Effects of Differential Shading on Summer Tea Quality and Tea Garden Microenvironment
Abstract
1. Introduction
2. Results
2.1. Photosynthesis and Tea Yield as Influenced by Different Shading Treatments
2.2. Nutrient Elements in Tea Leaves as Influenced by Different Shading
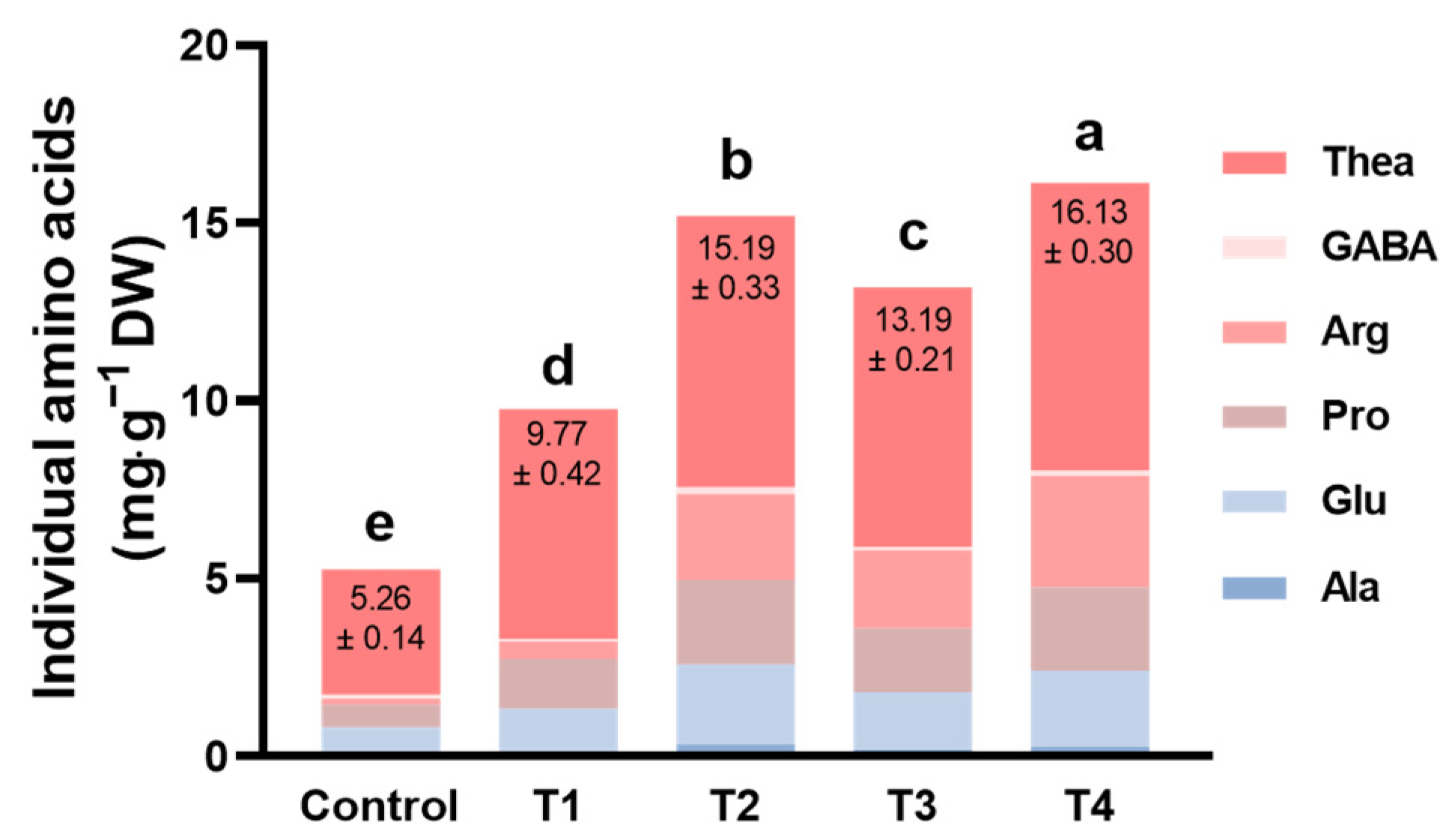
2.3. The Quality of Tea as Influenced by Different Shading
2.4. Temperature and Soil Water Content in the Tea Garden as Influenced by Different Shading
2.5. Light Environment in the Canopy of Tea Plants as Influenced by Different Shading
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Treatments, and Sample Collection
4.2. Gas Exchange and Chlorophyll Measurement
4.3. Nutrient Elements Measurement
4.4. Tea Quality Measurement
4.5. Temperature and Soil Water Content Measurement
4.6. Light Spectrum Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hazra, A.; Dasgupta, N.; Sengupta, C.; Bera, B.; Das, S. Tea: A worthwhile, popular beverage crop since time immemorial. Agron. Crops 2019, 1, 507–531. [Google Scholar]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Kumar, V.; Yadav, S.K. Tea caffeine: Metabolism, functions, and reduction strategies. Food Sci. Biotechnol. 2010, 19, 275–287. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Xu, W.; Song, Q.; Li, D.; Wan, X. Discrimination of the production season of Chinese green tea by chemical analysis in combination with supervised pattern recognition. J. Agric. Food Chem. 2012, 60, 7064–7070. [Google Scholar] [CrossRef]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; et al. Nontargeted analysis using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry uncovers the effects of harvest season on the metabolites and taste quality of tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Ma, L.; Yi, X.; Ruan, J. Metabolomic analysis using ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS) uncovers the effects of light intensity and temperature under shading treatments on the metabolites in tea. PLoS ONE 2014, 9, e112572. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Dong, F.; Li, J.; Zeng, L.; Tang, J.; Gu, D. Mechanism underlying the shading-induced chlorophyll accumulation in tea leaves. Front. Plant Sci. 2021, 12, 779819. [Google Scholar] [CrossRef]
- Ji, H.; Lee, Y.; Lee, M.; Hwang, K.; Park, C.; Kim, E.; Park, J.; Hong, Y. Diverse metabolite variations in tea (Camellia sinensis L.) leaves grown under various shade conditions revisited: A metabolomics study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, G.; Li, T.; Wang, Q.; Xing, Z.; LeCompte, J.; Harkess, R. Color shade nets affect plant growth and seasonal leaf quality of Camellia sinensis grown in mississippi, the United States. Front. Nutr. 2022, 9, 786421. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M. Chemical components and taste of green tea. Jpn. Agric. Res. Q. 1975, 9, 156–160. [Google Scholar]
- Yu, P.; Yeo, A.; Low, M.; Zhou, W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Choi, J.; Son, N.; Kim, S.; Park, J.; Jang, D.; Jeong, Y.; Kim, H. Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J. Agric. Food Chem. 2013, 61, 332–338. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of shading intensity on morphological and color traits and on chemical components of new tea (Camellia sinensis L.) shoots under direct covering cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, Z.; Sun, W.; Deng, T.; Chen, M. De novo sequencing of the leaf transcriptome reveals complex light-responsive regulatory networks in Camellia sinensis cv. Baijiguan. Front. Plant Sci. 2016, 7, 332. [Google Scholar] [CrossRef]
- Liu, G.; Han, Z.; Feng, L.; Gao, L.; Gao, M.; Gruber, M.; Zhang, Z.; Xia, T.; Wan, X.; Wei, S. Metabolic flux redirection and transcriptomic reprogramming in the albino tea cultivar ‘Yu-Jin-Xiang’ with an emphasis on catechin production. Sci. Rep. 2017, 7, srep45062. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, X.; Wang, H.; Tian, Y.; Xiang, Q.; Zhang, L. Study on physiological characteristics of leaves with different colors of ‘Huangjinya’. J. Tea Sci. 2019, 39, 530–536. [Google Scholar]
- Xu, P.; Su, H.; Jin, R.; Mao, Y.; Xu, A.; Cheng, H.; Wang, Y.; Meng, Q. Shading effects on leaf color conversion and biosynthesis of the major secondary metabolites in the albino tea cultivar “Yujinxiang”. J. Agric. Food Chem. 2020, 68, 2528–2538. [Google Scholar] [CrossRef]
- Shao, C.; Jiao, H.; Chen, J.; Zhang, C.; Liu, J.; Chen, J.; Li, Y.; Huang, J.; Yang, B.; Liu, Z. Carbon and nitrogen metabolism are jointly regulated during shading in roots and leaves of Camellia Sinensis. Front. Plant Sci. 2022, 13, 894840. [Google Scholar] [CrossRef]
- Li, Y.; Anburaj, J.; Yu, H.; Wang, Y.; Ma, Q.; Chen, X.; Sun, H.; Zhang, H.; Ding, Z.; Li, X. Metabolic regulation profiling of carbon and nitrogen in tea plants [Camellia sinensis (L.) O. Kuntze] in response to shading. J. Agric. Food Chem. 2020, 68, 961–974. [Google Scholar] [CrossRef]
- Liao, Q.; Liang, P.; Xing, Y.; Yao, Z.; Liu, Y.; Jiang, Z. Effects of different shading models on tea garden habitat and tea quality in summer. Southwest China J. Agric. Sci. 2022, 35, 2001–2008. [Google Scholar]
- Deng, W.; Fei, Y.; Wang, S.; Wan, X.; Zhang, Z.; Hu, X. Effect of shade treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Growth Regul. 2013, 71, 295–299. [Google Scholar] [CrossRef]
- Yang, T.; Xie, Y.; Lu, X.; Yan, X.; Wang, Y.; Ma, J.; Cheng, X.; Lin, S.; Bao, S.; Wan, X.; et al. Shading promoted theanine biosynthesis in the roots and allocation in the shoots of the tea Plant (Camellia sinensis L.) cultivar Shuchazao. J. Agric. Food Chem. 2021, 69, 4795–4803. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, B.; Chung, J.; Hwang, J.; Lee, S.; Lee, C.; Hong, Y. Geographical and climatic dependencies of green tea (Camellia sinensis) metabolites: A1H NMR-based metabolomics study. J. Agric. Food Chem. 2010, 58, 10582–10589. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, J.; Zhou, Y.; Zhou, S.; Zhang, S.; Tong, H.; Zhao, A. Transcriptome and metabolome profiling unveiled mechanisms of tea (Camellia sinensis) quality improvement by moderate drought on pre-harvest shoots. Phytochemistry 2020, 180, 112515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, W.; Lou, Y.; Shu, Z.; Ji, Q.; Shao, J. Effects of different shading treatments on microclimate of tea garden and the quality of flake tea. J. South. Agric. 2021, 52, 711–721. [Google Scholar]
- Lin, S.; Lin, J.; Liu, Q.; Ai, Y.; Ke, Y.; Chen, C.; Zhang, Z.; He, H. Time-course of photosynthesis and non-structural carbon compounds in the leaves of tea plants (Camellia sinensis L.) in response to deficit irrigation. Agric. Water Manag. 2014, 144, 98–106. [Google Scholar] [CrossRef]
- Ye, J.; Lv, Y.; Liu, S.; Jin, J.; Wang, Y.; Wei, C.; Zhao, S. Effects of light intensity and spectral composition on the transcriptome profiles of leaves in shade grown tea plants (Camellia sinensis L.) and regulatory network of flavonoid biosynthesis. Molecules 2021, 26, 5836. [Google Scholar] [CrossRef]
- Song, L.; Ma, Q.; Zou, Z.; Sun, K.; Yao, Y.; Tao, J.; Kaleri, N.; Li, X. Molecular link between leaf coloration and gene expression of flavonoid and carotenoid biosynthesis in Camellia sinensis cultivar ‘Huangjinya’. Front. Plant Sci. 2017, 8, 803. [Google Scholar] [CrossRef]
- Li, C.; Ma, J.; Huang, D.; Ma, C.; Jin, J.; Yao, M.; Chen, L. Comprehensive dissection of metabolic changes in albino and green tea cultivars. J. Agric. Food Chem. 2018, 66, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kobayashi, E.; Katsuno, T.; Asanuma, T.; Fujimori, T.; Ishikawa, T.; Tomomura, M.; Mochizuki, K.; Watase, T.; Nakamura, Y.; et al. Characterisation of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. Food Chem. 2012, 135, 2268–2276. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef]
- Bautista-Saraiva, A.; Bonomi-Barufi, J.; Figueroa, F.; Necchi, O. UV-radiation effects on photosynthesis, photosynthetic pigments and UV-absorbing substances in three species of tropical lotic macroalgae. Theor. Exp. Plant Physiol. 2018, 30, 181–192. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Xiong, Y.; Fu, Y.; Chen, Y. Effects of red-blue led light intensity on growth and biochemical components of tea plants. Mol. Plant Breed. 2020, 18, 1656–1660. [Google Scholar]
- Ge, S.; Jin, D.; Yang, M.; Wang, H.; Zhang, L.; Han, W.; Li, X. Effects and regulation mechanism of different proportions of red and blue light on quality components in tea (Camellia sinensis L.) plant. Acta Agric. Zhejiangensis 2022, 34, 2105–2111. [Google Scholar]
- Islam, M.; Tarkowská, D.; Clark, L.; Blystad, D.; Gislerod, H.; Torre, S.; Olsen, J. Impact of end-of-day red and far-red light on plant morphology and hormone physiology of poinsettia. Sci. Hortic. 2014, 174, 77–86. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Folta, K. Green light augments far-red-light-induced shade response. Plant Growth Regul. 2015, 77, 147–155. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Peron, T.; Corot, A.; Bertheloot, J.; Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Samuolienė, G.; Novičkovas, A.; Duchovskis, P. Nitrate, nitrite, protein, amino acid contents, and photosynthetic and growth characteristics of tatsoi cultivated under various photon flux densities and spectral light compositions. Sci. Hortic. 2019, 258, 108781. [Google Scholar] [CrossRef]
- Toldi, D.; Gyugos, M.; Darkó, É.; Szalai, G.; Gulyás, Z.; Gierczik, K.; Székely, A.; Boldizsár, A.; Galiba, G.; Müller, M.; et al. Light intensity and spectrum affect metabolism of glutathione and amino acids at transcriptional level. PLoS ONE 2019, 14, e0227271. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; He, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Far-red light suppresses glucosinolate profiles of Chinese kale through inhibiting genes related to glucosinolate biosynthesis. Environ. Exp. Bot. 2021, 188, 104507. [Google Scholar] [CrossRef]
- Ge, S.; He, L.; Jin, L.; Xia, X.; Li, L.; Ahammed, G.; Qi, Z.; Yu, J.; Zhou, Y. Light-dependent activation of HY5 promotes mycorrhizal symbiosis in tomato by systemically regulating strigolactone biosynthesis. New Phytol. 2021, 233, 1900–1914. [Google Scholar] [CrossRef]
- Delhaize, E.; Randall, P. Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol. 1995, 107, 207–213. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Deng, W.; Ahammed, G.; Wei, J.; Yan, P.; Zhang, L.; Fu, J.; Han, W. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef] [PubMed]







| Group Name | PPFD (μmol·m−2·s−1) | Light Transmittance (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | ||
| Control | 512.83 | 1110.40 | 761.27 | 1186.30 | 513.85 | 230.24 | 333.28 | / |
| T1 | 50.15 | 16.47 | 67.05 | 70.35 | 65.87 | 27.66 | 32.19 | 8.64 ± 3.87 b 1 |
| T2 | 7.75 | 2.23 | 10.32 | 11.45 | 8.89 | 3.87 | 5.99 | 1.32 ± 0.57 c 1 |
| T3 | 77.82 | 62.19 | 90.99 | 105.32 | 68.36 | 30.61 | 50.99 | 11.93 ± 3.54 a 1 |
| T4 | 11.81 | 3.75 | 19.50 | 16.96 | 12.11 | 6.41 | 7.00 | 1.98 ± 0.84 c 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Wang, Y.; Shen, K.; Wang, Q.; Ahammed, G.J.; Han, W.; Jin, Z.; Li, X.; Shi, Y. Effects of Differential Shading on Summer Tea Quality and Tea Garden Microenvironment. Plants 2024, 13, 202. https://doi.org/10.3390/plants13020202
Ge S, Wang Y, Shen K, Wang Q, Ahammed GJ, Han W, Jin Z, Li X, Shi Y. Effects of Differential Shading on Summer Tea Quality and Tea Garden Microenvironment. Plants. 2024; 13(2):202. https://doi.org/10.3390/plants13020202
Chicago/Turabian StyleGe, Shibei, Yameng Wang, Keyin Shen, Qianying Wang, Golam Jalal Ahammed, Wenyan Han, Zhifeng Jin, Xin Li, and Yuanzhi Shi. 2024. "Effects of Differential Shading on Summer Tea Quality and Tea Garden Microenvironment" Plants 13, no. 2: 202. https://doi.org/10.3390/plants13020202
APA StyleGe, S., Wang, Y., Shen, K., Wang, Q., Ahammed, G. J., Han, W., Jin, Z., Li, X., & Shi, Y. (2024). Effects of Differential Shading on Summer Tea Quality and Tea Garden Microenvironment. Plants, 13(2), 202. https://doi.org/10.3390/plants13020202










