.; Van Staden, J. Growth and phytochemical response in Eucomis autumnalis (Mill.) Chitt. Treated with phenolic biostimulants from brown alga, Ecklonia maxima. S. Afr. J. Bot. 2015, 98, 211. [Google Scholar] [CrossRef]
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in Kandelia obovata under cadmium and zinc stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef]
- Yahia, Y.; Bagues, M.; Zaghdoud, C.; Al-Amri, S.M.; Nagaz, K.; Guerfel, M. Phenolic profile, antioxidant capacity and antimicrobial activity of Calligonum arich L., desert endemic plant in Tunisia. S Afr. J. Bot. 2019, 124, 414–419. [Google Scholar] [CrossRef]
- Cueto, J.D.; Kosinska-Cagnazzo, A.; Stefani, P.; Heritier, J.; Roch, G.; Oberhansli, T.; Audergon, J.-M.; Christen, D. Phenolic compounds identified in apricot branch tissues and their role in the control of Monilinia laxa growth. Sci. Hortic. 2021, 275, 109707. [Google Scholar] [CrossRef]
- Jakubke, H.D.; Jeschkeit, H. Aminokwasy, Peptydy, Bialka, 2nd ed.; Polskie Wydawnictwo Naukowe: Warsaw, Poland, 1989. [Google Scholar]
- Shahrajabian, M.H.; Khoshkharam, M.; Sun, W.; Cheng, Q. Germination and seedlings growth of corn (Zea mays L.) to allelopathic effects of rice (Oryza sativa L.). Trop. Plant Res. 2019, 6, 152–156. [Google Scholar] [CrossRef]
- Del Bubba, M.; Ancillotti, C.; Checchini, L.; Ciofi, L.; Fibbi, D.; Gonnelli, C.; Mosti, S. Chromium accumulation and changes in plant growth, selected phenolics and sugars of wild type and genetically modified Nicotiana langsdorfii. J. Hazard. Mater. 2013, 262, 394–403. [Google Scholar] [CrossRef]
- Kucinska, J.K.; Magnucka, E.G.; Oksinska, M.P.; Pietr, S.J. Bioefficacy of hen feather keratin hydrolysate and compost on vegetable plant growth. Compost Sci. Utiliz. 2014, 22, 179–187. [Google Scholar] [CrossRef]
- Tejada, M.; Rodriguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of foliar fertilization of a biostimulant obtained from chicken feathers on maize yield. Eur. J. Agron. 2018, 96, 54–59. [Google Scholar] [CrossRef]
- Wong, F.-C.; Chai, T.-T. Bioactive peptides and protein hydrolysates as lipoxygenase inhibitors. Biology 2023, 12, 917. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).