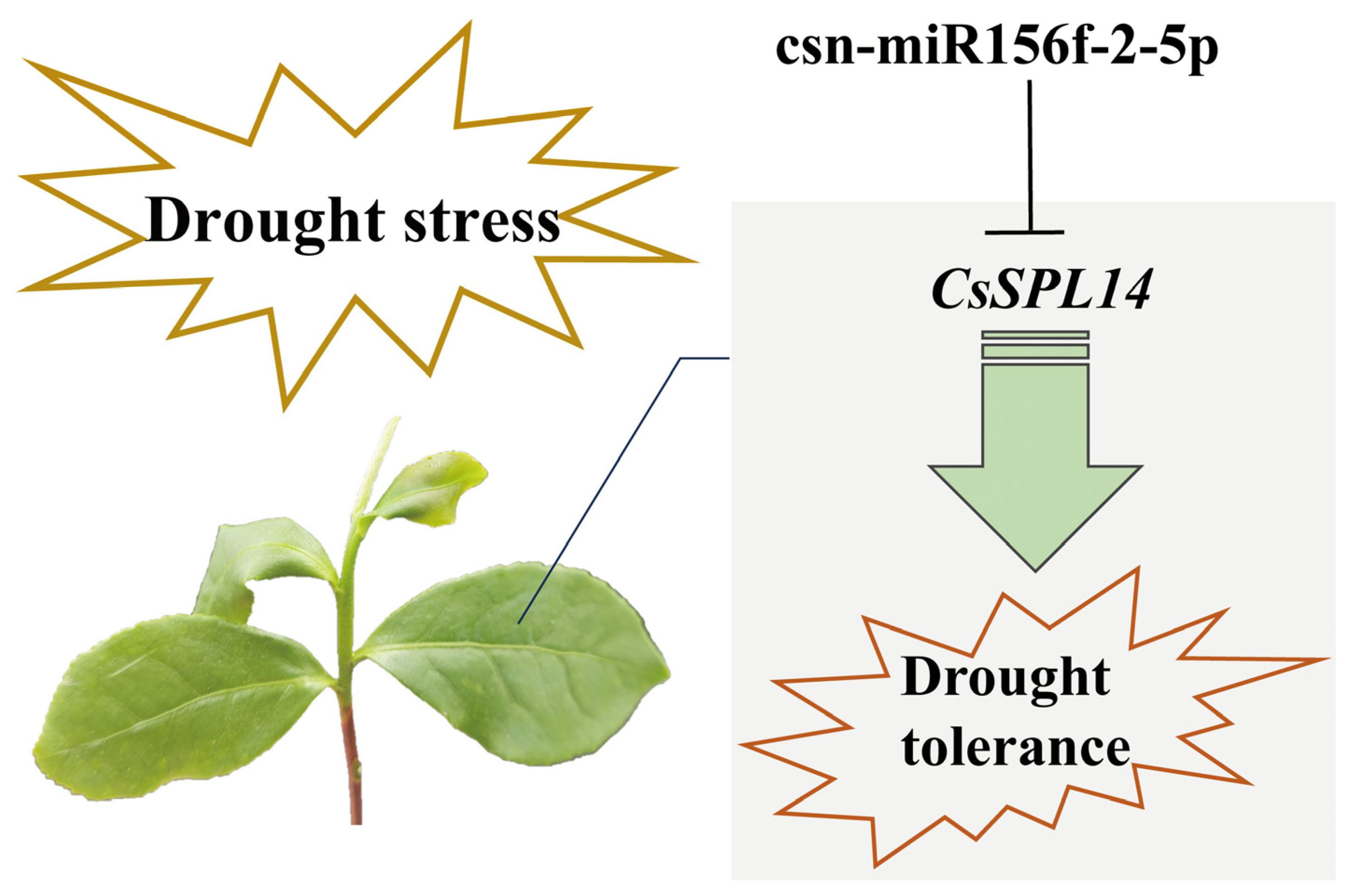
Abstract
The microRNA156 (miR156) family, one of the first miRNA families discovered in plants, plays various important roles in plant growth and resistance to various abiotic stresses. Previously, miR156s were shown to respond to drought stress, but miR156s in tea plants (Camellia sinensis (L.) O. Kuntze) have not been comprehensively identified and analyzed. Herein, we identify 47 mature sequences and 28 precursor sequences in tea plants. Our evolutionary analysis and multiple sequence alignment revealed that csn-miR156s were highly conserved during evolution and that the rates of the csn-miR156 members’ evolution were different. The precursor sequences formed typical and stable stem–loop structures. The prediction of cis-acting elements in the CsMIR156s promoter region showed that the CsMIR156s had diverse cis-acting elements; of these, 12 CsMIR156s were found to be drought-responsive elements. The results of reverse transcription quantitative PCR (RT-qPCR) testing showed that csn-miR156 family members respond to drought and demonstrate different expression patterns under the conditions of drought stress. This suggests that csn-miR156 family members may be significantly involved in the response of tea plants to drought stress. Csn-miR156f-2-5p knockdown significantly reduced the Fv/Fm value and chlorophyll content and led to the accumulation of more-reactive oxygen species and proline compared with the control. The results of target gene prediction showed that csn-miR156f-2-5p targeted SQUAMOSA promoter binding protein-like (SPL) genes. Further analyses showed that CsSPL14 was targeted by csn-miR156f-2-5p, as confirmed through RT-qPCR, 5′ RLM-RACE, and antisense oligonucleotide validation. Our results demonstrate that csn-miR156f-2-5p and CsSPL14 are involved in drought response and represent a new strategy for increasing drought tolerance via the breeding of tea plants.
1. Introduction
Drought stress is a devastating and unavoidable reality that is becoming increasingly prevalent in most parts of the world, and research data show that flash droughts occur frequently worldwide [1,2]. Drought not only causes serious agricultural production losses but also ecological damage, desertification, and soil erosion, among other things. As a result, drought has been recognized as an urgent global and environmental issue [3,4]. The tea plant (Camellia sinensis (L.) O. Kuntze) is an economically important crop grown all over the world. Drought stress is one of the most prominent natural challenges threatening major tea-producing areas in China; it remarkably affects the growth and development, yield, and quality of tea plants. Tea plants are sessile organisms that cannot escape drought when it occurs. Thus, to withstand drought stress, like other plants, tea plants display a series of interconnected response and defense mechanisms to safeguard their survival, which involve multiple processes. These may be morphological, physiological, or related to water stress sensing, stress signal transduction, and related gene expression regulation [5]. Recent studies have shown that (Z)-3-hexanol and eugenol can enhance the drought tolerance of tea plants by mediating glucosylation [6]. An exogenous application of 24-epibrassinolide can improve the drought resistance of tea plants by promoting the expression of genes involved in the biosynthesis of galactinol and abscisic acid [7]. In addition, several genes such as CsUGT71A59, CsGSTU8, and CsSnRK2.5 have shown the ability to enhance drought tolerance by regulating the accumulation of reactive oxygen species and altering abscisic acid homeostasis [8,9,10]. However, the specifics of the tea plant’s drought resistance mechanism are still unclear. Therefore, it is necessary to study said drought resistance mechanism and thereby improve the drought tolerance of tea plants.
MicroRNAs (miRNAs) are a class of 20–24-nucleotide (nt) endogenous non-coding RNAs in eukaryotes that regulate gene expression at the transcriptional, post-transcriptional, or translational level through complementary binding with target genes. As an important component of gene expression regulation, miRNAs play an important role in plant growth, development, and response to stress through the precise regulation of corresponding target genes [11,12]. Recently, the strategy of fine-tuning miRNAs based on the regulation of target gene transcription has been highlighted as an effective biotechnological method of improving tolerance to abiotic or biotic stresses in economically vital crops. Several studies have confirmed that many miRNAs, such as miR156 [13,14], miR166 [15], miR319 [16], and miR408 [17,18], can improve plant stress resistance to biotic or abiotic stresses by overexpressing or knocking out miRNAs. In apples, MIR156a overexpression weakens salt resistance [19]. The knock-down of miR166 can enhance abiotic stress resistance [20]. The overexpression of MIR319b and miR319 in the target simulated form (MIM319) demonstrated that miR319 positively regulates salt tolerance in switchgrass [21]. The drought resistance of Os-miR408 transgenic plants can be improved through changes in their leaf morphology [22]. miR156s are a family of small RNAs that were one of the first miRNAs discovered in Arabidopsis; they are highly conserved in plant species. In general, miR156s regulate the expression of the SQUAMOSA promoter binding protein-like (SPL) gene family through transcriptional cleavage or translation inhibition, thus inducing resistance to drought stress. SPLs are a family of plant-specific transcription factors with highly conserved SBP domains that play an essential role in plant growth and development and response to stress [14,23,24]. A recent study showed that the miR156-mediated silencing of SPLs improved drought stress resilience and promoted leaf gas exchange and abscisic acid sensitivity [25,26]. In alfalfa, miR156 was shown to improve drought resistance by downregulating SPL13 expression; in the same study, the overexpression of miR156 led to stronger drought resistance [13]. Therefore, we used an analysis of the csn-miR156 family as the starting point of our study, progressing to explore the regulating effect of csn-miR156s on drought resistance.
In this study, 47 mature sequences and 28 precursor sequences in tea plants were identified based on published small-RNA sequencing results. Herein, we will provide a detailed overview of the evolution of miR156s and pre-miR156s in tea plants. We detected the expression level of csn-miR156s while being affected by different degrees of drought using RT-qPCR; then, csn-miR156f-2-5p was screened and knocked down. Compared to the control, the csn-miR156f-2-5p-knockdown tea plants showed lower tolerance to drought stress. In order to further explore the drought resistance mechanism of csn-miR156f-2-5p, we also predicted and analyzed the csn-miR156f-2-5p targets. The relationship between csn-miR156f-2-5p and its target genes was verified using reverse transcription quantitative PCR (RT-qPCR) and 5′RACE, followed by antisense oligonucleotide (AsODN) treatment in tea plants. To summarize, we explored the mechanisms mediated by the miR156-SPL regulatory module during drought in tea plants to provide a reference for breeding drought resistance in tea plants.
2. Results
2.1. Identification and Analysis of miR156 in Plants
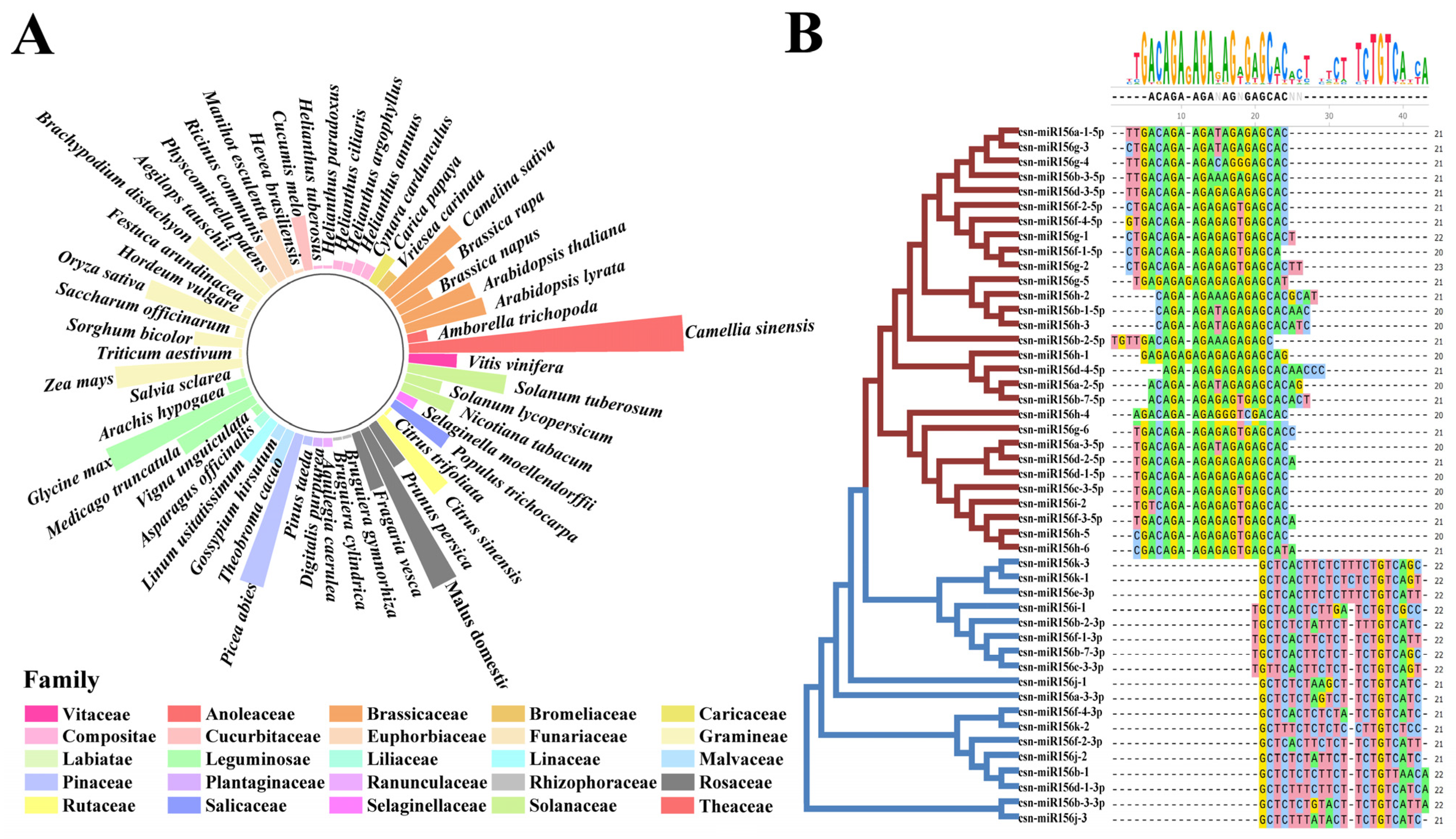
To understand the distribution of the miR156 family in plants, a total of 447 mature sequences of miR156 from 54 species were downloaded from miRbase, and 47 csn-miR156 mature sequences were identified from published small-RNA sequencing results [27,28,29,30,31]. The miR156s were widely distributed in angiosperms, gymnosperms, and mosses, and the number of miR156s in angiosperms was the largest. The number of miR156 members in each species ranged from 1 to 47 (Figure 1A). To further investigate the evolutionary relationship between miR156s in the plants, we used Physcomitrella patens (3), Selaginella moellendorffii (4), Oryza Sativa (18), Glycine max (28), Vitis vinifera (9), Arabidopsis thaliana (13), Medicago truncatula (15), Nicotiana tabacum (10), Brassica napus (7), Picea abies (27), and Camellia sinensis (47) to construct a phylogenetic tree (Figure S1). The phylogenetic tree was divided into three clades. All the miR156s derived from the 5′ end of the precursor, such as csn-miR156a-1-5p, osa-miR156l-5p, mtr-miR156i-5p, ath-miR156c-5p, and so on, belonged to clade I and clade II. Clade III consisted of miR156s derived from the 3′ end of the precursor and csn-miR156i-1, csn-miR156j-1/2/3, and csn-miR156k-2.
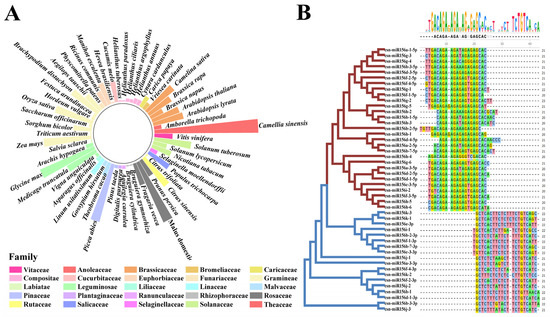
Figure 1.
Statistics of plants’ miR156 sequences alongside multiple sequence alignment of csn-miR156s. (A) Numeric distribution of miR156s in 55 plant species; the height of the column indicates their quantity. (B) Classification and multiple sequence alignment of miR156 family members in Camellia sinensis.
Alignment of the mature sequences of the miR156 family of the above 10 plants showed that miR156s were roughly divided into two groups based on whether they were derived from the 5′ or 3′ arms of the precursor (Figure S2). The results showed that most miR156 family members of different species were 21 nt, and a few were 20 nt or 22 nt. Except for a few base mutations, deletions, or insertions in the 12th, 15th, and 16th bases, the rest of the bases are highly conserved. Further comparison of the mature sequence of miR156 in tea plants showed that the csn-miR156s were divided into two types: the 5′-end sequence and the 3′-end sequence (Figure 1B). Except for the 1st, 12th, and 15th base differences at the 5′ end and the 5th, 13th, and 19th base differences at the 3′ end, the bases of the csn-miR156s were conserved.
2.2. Sequence Analysis and Chromosomal Localization of csn-MIR156s in the Tea Plant Genome
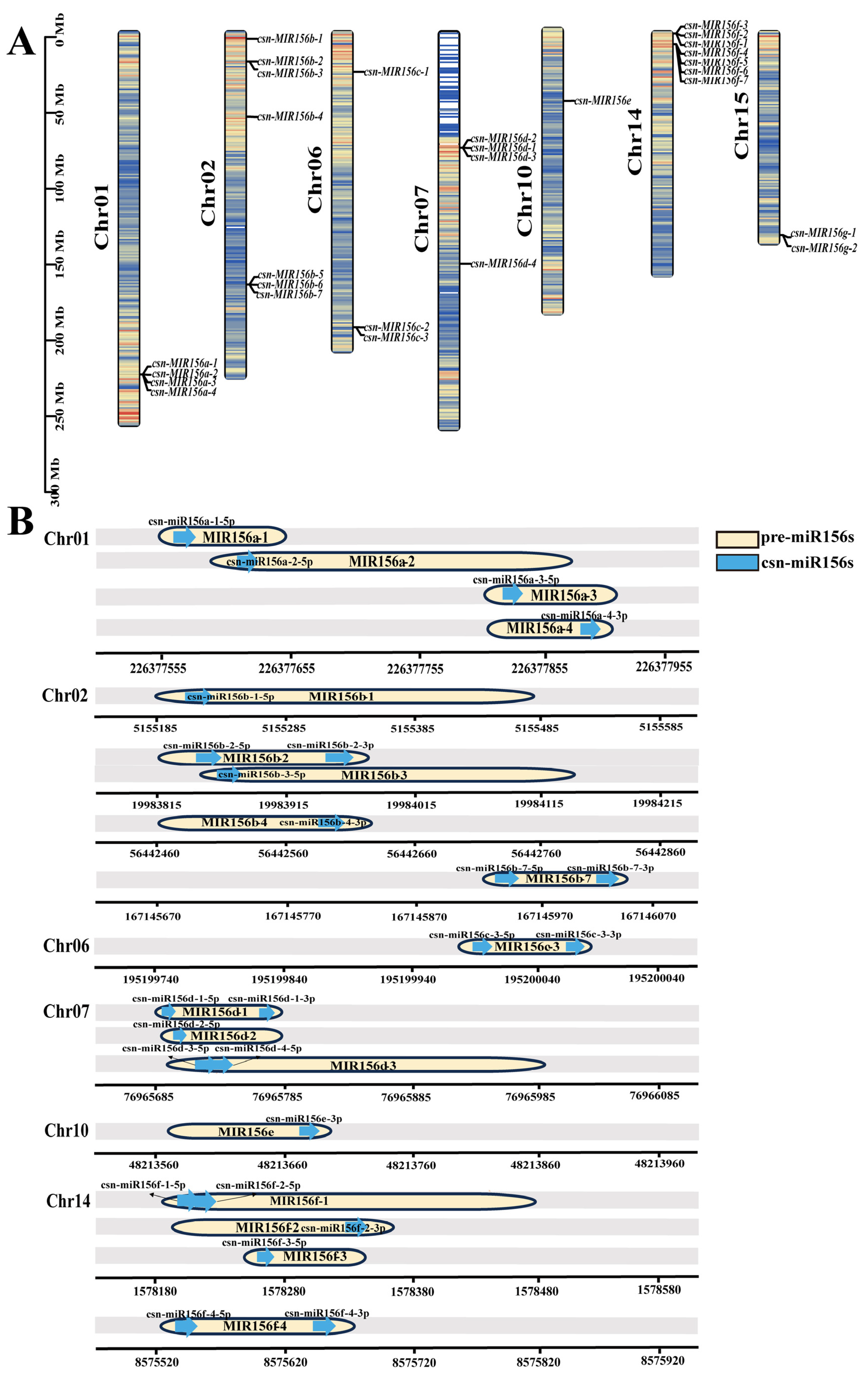
Based on published small-RNA sequencing results, 28 precursor sequences of the miR156 family were identified in the tea plants. The identified pre-miR156s were within 300 nt, meaning they were within the normal range of plant miRNA precursor length, and the sequences were conserved at the 5′ and 3′ ends (Figure S3). To determine the chromosomal locations of csn-miR156 family members, we aligned the precursor sequences of csn-miR156s (csn-MIR156s) with the ‘Tieguanyin’ genome (Figure 2A). The csn-MIR156s were located on chr01 (csn-MIR156a), chr02 (csn-MIR156b), chr06 (csn-MIR156c), chr07 (csn-MIR156d), chr10 (csn-MIR156e), chr14 (csn-MIR156f), and chr15 (csn-MIR156g). Csn-miR156a/b/c/d/e/f were named according to the position of the mature sequence on their corresponding precursor (Figure 2B). Among them, csn-miR156f-1-5p and csn-miR156f-2-5p were likely derived from csn-MIR156f-1 on chr14, while csn-miR156g/h/i/j/k did not correspond to the 28 precursor sequences.
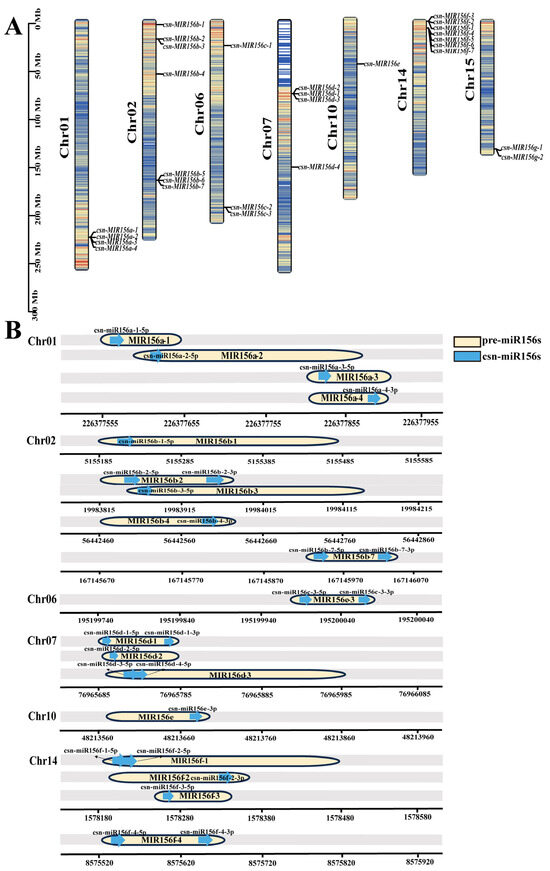
Figure 2.
Location of the csn-MIR156 family in the ‘Tieguanyin’ genome. (A) Location of csn-miR156s’ precursor sequences on chromosomes. The different colors in the chromosomes represent gene density. (B) The position of mature csn-miR156s on precursors. The blue arrow represents the mature sequence, and the yellow capsule represents the precursor sequence.
2.3. Predicted Secondary Structure Analysis and Phylogenetic Analysis of csn-MIR156s in the Tea Plant Genome
In order to understand the secondary structure of csn-MIR156s, the RNAfold web server (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi; accessed on 25 July 2023) was used, with default parameters, to analyze the secondary structures of the csn-MIR156s. The results showed that almost all the csn-MIR156s formed typical and stable structures with a stem–loop hairpin (Figure S4). In addition, we also labeled the location of the mature sequence on the secondary structure of the precursor and found that the csn-MIR156s’ secondary structures were highly stable in the region (arm) of the mature miRNA sequences, while the rest of the sequences (loop) were more unstable. The minimum folding free energy of the csn-MIR156s varied considerably, ranging from −95.60 to −13.40 kcal/mol. The minimum folding free energy was proportional to the sequence length of the csn-MIR156s.
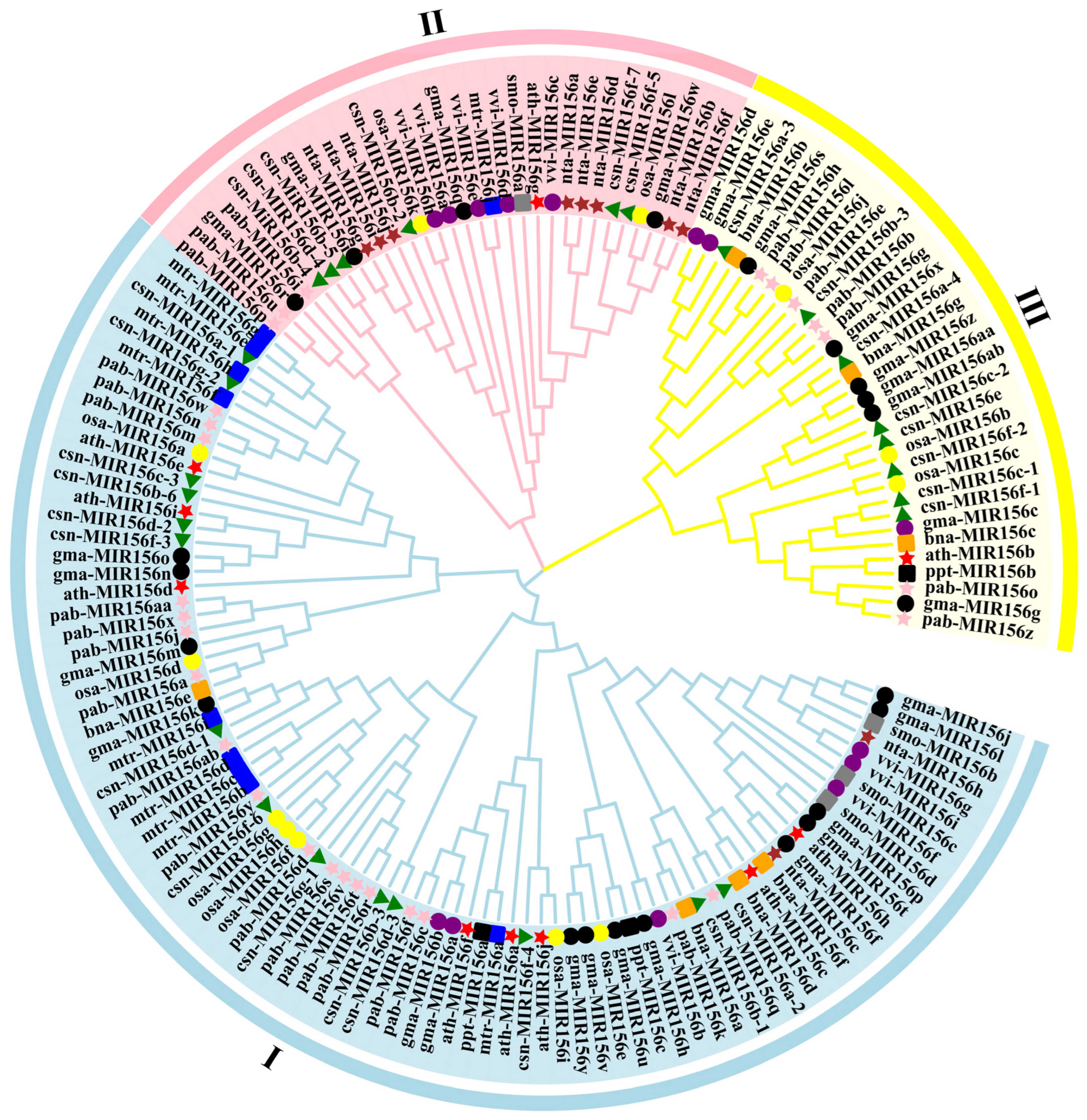
To further examine the evolutionary relationship among the 28 csn-MIR156s, we used MEGA 10.0 software to analyze the evolution of the pre-miR156 sequences from P. patens (3), S. moellendorffii (4), O. sativa (12), V. vinifera (nine), A. thaliana (10), G. max (28), M. truncatula (10), N. tabacum (10), C. sinensis (28), B. napus (seven), and P. abies (27). The MIR156s were mainly divided into three branches. Specifically, 18 pab-MIR156s (pabMIR156a/c/f/d/j/k/m/n/q/v/r/s/t/w/x/y/aa/ab/), 15 gam-MIR156 (gam-MIR156a/b/f/h/j/k/l/m/n/o/p/t/u/v/y), 14 csn-MIR156 (csn-MIR156a-1/a-2/b-1/b-3/b-6/c-3/d-1/d-2/d-3/f-3/f-4/f-6/g-1/g-2), 7 osa-MIR156 (osa-MIR156a/d/e/f/g/h/i), 4 vvi-MIR156s (vvi-MIR156b/f/g/i), 2 nta-MIR156 (nta-MIR156c/h), 8 mtr-MIR156 (mtr-MIR156b/c/d/e/f/g/h/i), 8 ath-MIR156 (ath-MIR156a/c/d/e/f/h/i/j), 4 bna-MIR156 (bna-MIR156a/d/e/f), and 2 ppt-MIR156 (ppt-MIR156a/c) members were clustered into a broad branch. The second branch consisted of six csn-MIR156s (csn-MIR156b-2/b-4/b-5/d-4/f-5/f-7), three pab-MIR156s (pab-MIR156i/p/u), four gam-MIR156s (gam-MIR156i/q/r/w), five vvi-MIR156s (vvi-MIR156a/c/d/e/h), two osa-MIR156s (osa-MIR156k/l), eight nta-MIR156s (nta-MIR156a/b/d/e/f/g/i/j), one mtr-MIR156 (mtr-MIR156j), and one ath-MIR156 (ath-MIR156g). Eight csn-MIR156s (csn-MIR156a-3/a-4/b-4/c-1/c-2/e/f-1/f-2), six pab-MIR156s (pab-MIR156b/e/g/h/l/o/z), nine gam-MIR156s, three osa-MIR156s, one ath-MIR156s, three bna-MIR156s, and one ppt-MIR156 (ppt-MIR156b) were grouped into the third branch (Figure 3).
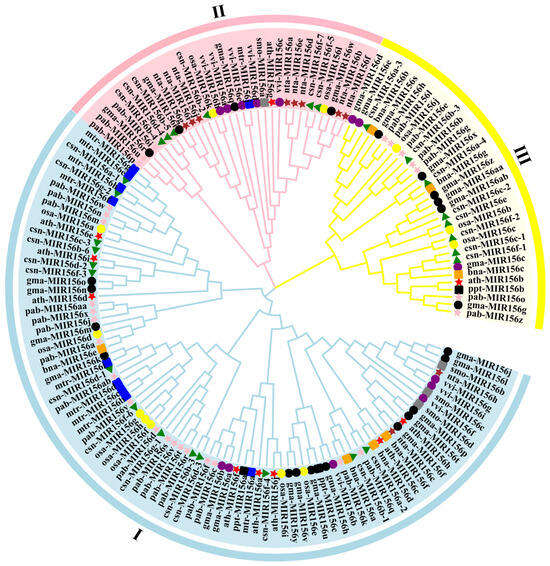
Figure 3.
Phylogenetic tree of plants’ MIR156 sequences. vvi. Vitis vinifera; smo. Selaginella moellendorffii; osa. Oryza sativa; csn. Camellia sinensis; ath. Arabidopsis thaliana; gma. Glycine max; mtr. Medicago truncatula; nta. Nicotiana tabacum; bna. Brassica napus; pab. Picea abies; ppt. Physcomitrella patens.
Further analysis revealed that the 28 miR156 precursors of the tea plant were present in three branches, as well as in P. abies, A. thaliana, M. truncatula, and G. max. Studies have shown that the evolutionary distance of miRNA families is not closely related to the genetic relationship between species [32,33,34]. In this study, members of csn-MIR156 and the MIR156s of monocotyledons, dicotyledons, gymnosperms, and ferns were widely distributed on the phylogenetic tree (Figure 3), suggesting that the csn-MIR156 family is a conserved and ancient miRNA family.
2.4. Analysis of Cis-Acting Elements in CsMIR156s Promoter Regions
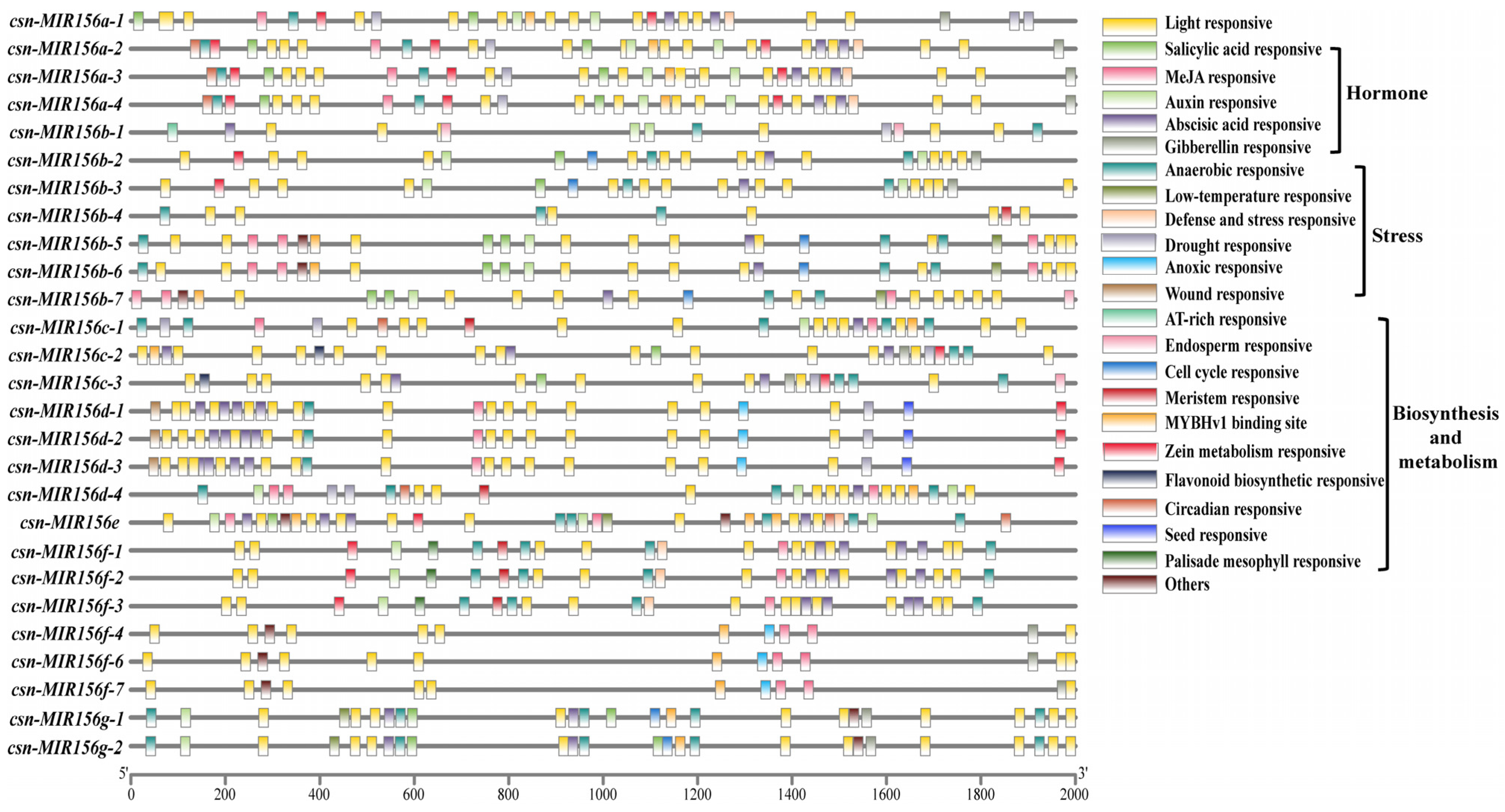
Transcription initiation is a vital stage of gene expression, and promoters are an important regulatory component of gene transcription initiation. The identification and analysis of the cis-acting elements of miRNA promoters will help to further our understanding of the transcriptional activation mechanism of miRNA, as well as its role in the overall regulatory network [35]. Therefore, to identify the function of CsMIR156s, 2000 bp upstream regions of CsMIR156s in tea plants were used as putative promoter regions for the prediction of cis-acting elements. In 28 CsmiR156s promoters, in addition to the core promoter element TATA-box and common CAAT-box elements, a total of 52 specific promoter cis-acting elements were identified. (Table S1), which were divided into the following five categories: light-responsive, hormone-responsive, stress-responsive, biosynthesis- and metabolism-related cis-acting elements, and others (A-box, 3-AF3 binding sites, 60K protein binding sites, and AT-rich sequences) (Figure 4). Among them, the ‘light-responsive’ (44.7%) category was shared among the 28 csn-MIR156s, and six stress-response elements, namely, ‘anaerobic-responsive element’ (63.6%), ‘drought-responsive element’ (15.0%), ‘defense and stress-responsive element’ (7.5%), ‘low-temperature-responsive element’ (5.6%), ‘anoxic-responsive element’ (5.6%), and ‘wound-responsive element’ (2.8%), were identified. A total of 12 CsMIR156s (CsMIR156a-1/a-2/a-3/a-4/b-3/c-1/c-2/c-3/d-1/d-2/d-3) were identified as possessing drought-responsive elements (MBS).
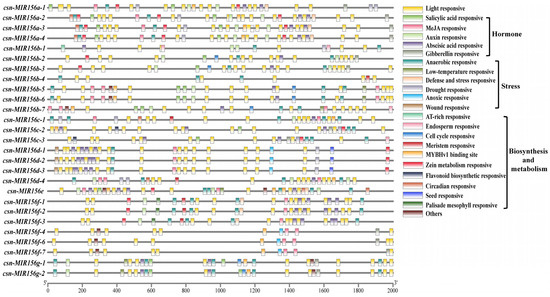
Figure 4.
Promoter analysis of CsMIR156 genes in C. sinensis.
2.5. Expression Analysis of csn-miR156s under Drought Stress
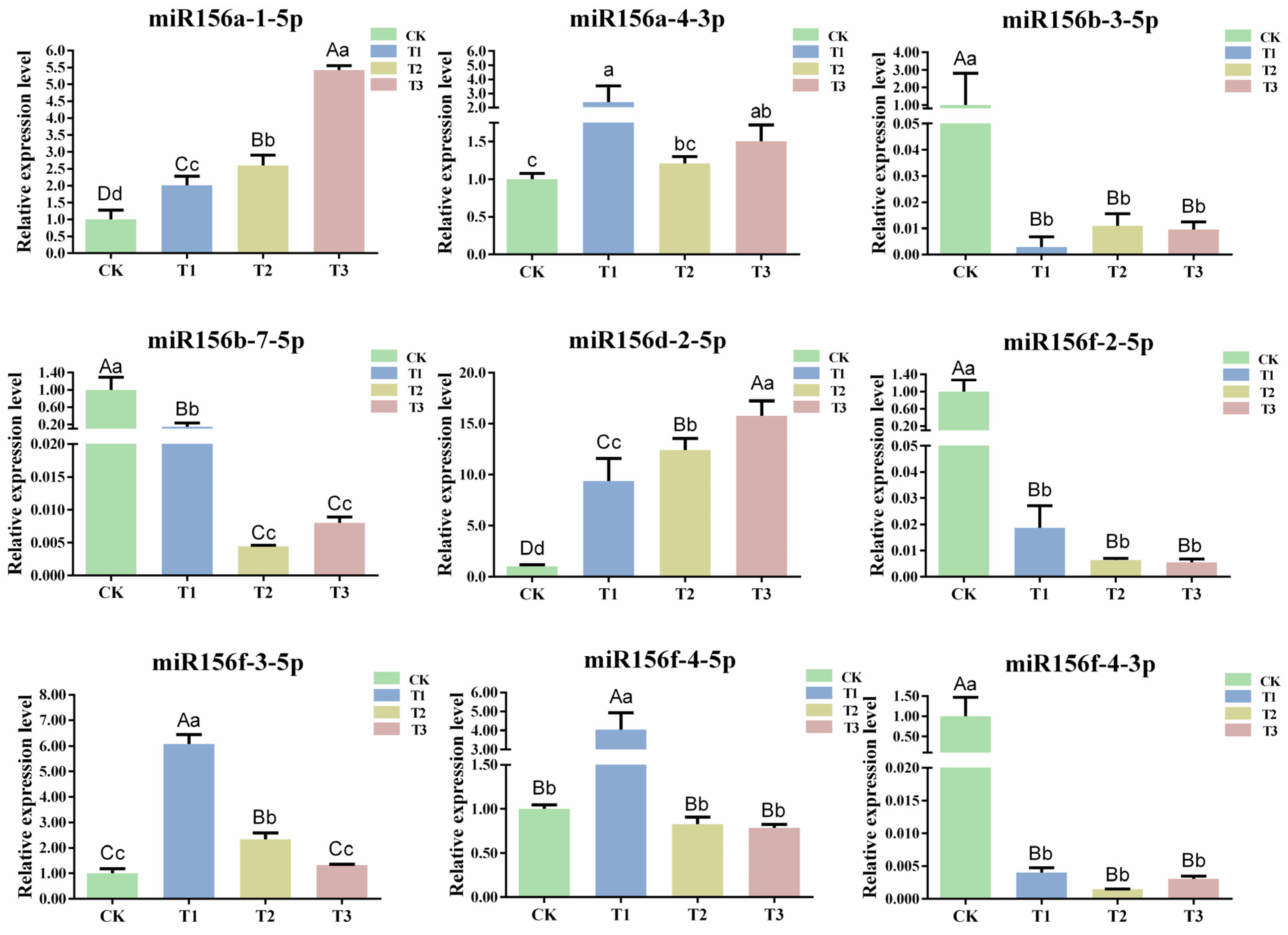
The length of miRNAs that guide AGO1 to cleave their target mRNAs in plants is generally 21 nt [12]. To explore the expression pattern of csn-miR156s under different drought degrees, nine 21 nt csn-miR156s that could localize on precursors (csn-miR156a-1-5p, csn-miR156a-4-3p, csn-miR156b-3-5p, csn-miR156b-7-5p, csn-miR156d-2-5p, csn-miR156f-2-5p, csn-miR156f-3-5p, csn-miR156f-4-5p, and csn-miR156f-4-3p) were chosen to detect expression patterns under drought stress via RT-qPCR. The expression patterns of the csn-miR156 members displayed a different trend in response to different degrees of drought. Specifically, the expression levels of miR156a-1-5p, miR156a-4-3p, miR156d-2-5p, and miR156f-3-5p showed a similar tendency. Their expression levels were upregulated compared with CK under T1, T2, and T3. The expression level of miR156f-4-5p increased significantly at T1 but decreased insignificantly at T2 and T3. Concurrently, the expression levels of miR156b-3-5p, miR156b-7-5p, miR156f-4-3p, and miR156f-2-5p decreased under different drought degrees compared with CK, and that of miR156f-2-5p declined continuously (Figure 5). Various studies have revealed that the expression levels of miR156s (mdmiR156a and ath-miR156) are continuously downregulated under abiotic stress, and miR156f-2-5p showed the same trend [19,36]. Therefore, miR156f-2-5p was selected for further analysis.
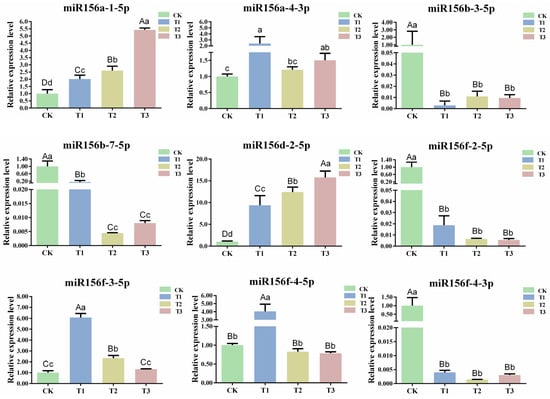
Figure 5.
Expression patterns of csn-miR156s under different degrees of drought. CK: normal water supply; T1: mild drought stress, T2: moderate drought stress, and T3: severe drought stress. Data are the means of three independent replicates ± standard deviation (SD). Different lowercase letters indicate significant differences (p < 0.05); different uppercase letters indicate highly significant differences (p < 0.01).
2.6. csn-miR156f-2-5p Participates in Drought Stress Response in Tea Plants
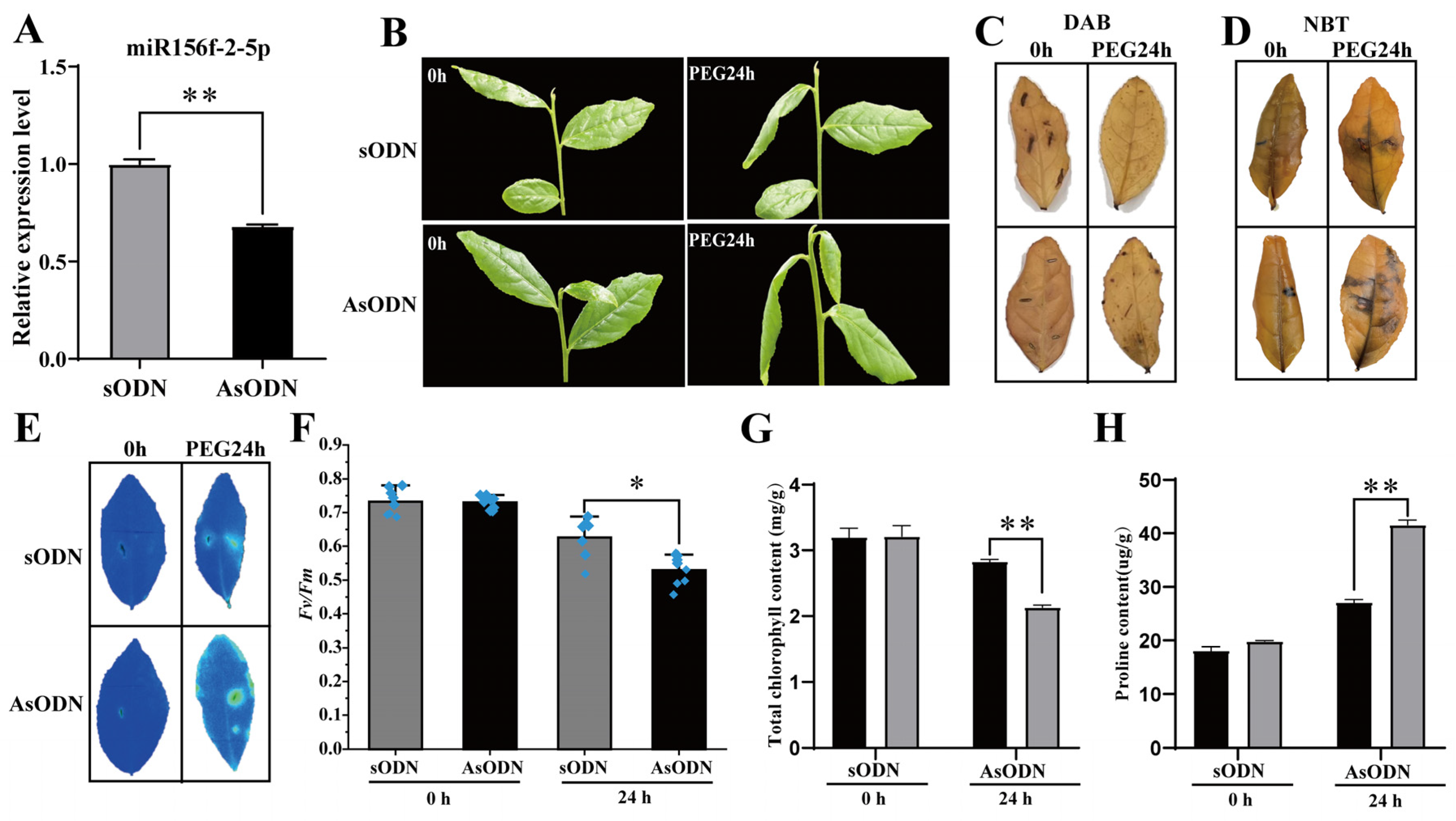
As there is no existing efficient and stable genetic transformation system for the tea plant, we used a gene-specific antisense oligodeoxynucleotide (AsODN) suppression strategy to knockdown csn-miR156f-2-5p in order to further confirm that csn-miR156f-2-5p participates in drought tolerance in tea plants. Drought stress usually causes osmotic stress in plants, which leads to restricted plant development [4]. Therefore, we intended to simulate drought with PEG 6000; firstly, we observed the expression pattern of csn-miR156f-2-5p during a 15% (w/v) polyethyleneglycol (PEG) treatment of tea plants. In contrast with previous natural drought results, csn-miR156f-2-5p increased significantly at 4 h and 12 h after PEG treatment and then decreased at 24 h (Figure S5). Subsequently, AsODN and sODN solutions were injected into the leaves of hydroponic tea plants, respectively. After 24 h of incubation after the injection of the AsODN solution, we successfully knocked down the expression of csn-miR156f-2-5p relative to the control (Figure 6A). The tea plants of the csn-miR156f-2-5p-knockdown (AsODN) and control (sODN) tea plants were immersed in a 15% PEG solution to assess drought tolerance. After 24 h of drought, the csn-miR156f-2-5p-knockdown tea plants showed a more severe degree of wilting than the controls (Figure 6B). Diaminobenzidine (DAB) staining (Figure 6C) and nitroblue tetrazolium (NBT) staining (Figure 6D) showed that more H2O2 and O2− accumulated in the AsODN leaves than in the sODN leaves after 24 h of drought. Consistent with their phenotypes, chlorophyll fluorescence imaging demonstrated that the leaves of the csn-miR156f-2-5p-knockdown plants maintained a lower level of chlorophyll fluorescence (Figure 6E), and the Fv/Fm ratio was significantly decreased in the csn-miR156f-2-5p-knockdown tea plants compared with that of the controls (Figure 6F). In addition, the total chlorophyll content of the csn-miR156f-2-5p-knockdown plants was significantly lower than that of the control plants (Figure 6G); the proline content was significantly higher (Figure 6H), indicating that csn-miR156f-2-5p plays a role in tea plants’ response to drought.
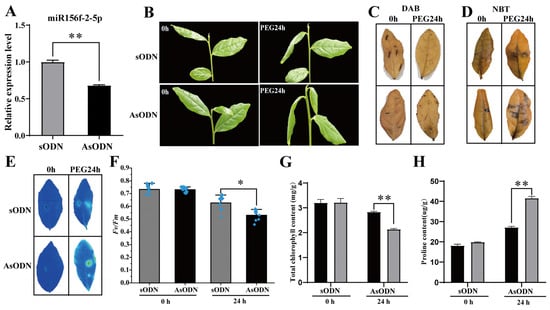
Figure 6.
Csn-miR156f-2-5p plays a role in the drought tolerance of tea plants. (A) Expression levels of csn-miR156f-2-5p control (sODN) and csn-miR156f-2-5p-knockdown (AsODN) tea plants. (B) Phenotypes of the control (sODN) and csn-miR156f-2-5p-knockdown (AsODN) tea plants after 0 h and 24 h of being subjected to drought conditions. (C,D) DAB (C) and NBT (D) staining of csn-miR156f-2-5p-knockdown (AsODN) and sODN tea leaves subjected to 15% PEG at 0 h and 24 h. (E,F) Phenotype of damage (E) and Fv/Fm values (F) of csn-miR156f-2-5p-knockdown (AsODN) and sODN tea leaves subjected to 15% PEG after 0 h and 24 h that were used to assess drought stress resistance. Blue indicates a normal state of the photosynthetic apparatus, while green and yellow indicate damage to photosystem II due to drought. (G,H) Total chlorophyll (G) and proline content (H) of csn-miR156f-2-5p-knockdown (AsODN) and sODN tea leaves subjected to 15% PEG after 0 h and 24 h (* p < 0.05 and ** p < 0.01).
2.7. Prediction and Expression Analysis of csn-miR156f-2-5p Target Genes in Tea Plants
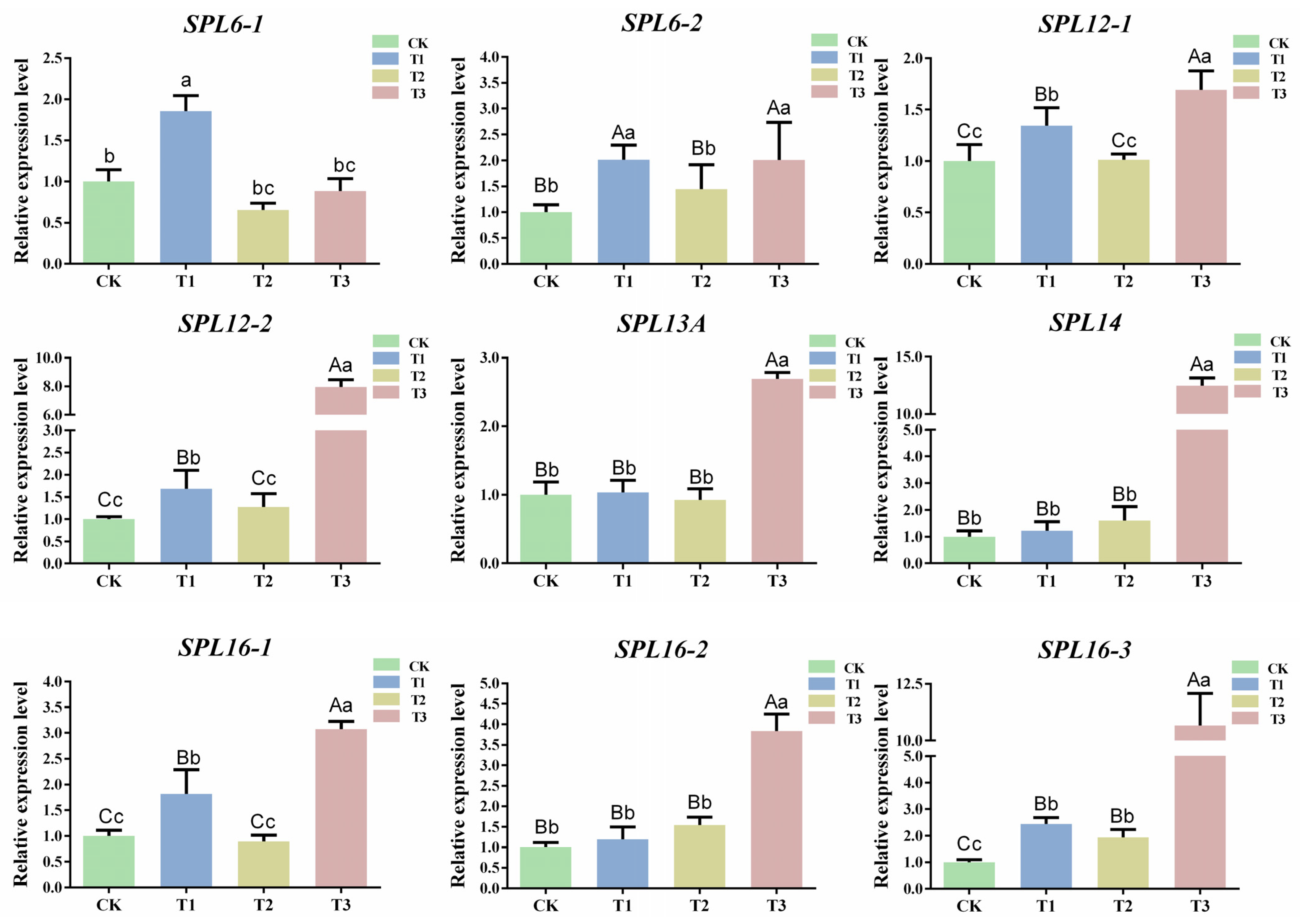
To further explore the mechanism of csn-miR156f-2-5p in the drought resistance of tea plants, the target genes of csn-miR156f-2-5p were predicted using the psRNATarget website (https://www.zhaolab.org/psRNATarget/; accessed on 3 August 2023), and the results showed that nine SPL genes were targeted by csn-miR156f-2-5p (Table S2). These SPL genes include two SPL6 (CsTGY06G0000542 and CsTGY15G0001546), two SPL12 (CsTGY06G0000121 and CsTGY10G0000091), one SPL13A (CsTGY15G0000933), one SPL14 (CsTGY10G0002410) and three SPL16 (CsTGY02G0000059, CsTGY02G0001323, and CsTGY10G0000081) genes (Table S3). In order to further understand the expression patterns of CsSPLs, the expression patterns of CsSPLs in different tissue parts of the tea plants (buds, flowers, stems, young leaves, old leaves, and roots) were analyzed (Figure S6). The results showed that the expression levels of SPL genes varied in different kinds of tissue, mainly in the buds, stems, and leaves. In particular, the expression levels of CsTGY06G0000121 (SPL12-1) and CsTGY10G0002410 (SPL14) were higher in buds and leaves. Subsequently, the expression patterns of nine SPL genes were explored under different degrees of drought (Figure 7). The expression levels of five CsSPL target genes (SPL6-2, SPL12-1, SPL12-2, SPL16-1, and SPL16-3) showed a similar upward trend, all of which were significantly upregulated compared with CK at T1 and T3. The expression levels of SPL6-1 and SPL13A increased significantly at T1. In addition, compared with CK, the expression levels of SPL14 and SPL16-2 continuously increased from T1 to T3. In most cases, miRNAs inhibited the expression of target genes. The results indicated that nine CsSPL genes were responding to drought stress, among which SPL14 and SPL16-2 were negatively correlated with the expression of csn-miR156f-2-5p during subjection to different degrees of drought stress.
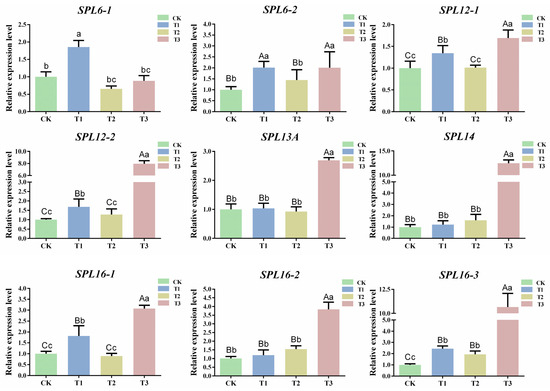
Figure 7.
Expression patterns of targets during subjection to different degrees of drought. Data are the means of three independent replicates ± standard deviation (SD). Different lowercase letters indicate significant differences (p < 0.05); different uppercase letters indicate highly significant differences (p < 0.01).
2.8. Csn-miR156f-2-5p Suppresses the Expression of CsSPL14 in Tea Plants
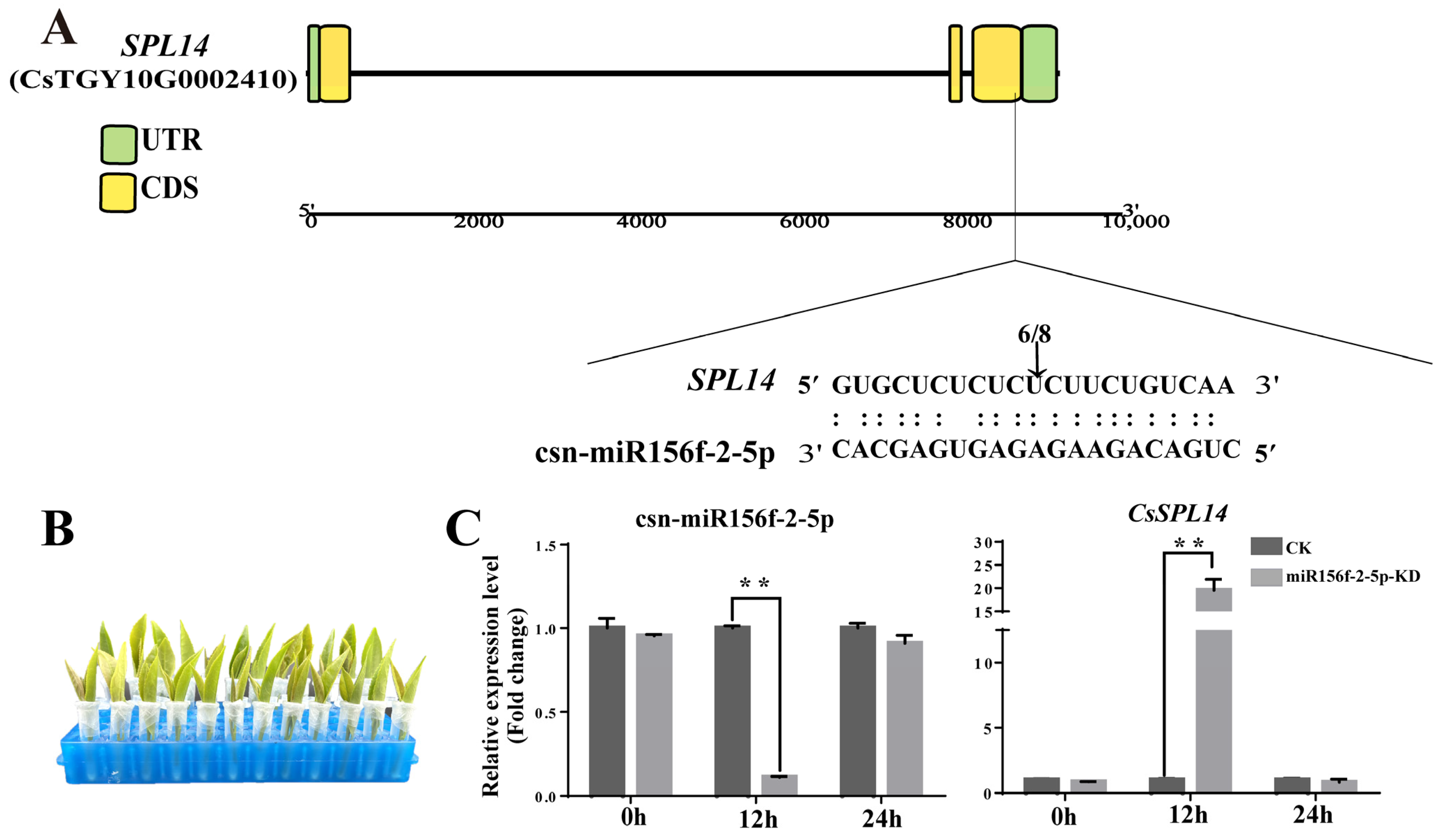
Due to its low background expression level, only CsSPL14 was successfully confirmed to contain cleaved sites. Multiple sequence alignment showed that the csn-miR156f-2-5p/CsSPL14 duplex is cleaved between the 10th and 11th nucleotide from the 5′ end of csn-miR156f-2-5p (Figure 8A). These results confirmed that CsSPL14 is the target gene of csn-miR156f-2-5p in tea plants. To explore the function of csn-miR156f-2-5p and its target gene CsSPL14 in tea plants, we further verified the relationship between csn-miR156f-2-5p and CsSPL14 expression. We incubated tender shoots of tea plants with either AsODN solution or sODN solution to knock down the expression of csn-miR156f-2-5p (Figure 8B). With the extension of incubation time, the expression level of csn-miR156f-2-5p decreased significantly in contrast to that of CK (sODN) at 24 h. The knockdown of csn-miR156f-2-5p (miR156f-2-5p-KD) expression led to increased levels of CsSPL14 expression compared with those of CK at 24 h after incubation, suggesting that CsSPL14 can be suppressed by csn-miR156f-2-5p (Figure 8C). These results illustrated that csn-miR156f-2-5p may play a role in the drought resistance mechanism of tea plants by inhibiting the expression levels of CsSPL14.
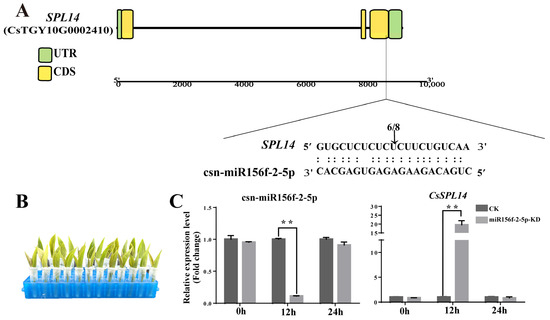
Figure 8.
Verification of the relationship between csn-miR156f-2-5p and CsSPL14. (A) CsSPL14 cleavage sites of csn-miR156f-2-5p, as identified using 5′RLM-RACE. (B) Knockdown of csn-miR156f-2-5p (miR156f-2-5p-KD) with AsODN and incubation, with a solution containing sense oligonucleotide (sODN) serving as a control. (C) RT-qPCR verification of csn-miR156f-2-5p knockdown (KD) and changes in the expression levels of CsSPL14 after 0 h, 12 h, and 24 h of incubation (** p < 0.01).
3. Discussion
3.1. Evolutionary Characteristics of the csn-miR156s Family
miR156s were some of the first miRNAs to be discovered; they are highly conserved [37] and widely distributed in 56 plant species, including angiosperms, gymnosperms, ferns, and bryophytes (Figure 1A). In recent years, with the rapid development of plant-genome-sequencing technology and the increasing abundance of plant genomes, research on miR156 in different plant species has gradually grown in prominence. For example, studies of the miR156 family in M. truncatula, Glycine max, A. thaliana, and O. sativa have shown that miR156s are an ancient miRNA family that play an important role in the regulation of plant growth, developmental stage transitions, flowering time, and stress responses [38,39,40,41,42]. Numerous studies have shown that the miR156 family is highly conserved in monocotyledons and dicotyledons [34]. In the present study, the evolutionary characteristics of the csn-miR156 family were analyzed in tea plants. In accordance with previous studies [14], our multiple sequence alignment results showed that miR156s were highly conserved in different species (Figure 1B). Sequences of csn-miR156s were relatively conserved among different plant species, but they also showed a degree of diversity. Studies have shown that the evolutionary distance of miRNA families is not closely related to the genetic relationship between species [33,37]. On the evolutionary tree, members of the csn-miR156 family of tea plants are distributed across various species (Figure 3), indicating that the csn-miR156 family of plants is highly complex in terms of its evolution; the evolution rates of each member of the csn-miR156 family are also different. This high level of complexity may explain why the evolutionary distance of the csn-miR156 gene family has little to do with how closely related species are to each other. Gene polymorphism involves gene replication, mutation, deletion, and duplication [43]. The mature sequences of csn-miR156 from the 5′ or 3′ arm of the precursors have a few base mutations, deletions, or insertions in addition to their most base-conserved sequences. We speculate that the diversity of the csn-miR156s family may also be a consequence of random mutations, which result in sequence diversity among csn-miR156s.
Based on small-RNA sequencing results and the NCBI database, the number of mature sequences of csn-miR156s was determined to be 47, while the number of corresponding precursors was 28. In the miRBase database, the quantities of mature sequences and precursors of A. thaliana, M. truncatula, and O. sativa were also inconsistent. The quantities of their mature sequences were 15, 15, and 18, respectively, while those of the precursors were 10, 10, and 12, respectively. The CsMIR156s were located on chr01, chr02, chr06, chr07, chr10, chr14, and chr15. Csn-miR156a/b/c/d/e/f were named according to the position of the mature sequence on their corresponding precursors (Figure 2B), while csn-miR156g/h/i/j/k did not correspond to the 28 precursor sequences. We observed that there was no one-to-one correspondence between the number of mature sequences and the number of precursor sequences in the same species; moreover, precursor genes may undergo mutations such as base substitution or deletion during the cutting and processing process to produce one or more mature sequences. It is also possible that some precursors were not detected due to the use of inadequate genome-sequencing techniques.
3.2. Different csn-miR156 Members May Respond to Drought Stress with Different Strategies
Cis-regulatory elements are important for studying the transcriptional regulation of genes. The prediction of cis-acting elements in the CsMIR156s promoter of the tea plant showed the existence of cis elements responsive to drought (MBS) (Figure 4). As expected, the expression patterns of csn-miR156s were affected by drought stress. The expression patterns of miR156 family members were different during subjection to drought stress, among which csn-miR156a-1-5p, csn-miR156a-4-3p, csn-miR156f-3-5p, and csn-miR156d-2-5p showed a pattern of upregulation during drought stress, while csn-miR156b-3-5p, csn-miR156b-7-5p, csn-miR156f-2-5p, csn-miR156f-4-5p, and csn-miR156f-4-3p showed a pattern of downregulation (Figure 5). It has been reported that different members of the same miRNA family may play various modal or regulatory roles in resistance to drought stress [44,45]. This may be due to the fact that the different csn-miR156 family members are derived from different precursors with different cis-responsive elements. These results indicate that members of the csn-miR156 family have a multitude of different roles in the drought resistance mechanism of tea plants, and the regulation of these plants’ genes during different degrees of drought may be complex and diverse. In fact, even within the same plant species, miRNAs can exhibit diverse responses to drought depending on specific conditions. For example, one study observed an increase in the expression level of miR398a/b in M. truncatula during drought stress [46], while another study reported a decrease in the expression level of this identical miRNA within the same plant species under similar drought conditions [47]. Indeed, the expression of csn-miR156f-2-5p in tea plants under PEG-simulated drought conditions was different from that found in a previous study. This difference may reflect different degrees of drought stress as well as the high sensitivity of some miRNAs to subtle differences in growth conditions. The differential expression of the same miRNA in the same plant species under drought conditions may be the result of different spatial–temporal behavior.
3.3. The csn-miR156 Family May Be Play a Role in the Drought Tolerance Mechanism by Cleaving CsSPLs
In the present study, we successfully knocked down csn-miR156f-2-5p and found that tea plants with csn-miR156f-2-5p knocked down might be more sensitive to drought (Figure 6). This is consistent with previous findings showing that plants with miR156silence are more susceptible to drought stress than the corresponding WT with regard to Arabidopsis and apples [48,49].MicroRNAs function by cleaving the corresponding mRNA or inhibiting translation to regulate the expression of their target genes [12]. SPLs are genes with crucial roles in plants’ response to abiotic stress and their development, and they are the main target genes of miR156s [50]. Prediction of target genes revealed that csn-miR156f-2-5p targets SPLs, while RT-qPCR showed that SPLs respond to drought stress (Figure 7). This prediction was verified using 5′ RLM-RACE, which indicated that csn-miR156f-2-5p cleaved CsSPL14 genes (Figure 8A). We also observed that the expression level of csn-miR156f-2-5p was inhibited by AsODN technology, and the expression level of CsSPL14 was significantly increased (Figure 8B). The miR156ab-SPL module has been shown to improve drought resistance by accumulating auxin to maintain growth and by enhancing the activities of antioxidant enzymes in Malus sieversii [49]. Moreover, the drought tolerance of miR156-overexpressing and SPL13-silenced plants was significantly improved in alfalfa. Their survival rate and antioxidant enzyme activity were higher than those of the control [13]. In cassava, miR156-MeSPL9 affected drought resistance by regulating the levels of protective metabolites and the jasmonic acid (JA) signaling pathway [51]. Therefore, we speculated that the cleaving of CsSPL14 by csn-miR156f-2-5p could be an important mechanism involved in the mediation of drought resistance in tea plants (Figure 9).
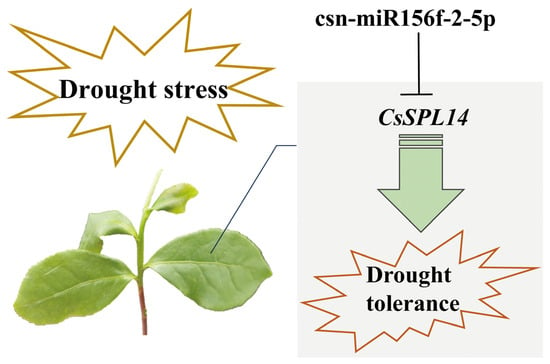
Figure 9.
The potential drought tolerance mechanisms of csn-miR156f-2-5p-CsSPL14 in tea plants.
Although it has been demonstrated that miR156-SPL modules are involved in plant responses to drought stresses, miR156-SPL combinations involving different SPLs may have different functions in different species. Therefore, it is necessary for us to further explore how the csn-miR156f-2-5p-CsSPL14 module participates in the drought resistance mechanism of tea plants. In summary, our results demonstrate that the csn-miR156f-2-5p-CsSPL14 module participates in drought tolerance in tea plants, providing a new research idea for improving tea plants’ drought resistance and productivity in terms of both yield and quality.
4. Materials and Methods
4.1. Plant Materials
The experimental materials were specimens of C. sinensis ‘Tieguanyin’. The experiment was conducted in the greenhouse of the College of Horticulture of Fujian Agriculture and Forestry University in June 2022 (119°14′ E, 26°05′ N). The daily temperature range of the greenhouse was 28–35 °C, and the atmospheric relative humidity was 50–75%. The soil field water capacity was 24%. Drought treatment was performed according to the method reported by Guo et al. [30]. The experimental design comprised treatment in four different forms: normal water supply (in which the soil water content was 80% ± 5% of the field water capacity; CK = 18.87%); mild drought stress treatment (wherein the soil water content was 60% ± 5% of field water capacity; T1 = 14.28%), moderate drought stress treatment (in which the soil water content was 40% ± 5% of the field water capacity; T2 = 9.96%); and severe drought stress (in which the soil water content was 20% ± 5% of the field water capacity; T3 = 5.90%). Each treatment was repeated 10 times. Tender C. sinensis leaves were fixed with liquid nitrogen and stored in a refrigerator at −80 °C until further analysis.
The relative water content of the soil was determined using the NY/T 52-1987 method. The relative water content of leaves was determined using the GB/T 8304-2002 method [45].
4.2. Bioinformatic Analyses of csn-miR156s and Target Gene Prediction
A total of 447 mature sequences of miR156 from 54 species were downloaded from miRbase, and we visualized the distribution of miR156 in 56 plant species using Origin (v2021) software. Some 47 mature sequences and 28 precursor sequences of csn-miR156s were obtained from the NCBI website (https://www.ncbi.nlm.nih.gov/; accessed on 18 June 2023) and named (Table S4). Subsequently, P. patens, S. moellendorffii, O. Sativa, G. max, V. vinifera, A. thaliana, M. truncatula, N. tabacum, B. napus, and P. abies members of the miR156 family were downloaded using the miRbase (Release 22.1) (http://www.mirbase.org/; accessed on 18 June 2023) database, which was used for subsequent analysis. DNAMAN 6.0 was used to analyze the csn-miR156 and pre-miR156 sequences. MEGA 10.0 software was used to construct a phylogenetic tree for the above sequences. The proximity neighbor-joining (NJ) method was implemented, and the Bootstrap coefficient was set to 1000. Evolution trees of csn-miR156s were visualized using Evolview (https://www.evolgenius.info/; accessed on 20 June 2023).
The secondary structure of csn-miR156 precursor sequences was predicted using RNAfold online website (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi; accessed on 25 July 2023). The 2000 bp upstream sequences of each csn-MIR156 and their cis-elements were predicted using the PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; accessed on 28 July 2023).
Based on genome sequences of ‘Tieguanyin’ cultivars [52], the csn-miR156 targets were predicted using the psRNATarget (http://plantgrn.noble.org/psRNATarget/?function=3; accessed on 3 August 2023) online tool. The default parameters were applied, but the expected value was set to 2.0.
4.3. Suppression of csn-miR156f-2-5p Expression in Tea Leaves Treated with AsODN
Candidate AsODNs were selected using Soligo online website (http://sfold.wadsworth.org/cgi-bin/soligo.pl; accessed on 4 August 2023), using csn-miR156f-2-5p as the input sequence (Table S5). To knock down csn-miR156f-2-5p expression, 1 mL of 50 μM AsODN-csn-miR156f-2-5p solution was injected into the leaves of hydroponic tea plant seedlings, and seedlings injected with sODN were used as controls. After incubation for 24 h, tea plants were treated with 15% (w/v) polyethyleneglycol (PEG) 6000 for 24 h (sODN/AsODN) [6]. Three independent biological replicates were established for each treatment. The obtained leaves were immediately frozen in liquid nitrogen and stored at 80 °C for further analysis.
Using Soligo online website (http://sfold.wadsworth.org/cgi-bin/soligo.pl; accessed on 4 August 2023), the antisense oligonucleotides for csn-miR156f-2-5p were designed, with csn-MIR156f-1 being used as an input sequence (Table S5). To knock down csn-miR156f-2-5p expression, one bud and two leaves of a freshly detached healthy plant of the variety ‘Tieguanyin’ were incubated in a 1.5 mL microcentrifuge tube that contained 50 μM of AsODN solution (containing 80 mM of sucrose solution) for various times (24 h and 48 h). Healthy tea branches incubated in sense ODNs (sODNs) together with 80 mM sucrose solution were used as controls [53]. The leaves were sampled at different time intervals to analyze gene expression levels. Three independent biological replicates were established for each treatment. The obtained leaves were immediately frozen in liquid nitrogen and stored at 80 °C for further analysis.
4.4. DAB and NBT Staining
Leaves were placed in a staining solution of 1 mg/mL of DAB or NBT (Solarbio, Beijing, China) prepared with 0.05 mol/L of sodium phosphate buffer (pH 7.5), evacuated for 5 min, and incubated in the dark at room temperature for 8 h before decolorization in 95% ethanol.
4.5. Determination of Fv/Fm, Chlorophyll, and Proline Content
The maximum efficiency of the photosystem II photochemistry (Fv/Fm) was measured using a chlorophyll fluorescence imaging system (IMAGING-PAM, Walz) after 30 min of dark adaptation and quantified using Origin (v2021) software. The chlorophyll content and proline content were determined using a chlorophyll content assay kit (HERUI, Fuzhou, China) and proline content kit (HERUI, Fuzhou, China), respectively.
4.6. RNA Extraction and RT-qPCR Analysis
Total RNA was extracted using Transzol UP (TransGen Biotech, Beijing, China). CsSPL genes and the first-strand cDNA of miRNA were synthesized using the TransScript® Uni One-Step gDNA Removal, cDNA Synthesis SuperMix (TransGen, Beijing, China), and TranScript® miRNA First-Strand cDNA Synthesis SuperMix (TransGen, Beijing, China) kits, respectively. The csn-miR156s, CsSPLs, and reference gene primers were designed using the Primer3 (https://bioinfo.ut.ee/primer3/; accessed on 5 August 2023) website (Table S5). RT-qPCR analysis of the csn-miR156s and their targets was conducted using TransStart® Tip Green qPCR SuperMix (TransGen, Beijing, China) via an ABI StepOnePlus™ Real-Time PCR System (Applied Biosystems, Waltham, MA). CsmiR222 and CsGAPDH were used as internal controls for the csn-miR156s and CsSPLs, respectively. The 2−ΔΔCt method was used to calculate relative expression. Finally, Tbtools and Prism 5 software were used to draw heat maps and bar charts.
4.7. Cleavage Site Identification with Modified 5′ RLM-RACE
Experimental validation of the predicted targets was conducted using a FirstChoice™ RLM-RACE Kit (Thermo Fisher Scientific, Carlsbad, CA, USA). The target gene cleavage primers were designed using DNAMAN 6.0 (Table S5). Two rounds of nested PCR amplification were performed, using the 5′ RACE outer primer and inner primer alongside target-gene-specific outer primer and inner primers, respectively. The PCR products were purified using an EasyPure® Quick Gel Extraction Kit (TransGen, Beijing, China) and cloned into a Blunt Zero cloning vector (TransGen, Beijing, China); subsequently, single colonies of putative transformants of at least eight independent clones were sequenced.
4.8. Statistical Analysis
Data were analyzed using SPSS 25 software and are presented as the means ± SD of three independent biological replicates unless otherwise indicated. Significances were determined at p < 0.05 via one-way ANOVA analysis.
5. Conclusions
In this study, a total of 47 mature members and 28 precursor members of the miR156 family were identified in tea plants; they were found to be evolutionarily conservative and diverse. Moreover, our RT-qPCR analysis indicated that csn-miR156 family members are active in response to drought stress. In knocking down csn-miR156f-2-5p, we found that affected tea plants might be more sensitive to drought. The prediction and expression levels of target genes indicated that CsSPLs were involved in drought response. The 5′RACE and AsODN results demonstrated that csn-miR156f-2-5p cleaved and suppressed CsSPL14. Furthermore, AsODN confirmed that csn-miR156f-2-5p negatively regulated the expression of CsSPL14.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13020201/s1. Figure S1: Phylogenetic tree of plants’ miR156 sequences. vvi, Vitis vinifera; smo, Selaginella moellendorffii; osa, Oryza sativa; csn, Camellia sinensis; ath, Arabidopsis thaliana; gma, Glycine max; mtr, Medicago truncatula; nta, Nicotiana tabacum; bna, Brassica napus; pab, Picea abies; ppt, Physcomitrella patens. Figure S2: Sequence alignment analysis of miR156s. Different highlight colors represent different homology levels of sequence. Black, ≥100%; red, ≥75%; green, ≥50%. Figure S3: Sequence alignment among C. sinensis pre-miR156s. Different highlight colors represent different homology levels of sequence. Black, ≥100%; red, ≥75%; green, ≥50%. Figure S4: Stem–loop structures of csn-MIR156s in C. sinensis. The mature miRNA sequences are marked with a black line. Figure S5: Expression patterns of the csn-miR156f-2-5p at 0 h, 2 h, 4 h, 12 h, and 24 h after 15% PEG treatment. Data are the mean of three independent replicates ± standard deviation (SD). Different lowercase letters indicate significant differences (p < 0.05); different uppercase letters indicate highly significant differences (p < 0.01). Figure S6: Tissue-specific gene expression patterns of nine SPL genes targeted by csn-miR156f-2-5p in buds, flowers, stems, leaves, and roots. Table S1: Cis-Acting regulatory element analysis of MIR156 promoters. Table S2: Summary of target genes corresponding to csn-miR156f-2-5p. Table S3: Identification of target genes corresponding to csn-miR156f-2-5p. Table S4: Identification of 47 mature miRNA sequences and 28 precursor sequences. Table S5: The gene primers presented in this paper.
Author Contributions
Conceptualization, S.W. and Y.G.; methodology, S.W. and C.Z. (Chengzhe Zhou); software, S.W. and C.Z. (Cheng Zhang); validation, S.W., C.T., N.Y., and A.Z.; formal analysis, S.W.; data curation, S.W. and Y.C.; writing—original draft preparation, S.W.; writing—review & editing, S.W., C.Z. (Chengzhe Zhou), and Y.G.; visualization, S.W.; supervision, Y.G. and Z.L.; funding acquisition, Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Construction Project for Technological Innovation and Service System (K1520005A01), the Rural Revitalization Tea Industry Technical Service Project of Fujian Agriculture and Forestry University (11899170145), the “Double First-class” Scientific and Technological Innovation Capacity and Enhancement Cultivation Plan of Fujian Agriculture and Forestry University (KSYLP004), the Construction of Plateau Discipline of Fujian Province (102/71201801101), the Tea Industry Branch of Collaborative Innovation Institute of Fujian Agriculture and Forestry University (K1521015A), and the Special Fund for Science and Technology Innovation of Fujian Zhang Tianfu Tea Development Foundation (FJZTF01).
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Christian, J.I.; Basara, J.B.; Hunt, E.D.; Otkin, J.A.; Furtado, J.C.; Mishra, V.; Xiao, X.; Randall, R.M. Global distribution, trends, and drivers of flash drought occurrence. Nat. Commun. 2021, 12, 6330. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Nadarajah, K.; Kumar, I.S. Drought response in rice: The miRNA story. Int. J. Mol. Sci. 2019, 20, 3766. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, M.; Jing, T.; Wang, J.; Lu, M.; Pan, Y.; Du, W.; Zhao, C.; Bao, Z.; Zhao, W.; et al. (Z)-3-Hexenol integrates drought and cold stress signaling by activating abscisic acid glucosylation in tea plants. Plant Physiol. 2023, 193, 1491–1507. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Dong, Y.; Liu, Y.; Liu, L.; Wan, S.; He, J.; Yu, Y. Accumulation of galactinol and ABA is involved in exogenous EBR-induced drought tolerance in tea plants. J. Agric. Food Chem. 2022, 70, 13391–13403. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol functions as a signal mediating cold and drought tolerance via UGT71A59-mediated glucosylation in tea plants. Plant J. 2022, 109, 1489–1506. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Xiao, Y.; Zhang, Y.; Liu, Y.; Wan, S.; Liu, L.; Dong, Y.; Liu, H.; Yu, Y. CsGSTU8, a glutathione S-transferase from Camellia sinensis, is regulated by CsWRKY48 and plays a positive role in drought tolerance. Front. Plant Sci. 2021, 12, 795919. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, S.; Liu, X.; He, J.; Cheng, L.; Duan, M.; Liu, H.; Wang, W.; Yu, Y. Overexpression of CsSnRK2.5 increases tolerance to drought stress in transgenic Arabidopsis. Plant Physiol. Bioch. 2020, 150, 162–170. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant noncoding RNAs: Hidden players in development and stress responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and their regulatory roles in plant-environment interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Feyissa, B.A.; Arshad, M.; Gruber, M.Y.; Kohalmi, S.E.; Hannoufa, A. The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. Bmc Plant Biol. 2019, 19, 434. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, J.; Huang, J.; Wang, C.; Zhang, L.; Feng, S. Genome-wide identification of miR156 and SPL family genes and phenotypic analysis of vegetative phase change in pepper (Capsicum annuum L.). Gene 2023, 877, 147542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Su, L.Y.; Zhang, S.T.; Xu, X.P.; Chen, X.H.; Li, X.; Jiang, M.Q.; Huang, S.Q.; Chen, Y.K.; Zhang, Z.H.; et al. Analyses of microRNA166 gene structure, expression, and function during the early stage of somatic embryogenesis in Dimocarpus longan Lour. Plant Physiol. Bioch. 2020, 147, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, H.; Zhu, J.; Tao, L.; Wei, C. miR319a targeting of CsTCP10 plays an important role in defense against gray blight disease in tea plant (Camellia sinensis). Tree Physiol. 2022, 42, 1450–1462. [Google Scholar] [CrossRef]
- Hajyzadeh, M.; Turktas, M.; Khawar, K.M.; Unver, T. miR408 overexpression causes increased drought tolerance in chickpea. Gene 2015, 555, 186–193. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Yu, K.; Wang, H.L.; Xu, H.; Song, C.; Zhao, Y.; Wen, J.; Fu, C.; Li, Y.; et al. Manipulating microRNA miR408 enhances both biomass yield and saccharification efficiency in poplar. Nat. Commun. 2023, 14, 4285. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Guo, Q.; Zhang, X.; Zhou, L.; Zhang, Y.; Zhang, C. Conservation and diversity of miR166 family members from highbush blueberry (Vaccinium corymbosum) and their potential functions in abiotic stress. Front. Genet. 2022, 13, 919856. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yan, J.; Wang, K.; Luo, H.; Zhang, W. MiR319-mediated ethylene biosynthesis, signalling and salt stress response in switchgrass. Plant Biotechnol. J. 2019, 17, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Hang, N.; Shi, T.; Liu, Y.; Ye, W.; Taier, G.; Sun, Y.; Wang, K.; Zhang, W. Overexpression of Os-microRNA408 enhances drought tolerance in perennial ryegrass. Physiol. Plantarum. 2021, 172, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.Y.; Felippes, F.F.; Liu, C.J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.R.; Baurle, I. Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef]
- Visentin, I.; Pagliarani, C.; Deva, E.; Caracci, A.; Tureckova, V.; Novak, O.; Lovisolo, C.; Schubert, A.; Cardinale, F. A novel strigolactone-miR156 module controls stomatal behaviour during drought recovery. Plant Cell Environ. 2020, 43, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Brun, G. At the crossroads of strigolactones and abscisic acid pathways: A role for miR156. Plant Cell Environ. 2020, 43, 1609–1612. [Google Scholar] [CrossRef]
- Jeyaraj, A.; Zhang, X.; Hou, Y.; Shangguan, M.; Gajjeraman, P.; Li, Y.; Wei, C. Genome-wide identification of conserved and novel microRNAs in one bud and two tender leaves of tea plant (Camellia sinensis) by small RNA sequencing, microarray-based hybridization and genome survey scaffold sequences. Bmc Plant Biol. 2017, 17, 212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Chen, X.; Song, C.; Zou, Z.; Wang, Y.; Wang, M.; Fang, W.; Li, X. Identification and characterization of cold-responsive microRNAs in tea plant (Camellia sinensis) and their targets using high-throughput sequencing and degradome analysis. Bmc Plant Biol. 2014, 14, 271. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, X.; Guo, R.; Xia, X.; Liu, L.; An, Y.; Yan, X.; Wang, S.; Guo, L.; Wei, C. The biosynthesis of main taste compounds Is coordinately regulated by miRNAs and phytohormones in tea plant (Camellia sinensis). J. Agric. Food Chem. 2020, 68, 6221–6236. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, S.; Zhu, C.; Chang, X.; Yue, C.; Wang, Z.; Lin, Y.; Lai, Z. Identification of drought-responsive miRNAs and physiological characterization of tea plant (Camellia sinensis L.) under drought stress. Bmc Plant Biol. 2017, 17, 211. [Google Scholar] [CrossRef]
- Fan, K.; Fan, D.; Ding, Z.; Su, Y.; Wang, X. Cs-miR156 is involved in the nitrogen form regulation of catechins accumulation in tea plant (Camellia sinensis L.). Plant Physiol. Bioch. 2015, 97, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Meyers, B.C. Revisiting criteria for plant microRNA annotation in the era of big data. Plant Cell 2018, 30, 272–284. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Q.; Zeng, Y.; Chen, X.; Zhang, Z.; Chen, Y.; Lai, Z. Molecular evolution and spatiotemporal expression of longan miR166 family. J. Hortic. 2017, 44, 2285–2295. [Google Scholar]
- Xie, Q.; Wang, X.; He, J.; Lan, T.; Zheng, J.; Li, Y.; Pan, J.; Lin, L.; Zhao, J.; Li, J.; et al. Distinct evolutionary profiles and functions of microRNA156 and microRNA529 in land plants. Int. J. Mol. Sci. 2021, 22, 11100. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Liu, H.; Wei, T.; Jin, H.; Yu, Y.; Ma, M.; Song, X.; Dai, R.; Guo, D. Identification, characterization, and verification of miR399 target gene in grape. Hortic. Plant J. 2023, 10, 91–102. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, H.; Jiang, H.L.; Wang, H.S.; Chen, K.X.; Duan, J.J.; Feng, S.J.; Wu, G. Regulation of cadmium tolerance and accumulation by miR156 in Arabidopsis. Chemosphere 2020, 242, 125168. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Theissen, G. Plant miRNA conservation and evolution. Methods Mol. Biol. 2019, 1932, 41–50. [Google Scholar]
- Wang, H.; Lu, Z.; Xu, Y.; Kong, L.; Shi, J.; Liu, Y.; Fu, C.; Wang, X.; Wang, Z.Y.; Zhou, C.; et al. Genome-wide characterization of SPL family in Medicago truncatula reveals the novel roles of miR156/SPL module in spiky pod development. Bmc Genom. 2019, 20, 552. [Google Scholar] [CrossRef]
- Naya, L.; Khan, G.A.; Sorin, C.; Hartmann, C.; Crespi, M.; Lelandais-Briere, C. Cleavage of a non-conserved target by a specific miR156 isoform in root apexes of Medicago truncatula. Plant Signal Behav. 2010, 5, 328–331. [Google Scholar] [CrossRef]
- Ding, X.; Guo, J.; Lv, M.; Wang, H.; Sheng, Y.; Liu, Y.; Gai, J.; Yang, S. The miR156b-GmSPL2b module mediates male fertility regulation of cytoplasmic male sterility-based restorer line under high-temperature stress in soybean. Plant Biotechnol. J. 2023, 21, 1542–1559. [Google Scholar] [CrossRef]
- Yin, H.; Hong, G.; Li, L.; Zhang, X.; Kong, Y.; Sun, Z.; Li, J.; Chen, J.; He, Y. miR156/SPL9 regulates reactive oxygen species accumulation and immune response in Arabidopsis thaliana. Phytopathology 2019, 109, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ming, L.; Liao, K.; Xia, C.; Sun, S.; Chang, Y.; Wang, H.; Fu, D.; Xu, C.; Wang, Z.; et al. Bract suppression regulated by the miR156/529-SPLs-NL1-PLA1 module is required for the transition from vegetative to reproductive branching in rice. Mol. Plant 2021, 14, 1168–1184. [Google Scholar] [CrossRef]
- Ellis, J.; Dodds, P.; Pryor, T. Structure, function and evolution of plant disease resistance genes. Curr. Opin. Plant Biol. 2000, 3, 278–284. [Google Scholar] [CrossRef]
- Eldem, V.; Celikkol, A.U.; Ozhuner, E.; Bakir, Y.; Uranbey, S.; Unver, T. Genome-wide identification of miRNAs responsive to drought in peach (Prunus persica) by high-throughput deep sequencing. PLoS ONE 2012, 7, e50298. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhou, C.; Zhu, C.; Chen, L.; Shi, B.; Lin, Y.; Lai, Z.; Guo, Y. Genome-wide investigation of the miR166 family provides new insights into Its involvement in the drought stress responses of tea plants (Camellia sinensis (L.) O. Kuntze). Forests 2022, 13, 628. [Google Scholar] [CrossRef]
- Trindade, I.; Capitao, C.; Dalmay, T.; Fevereiro, M.P.; Santos, D.M. miR398 and miR408 are up-regulated in response to water deficit in Medicago truncatula. Planta 2010, 231, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Zhao, M.; Tian, Q.; Zhang, W.H. Identification of drought-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Bmc Genom. 2011, 12, 367. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Liu, X.; Duan, S.; Jiang, S.; Zhu, J.; Zhang, Y.; Hou, H. The mdmiR156n regulates drought tolerance and flavonoid synthesis in apple calli and Arabidopsis. Int. J. Mol. Sci. 2023, 24, 6049. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, X.; Du, B.; Xiao, Y.; Wang, Y.; Sun, Y.; Zhou, X.; Wang, C.; Liu, Y.; Li, T.H. MicroRNA156ab regulates apple plant growth and drought tolerance by targeting transcription factor MsSPL13. Plant Physiol. 2023, 192, 1836–1857. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Wang, J.; Zhang, C.; Xiao, F.; Ye, J.; Li, C.; Ye, Z.; Zhang, J. miR156a-targeted SBP-Box transcription factor SlSPL13 regulates inflorescence morphogenesis by directly activating SFT in tomato. Plant Biotechnol. J. 2020, 18, 1670–1682. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Z.; Li, Z.; Dong, S.; Yu, X.; Zhao, P.; Liao, W.; Yu, X.; Peng, M. MeSPL9 attenuates drought resistance by regulating JA signaling and protectant metabolite contents in cassava. Theor. Appl. Genet. 2022, 135, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Shi, L.; Gong, D.; Zhang, S.; Zhao, Q.; Zhan, D.; Vasseur, L.; Wang, Y.; Yu, J.; et al. Haplotype-resolved genome assembly provides insights into evolutionary history of the tea plant Camellia sinensis. Nat. Genet. 2021, 53, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, S.; Liu, L.; Li, R.; Guo, R.; Xia, X.; Wei, C. miR477 targets the phenylalanine ammonia-lyase gene and enhances the susceptibility of the tea plant (Camellia sinensis) to disease during Pseudopestalotiopsis species infection. Planta 2020, 251, 59. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).