Antioxidative, Metabolic and Vascular Medicinal Potentials of Natural Products in the Non-Edible Wastes of Fruits Belonging to the Citrus and Prunus Genera: A Review
Abstract
1. Introduction
2. Review Strategy
3. Ethnomedicinal, Pharmacological and Phytochemical Properties of the Non-Edible Fruit Parts Selected Fruits
3.1. The Citrus Fruits
3.1.1. Orange (Citrus × sinensis (L.) Osbeck)
3.1.2. Naartjie (Citrus unshiu (Yu.Tanaka ex Swingle) Marcow)
3.1.3. Grapefruit (Citrus × paradisi Macfad)
3.1.4. Lemon (Citrus limon (L.) Osbeck) and Key Lime (Citrus × aurantiifolia (Christm.) Swingle)
3.2. Fruits Belonging to the Prunus Genus
3.2.1. Apricot (Prunus armeniaca L.)
3.2.2. Sour Cherry (Prunus cerasus L.)
3.2.3. Peach (Prunus persica (L.) Batsch 1801)
3.2.4. European Plum (Prunus domestica L.)
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 16 August 2023).
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Matsabisa, M.G.; Ibrahim, M.A.; Erukainure, O.L.; Chabalala, M.H.; Islam, M.S. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: A review. J. Ethnopharmacol. 2019, 235, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sifunda, S.; Mbewu, A.D.; Mabaso, M.; Manyaapelo, T.; Sewpaul, R.; Morgan, J.W.; Harriman, N.W.; Williams, D.R.; Reddy, S.P. Prevalence and Psychosocial Correlates of Diabetes Mellitus in South Africa: Results from the South African National Health and Nutrition Examination Survey (SANHANES-1). Int. J. Environ. Res. Public Health 2023, 20, 5798. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B.M.; Flisher, A.J.; Lalloo, U.G.; Sitas, F.; Tollman, S.M.; Bradshaw, D. The burden of non-communicable diseases in South Africa. Lancet 2009, 374, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Mutyambizi, C.; Pavlova, M.; Hongoro, C.; Groot, W. Inequalities and factors associated with adherence to diabetes self-care practices amongst patients at two public hospitals in Gauteng, South Africa. BMC Endocr. Disord. 2020, 20, 15. [Google Scholar] [CrossRef]
- Mothibe, M.E.; Sibanda, M. African Traditional Medicine: South African Perspective. In Traditional and Complementary Medicine; Mordeniz, C., Ed.; IntechOpen, Ltd.: London, UK, 2019. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.S.; Makkar, H.P.S. Wastes to worth: Value added products from fruit and vegetable wastes. CAB Rev. 2015, 10, 1–25. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Le Roes-Hill, M.; Welz, P.J.; Grandin, K.A.; Kudanga, T.; Van Dyk, S.J.; Ohlhoff, C.; Van Zyl, W.H.E.; Pletschke, B.I. Fruit waste streams in South Africa and their potential role in developing a bio-economy. S. Afr. J. Sci. 2015, 111, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, K.; Luo, M.; Wei, S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Micek, A.; Currenti, W.; Godos, J. Plant-based polyphenol-rich foods and beverages influence metabolic health in a Mediterranean cohort. Eur. J. Public Health 2021, 31, ckab164. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Ke, Z.; Zhao, Y.; Tan, S.; Chen, H.; Li, Y.; Zhou, Z.; Huang, C. Citrus reticulata Blanco peel extract ameliorates hepatic steatosis, oxidative stress and inflammation in HF and MCD diet-induced NASH C57BL/6 J mice. J. Nutr. Biochem. 2020, 83, 108426. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef]
- Long, X.; Zeng, X.; Yan, H.; Xu, M.; Zeng, Q.; Xu, C.; Xu, Q.; Liang, Y.; Zhang, J. Flavonoids composition and antioxidant potential assessment of extracts from Gannanzao Navel Orange (Citrus sinensis Osbeck Cv. Gannanzao) peel. Nat. Prod. Res. 2021, 35, 702–706. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Ebuehi, O.A.; Iqbal Chaudhary, M.; Mesaik, M.A.; Shukralla, A.; Muhammad, A.; Zaruwa, M.Z.; Elemo, G.N. Orange Peel Extracts: Chemical Characterization, Antioxidant, Antioxidative Burst, and Phytotoxic Activities. J. Diet. Suppl. 2016, 13, 585–594. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, P.S.; Chen, Y.F.; Ho, C.T.; Pan, M.H. Suppression of Adipogenesis by 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone from Orange Peel in 3T3-L1 Cells. J. Med. Food 2016, 19, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, R.G.; Rajiv Gandhi, G.; Denadai, M.; Sridharan, G.; Jothi, G.; Sasikumar, P.; Siqueira Quintans, J.S.; Narain, N.; Cuevas, L.E.; Coutinho, H.; et al. Evidence of insulin-dependent signalling mechanisms produced by Citrus sinensis (L.) Osbeck fruit peel in an insulin resistant diabetic animal model. Food Chem. Toxicol. 2018, 116 Pt B, 86–99. [Google Scholar] [CrossRef]
- Parkar, N.; Addepalli, V. Amelioration of diabetic nephropathy by orange peel extract in rats. Nat. Prod. Res. 2014, 28, 2178–2181. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Abdel Azeem, M.N. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205. [Google Scholar] [CrossRef]
- Chen, Z.T.; Chu, H.L.; Chyau, C.C.; Chu, C.C.; Duh, P.D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ansari, M.N.; Alam, A.; Khan, T.H. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pak. J. Biol. Sci. 2013, 16, 1086–1094. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Fahim, H.I.; Ahmed, H.Y.; Al-Muzafar, H.M.; Ahmed, R.R.; Amin, K.A.; El-Nahass, E.S.; Abdelazeem, W.H. The Preventive Effects and the Mechanisms of Action of Navel Orange Peel Hydroethanolic Extract, Naringin, and Naringenin in N-Acetyl-p-aminophenol-Induced Liver Injury in Wistar Rats. Oxidative Med. Cell. Longev. 2019, 2019, 2745352. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.; Silva, A.C.; Aranha, C.P. Antioxidant activity of oils extracted from orange (Citrus sinensis) seeds. An. Da Acad. Bras. De Cienc. 2016, 88, 951–958. [Google Scholar] [CrossRef]
- Shakthi Deve, A.; Sathish Kumar, T.; Kumaresan, K.; Rapheal, V.S. Extraction process optimization of polyphenols from Indian Citrus sinensis—As novel antiglycative agents in the management of diabetes mellitus. J. Diabetes Metab. Disord. 2014, 13, 11. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Ghafoor, K. The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus seed and oils. J. Food Sci. Technol. 2018, 55, 190–197. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Mora, L.; Aristoy, M.C.; Mahoonak, A.S.; Ghorbani, M.; Houshmand, G.; Toldrá, F. Impact of Simulated Gastrointestinal Digestion on the Biological Activity of an Alcalase Hydrolysate of Orange Seed (Siavaraze, Citrus sinensis) by-Products. Foods 2020, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yang, D.S.; Han, S.I.; Yun, J.H.; Kim, I.W.; Kim, S.J.; Kim, J.H. Aqueous Extraction of Citrus unshiu Peel Induces Proangiogenic Effects through the FAK and ERK1/2 Signaling Pathway in Human Umbilical Vein Endothelial Cells. J. Med. Food 2016, 19, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Song, S.; Lee, J.; Chang, H.; Lee, S. Clinical Investigations of the Effect of Citrus unshiu Peel Pellet on Obesity and Lipid Profile. Evid.-Based Complement. Altern. Med. eCAM 2018, 2018, 4341961. [Google Scholar] [CrossRef]
- Shimamura, Y.; Sei, S.; Nomura, S.; Masuda, S. Protective effects of dried mature Citrus unshiu peel (Chenpi) and hesperidin on aspirin-induced oxidative damage. J. Clin. Biochem. Nutr. 2021, 68, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Jung, S.Y.; Lee, G.H.; Cho, J.H.; Choi, I.Y. Neuroprotective effect of Citrus unshiu immature peel and nobiletin inhibiting hydrogen peroxide-induced oxidative stress in HT22 murine hippocampal neuronal cells. Pharmacogn. Mag. 2015, 11 (Suppl. 2), S284–S289. [Google Scholar] [CrossRef]
- Kim, C.; Ji, J.; Ho Baek, S.; Lee, J.H.; Ha, I.J.; Lim, S.S.; Yoon, H.J.; Je Nam, Y.; Ahn, K.S. Fermented dried Citrus unshiu peel extracts exert anti-inflammatory activities in LPS-induced RAW264.7 macrophages and improve skin moisturizing efficacy in immortalized human HaCaT keratinocytes. Pharm. Biol. 2019, 57, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, Y.; Shen, S.; Zhi, Z.; Cheng, H.; Chen, S.; Ye, X. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chem. 2020, 326, 126785. [Google Scholar] [CrossRef]
- El Kamali, H.H.; Burham, B.O.; El-Egami, A.A. Essential oil composition of internal fruit peel of Citrus paradisi from Sudan. Am. Res. Thoughts 2015, 1, 2079–2085. [Google Scholar]
- Díaz-Juárez, J.A.; Tenorio-López, F.A.; Zarco-Olvera, G.; Valle-Mondragón, L.D.; Torres-Narváez, J.C.; Pastelín-Hernández, G. Effect of Citrus paradisi extract and juice on arterial pressure both in vitro and in vivo. Phytother. Res. PTR 2009, 23, 948–954. [Google Scholar] [CrossRef]
- Maurer, L.H.; Cazarin, C.; Quatrin, A.; Minuzzi, N.M.; Nichelle, S.M.; Lamas, C.A.; Cagnon, V.; Morari, J.; Velloso, L.A.; Maróstica Júnior, M.R.; et al. Grape peel powder attenuates the inflammatory and oxidative response of experimental colitis in rats by modulating the NF-κB pathway and activity of antioxidant enzymes. Nutr. Res. 2020, 76, 52–70. [Google Scholar] [CrossRef]
- Shaikh, S.; Bhatt, L.K.; Barve, K. Attenuation of isoproterenol-induced cardiotoxicity in rats by Narirutin rich fraction from grapefruit. Phytomedicine 2019, 55, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Adeneye, A.A. Methanol seed extract of Citrus paradisi Macfad lowers blood glucose, lipids and cardiovascular disease risk indices in normal Wistar rats. Niger. Q. J. Hosp. Med. 2008, 18, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Dembinski, A.; Warzecha, Z.; Konturek, S.J.; Ceranowicz, P.; Dembinski, M.; Pawlik, W.W.; Kusnierz-Cabala, B.; Naskalski, J.W. Extract of grapefruit-seed reduces acute pancreatitis induced by ischemia/reperfusion in rats: Possible implication of tissue antioxidants. J. Physiol. Pharmacol. 2004, 55, 811–821. [Google Scholar]
- Udom, G.J.; Yemitan, O.K.; Umoh, E.E.; Mbagwu, H.O.C.; Ukpe, E.E.; Thomas, P.S. Hepatoprotective Properties of Ethanol Seed Extract of Citrus paradisi Macfad (Grape Fruit) Against Paracetamol-Induced Hepatotoxicity in Wistar Rats. J. Herb. Drugs 2018, 8, 219–225. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Adebayo, A.A.; Oboh, G. Modulatory effect of some citrus (Citrus limon, Citrus reticulata, Citrus maxima) peels on monoamine oxidase, phosphodiesterase-5 and angiotensin-1 converting enzyme activities in rat heart homogenate. J. Complement. Integr. Med. 2019, 16, 20180067. [Google Scholar] [CrossRef] [PubMed]
- Diab, K.A. In Vitro Studies on Phytochemical Content, Antioxidant, Anticancer, Immunomodulatory, and Antigenotoxic Activities of Lemon, Grapefruit, and Mandarin Citrus Peels. Asian Pac. J. Cancer Prev. 2016, 17, 3559–3567. [Google Scholar] [PubMed]
- Demir, A.; Celik, I. Investigation of healing effects of lemon (Citrus limonum) seeds lyophilized extracts on experimental diabetic rats. Arch. Physiol. Biochem. 2019, 128, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Enejoh, O.S.; Ogunyemi, I.O.; Bala, M.S.; Oruene, I.S.; Suleiman, M.M.; Ambali, S.F. Ethnomedical importance of Citrus aurantifolia (Christm) Swingle. Pharma Innov. J. 2015, 4, 1–6. [Google Scholar]
- Lin, L.Y.; Chuang, C.H.; Chen, H.C.; Yang, K.M. Lime (Citrus aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef]
- Hashemipour, M.; Kargar, M.; Ghannadi, A.; Kelishadi, R. The effect of Citrus aurantifolia (Lemon) peels on cardiometabolic risk factors and markers of endothelial function in adolescents with excess weight: A triple-masked randomized controlled trial. Med. J. Islam. Repub. Iran 2016, 30, 429. [Google Scholar]
- Boshtam, M.; Asgary, S.; Moshtaghian, J.; Naderi, G.; Jafari-Dinani, N. Impacts of fresh lime juice and peel on atherosclerosis progression in an animal model. ARYA Atheroscler. 2013, 9, 357–362. [Google Scholar]
- Youn, K.; Lee, S.; Jun, M. Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity. Nutrients 2019, 11, 2648. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of In Vitro and In Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Inaba, N.; Kuwahara, S.; Kuki, W. Effects of gamma-terpinene on lipid concentrations in serum using Triton WR1339-treated rats. Biosci. Biotechnol. Biochem. 2003, 67, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013, 715, 46–55. [Google Scholar] [CrossRef]
- Raafat, K.; El-Darra, N.; Saleh, F.A.; Rajha, H.N.; Maroun, R.G.; Louka, N. Infrared-Assisted Extraction and HPLC-Analysis of Prunus armeniaca L. Pomace and Detoxified-Kernel and their Antidiabetic Effects. Phytochem. Anal. 2018, 29, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.K. Can apricot kernels fatty acids delay the atrophied hepatocytes from progression to fibrosis in dimethylnitrosamine (DMN)-induced liver injury in rats? Lipids Health Dis. 2011, 10, 114. [Google Scholar] [CrossRef]
- Kopčeková, J.; Kováčiková, E.; Kováčik, A.; Kolesárová, A.; Mrázová, J.; Chlebo, P.; Kolesárová, A. Consumption of bitter apricot seeds affects lipid and endocrine profile in women. J. Environ. Sci. Health. Part B 2021, 56, 378–386. [Google Scholar] [CrossRef]
- González-García, E.; García, M.C.; Marina, M.L. Capillary liquid chromatography-ion trap-mass spectrometry methodology for the simultaneous quantification of four angiotensin-converting enzyme-inhibitory peptides in Prunus seed hydrolysates. J. Chromatogr. A 2018, 1540, 47–54. [Google Scholar] [CrossRef]
- Karaboğa, I.; Ovalı, M.A.; Yılmaz, A.; Alpaslan, M. Gastroprotective effect of apricot kernel oil in ethanol-induced gastric mucosal injury in rats. Biotech. Histochem. 2018, 93, 601–607. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Musa Özcan, M.; Ghafoor, K.; Babiker, E.E. The effect of microwave roasting on bioactive compounds, antioxidant activity and fatty acid composition of apricot kernel and oils. Food Chem. 2018, 243, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, B.; Modgil, R.; Goyal, A. Antinutritional factors and hypocholesterolemic effect of wild apricot kernel (Prunus armeniaca L.) as affected by detoxification. Food Funct. 2018, 9, 2121–2135. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; González-García, E.; Vásquez-Villanueva, R.; Marina, M.L. Apricot and other seed stones: Amygdalin content and the potential to obtain antioxidant, angiotensin I converting enzyme inhibitor and hypocholesterolemic peptides. Food Funct. 2016, 7, 4693–4701. [Google Scholar] [CrossRef]
- Hooper, D.; Field, H. Useful Plants and Drugs of Iran and Iraq; Steinmetz, E.F., Ed.; Field Museum of Natural History: Chicago, IL, USA; Amsterdam, The Netherlands, 1954; Volume 9. [Google Scholar]
- Micioni Di Bonaventura, M.V.; Martinelli, I.; Moruzzi, M.; Micioni Di Bonaventura, E.; Giusepponi, M.E.; Polidori, C.; Lupidi, G.; Tayebati, S.K.; Amenta, F.; Cifani, C.; et al. Brain alterations in high fat diet induced obesity: Effects of tart cherry seeds and juice. Nutrients 2020, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Moruzzi, M.; Martinelli, I.; Maggi, F.; Micioni Di Bonaventura, M.V.; Cifani, C.; Mosconi, G.; Tayebati, S.K.; Damiano, S.; Lupidi, G.; et al. Tart cherry (Prunus cerasus L.) dietary supplement modulates visceral adipose tissue CB1 mRNA levels along with other adipogenesis-related genes in rat models of diet-induced obesity. Eur. J. Nutr. 2021, 60, 2695–2707. [Google Scholar] [CrossRef]
- Raafat, K.; El-Darra, N.; Saleh, F.A. Gastroprotective and anti-inflammatory effects of Prunus cerasus phytochemicals and their possible mechanisms of action. J. Tradit. Complement. Med. 2020, 10, 345–353. [Google Scholar] [CrossRef]
- Varga, B.; Priksz, D.; Lampé, N.; Bombicz, M.; Kurucz, A.; Szabó, A.M.; Pósa, A.; Szabó, R.; Kemény-Beke, Á.; Remenyik, J.; et al. Protective Effect of Prunus cerasus (Sour Cherry) Seed Extract on the Recovery of Ischemia/Reperfusion-Induced Retinal Damage in Zucker Diabetic Fatty Rat. Molecules 2017, 22, 1782. [Google Scholar] [CrossRef]
- Saleh, F.A.; El-Darra, N.; Raafat, K. Hypoglycemic effects of Prunus cerasus L. pulp and seed extracts on Alloxan-Induced Diabetic Mice with histopathological evaluation. Biomed. Pharmacother. 2017, 88, 870–877. [Google Scholar] [CrossRef]
- Mahmoud, F.F.; Al-Awadhi, R.; Haines, D.D.; Dashti, A.; Dashti, H.; Al-Ozairi, E.; Bak, I.; Tosaki, A. Sour cherry seed kernel extract increases heme oxygenase-1 expression and decreases representation of CD3+ TNF-α+ and CD3+IL-8+ subpopulations in peripheral blood leukocyte cultures from type 2 diabetes patients. Phytother. Res. 2013, 27, 767–774. [Google Scholar] [CrossRef]
- Juhasz, B.; Kertész, A.; Balla, J.; Balla, G.; Szabo, Z.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Varga, B.; Haines, D.D.; et al. Cardioprotective effects of sour cherry seed extract (SCSE) on the hypercholesterolemic rabbit heart. Curr. Pharm. Des. 2013, 19, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Corroto, E.; Marina, M.L.; García, M.C. Multiple protective effect of peptides released from Olea europaea and Prunus persica seeds against oxidative damage and cancer cell proliferation. Food Res. Int. 2018, 106, 458–467. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Orellana, J.M.; Marina, M.L.; García, M.C. Isolation and Characterization of Angiotensin Converting Enzyme Inhibitory Peptides from Peach Seed Hydrolysates: In Vivo Assessment of Antihypertensive Activity. J. Agric. Food Chem. 2019, 67, 10313–10320. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Pacetti, D.; Lucci, P.; Núñez, O.; Menichini, F.; Frega, N.G.; Tundis, R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive Compounds and Antioxidant Activity of Pulp, Peel and Seed Ethanolic Extracts. Plant Foods Hum. Nutr. 2015, 70, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Nazar, R.; Butt, A.M.; Ijaz, B.; Tasawar, N.; Sheikh, A.K.; Shahid, I.; Shah, S.M.; Qamar, R. Phytochemical Screening and Protective Effects of Prunus persica Seeds Extract on Carbon Tetrachloride-Induced Hepatic Injury in Rats. Curr. Pharm. Biotechnol. 2021, 23, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Choi, H.G.; Kim, J.H.; Kim, S.H.; Kim, J.A.; Lee, S.H. Anti-allergic inflammatory effects of cyanogenic and phenolic glycosides from the seed of Prunus persica. Nat. Prod. Commun. 2013, 8, 1739–1740. [Google Scholar] [CrossRef]
- Khallouki, F.; Haubner, R.; Erben, G.; Ulrich, C.M.; Owen, R.W. Phytochemical composition and antioxidant capacity of various botanical parts of the fruits of Prunus × domestica L. from the Lorraine region of Europe. Food Chem. 2012, 133, 697–706. [Google Scholar] [CrossRef]
- Sompong, W.; Meeprom, A.; Cheng, H.; Adisakwattana, S. A comparative study of ferulic acid on different monosaccharide-mediated protein glycation and oxidative damage in bovine serum albumin. Molecules 2013, 18, 13886–13903. [Google Scholar] [CrossRef]

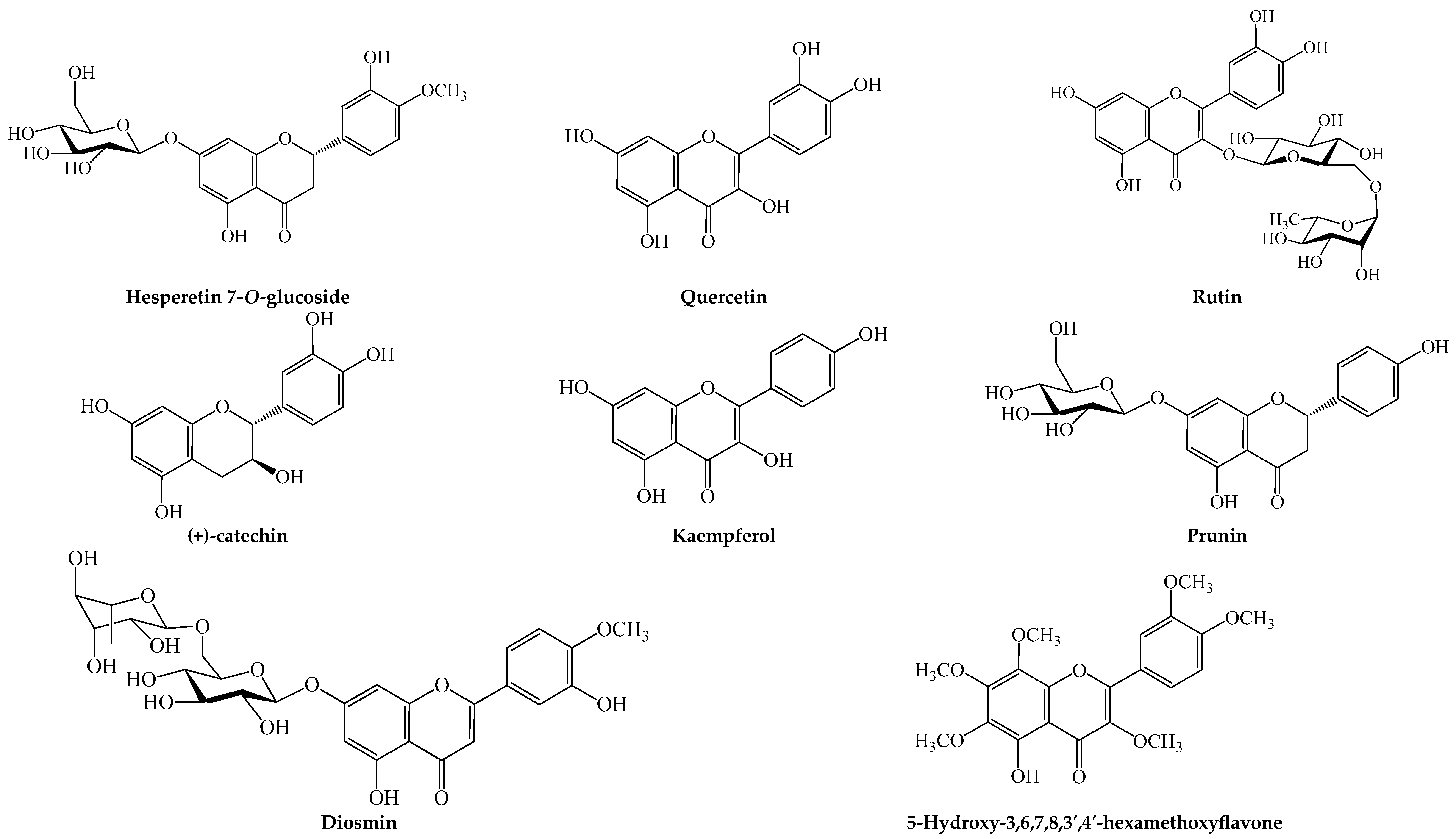


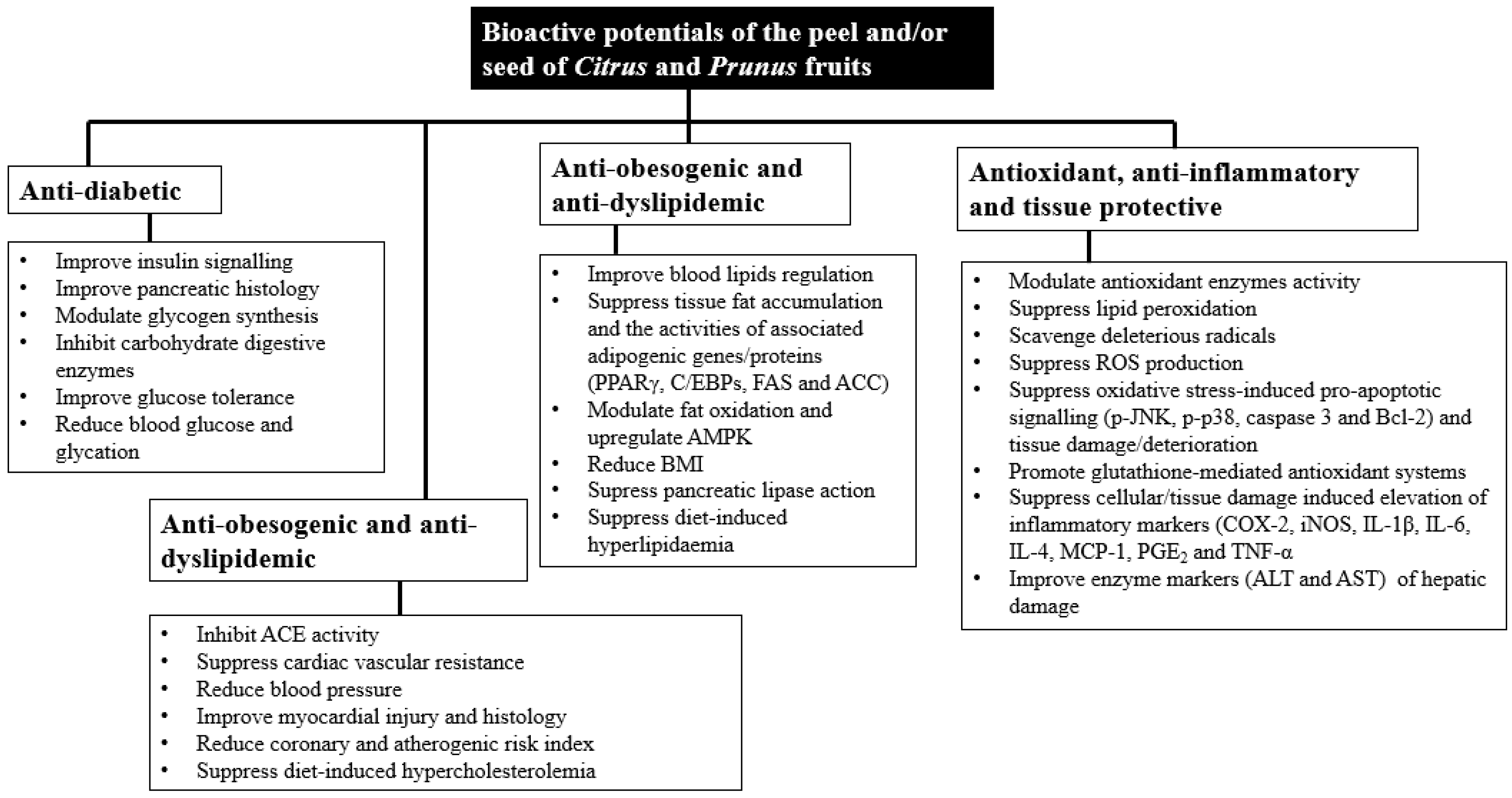
| Fruit Scientific Name (Common Name) | Parts Used | Traditional Use | Pharmacological Action | Material, Extract, Fraction, Isolate or Formulation Used | Phytochemical Profile of Material, Extract, Fraction, Isolate or Formulation Used (Method of Analysis) | Remarks | References |
|---|---|---|---|---|---|---|---|
| Citrus × sinensis (L.) Osbeck (sweet orange) | Peel | Used to treat symptoms of indigestion and respiratory tract inflammation [22]. | Inhibited adipogenesis and fat/lipid accumulation in 3T3-L1 adipocytes by downregulating PPARγ, C/EBPs, FAS and ACC and upregulating AMPK. | 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone isolated from the peel extract | 5-Hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone (HPLC) | Up to 100 µM of isolate showed no notable toxicity on the 3T3-L1 cells. | Wang et al., 2016 [23] |
| 30 days adm. (50 and 100 mg/kg bw, p.o.) of extracts in STZ and HFD-induced diabetic male Wistar rats reduced FBG, serum ALT and AST, improved GT, pancreatic histology and immunohistochemistry, IR and serum lipid profile and modulated the expression of adipose tissue PPARγ, GLUT-4 and InRec. | Methanol extract | Acacetin, apigenin, artepillin C, caffeic acid, (+)-catechin, chlorogenic acid, cinnamic acid, ferulic acid, kaempferide, naringenin, p-coumaric acid, protocatechuic acid, rutin, vanillin and vanillic acid (UHPLC-MS/MS) |
| Sathiyabama et al., 2018 [24] | |||
| 4 wks adm. (100 and 200 mg/kg bw, p.o.) ameliorated STZ-induced diabetic nephropathy in rats by increasing creatinine clearance, reducing renal hypertrophy and improving renal histology. | Ethanol extract | Parkar and Addepalli, 2014 [25] | |||||
| 4 wks adm. (100 mg/kg bw, p.o.) in nicotinamide and STZ-induced diabetic rats improved GT, increased serum insulin and C-peptide levels, hepatic glycogen content and glucose-6-phosphatatse activity, adipose tissue InRec, GLUT-4 and adiponectin expression, reduced hepatic glycogen phosphorylase activity and improved serum lipid profile and antioxidant status. | Ethanol extract | The effect of extract was comparable to that of naringin (100 mg/kg bw) and naringenin (100 mg/kg bw). | Ahmed et al., 2017 [26] | ||||
| Ethyl acetate fraction of 95% ethanol extract | Narirutin, sinensetin, nobiletin, 4′,5,6,7-tetramethoxyflavone and 3,3′,4′,5,6,7-hexamethoxyflavone | Long et al., 2021 [21] | ||||
| Boiling water extract | Narirutin, hesperidin, nobiletin and tangeretin (HPLC) |
| Chen et al., 2017 [18] | |||
| Suppressed t-BPH-induced oxidative stress and cytotoxicity in HepG2 cells by increasing antioxidant enzymes and GHS and suppressing ROS production, LPO, caspase-3 activation and pro-apoptotic signaling. | Boiling water extract | Hesperetin, hesperidin, nobiletin and tangeretin (HPLC) |
| Chen et al., 2012 [27] | |||
| 12-day treatment (400 mg/kg bw, p.o.) in STZ-induced diabetic male Wistar rats reduced BG and improved wound healing by promoting tissue growth and collagen synthesis. | Ethanol extracts | Carotenoids and vitamin C (non-specialized in vitro spectrometric methods) | Ahmad et al., 2013 [28] | ||||
| 4 wks adm. (50 mg/kg bw, p.o.) of extracts in male Wistar rats ameliorated N-acetyl-p-aminophenol-induced liver damage by reducing serum ALT, AST, ALP, inflammatory maker (TNF-α and IL-4), hepatic LPO and pro-apoptotic activity, increasing hepatic GSH and antioxidant enzyme activity and improving hepatic histopathological deteriorations. | 30% ethanol extract | Diosmin, gallic acid, naringin, rutin, hesperidin, quercetin, naringenin and hesperetin (HPLC-MS) | The effect of extract was comparable to that of naringin (20 mg/kg bw) and naringenin (20 mg/kg bw). | Ahmed et al., 2019 [29] | |||
| Seed | In vitro DPPH radical scavenging activity. | Essential oil | Cholestanol and β-sitosterol (GC) | Jorge et al., 2016 [30] | |||
| In vitro inhibitory activity on BSA glycation. | Pressurized hot water extract and water extract obtained from sequential extraction in increasing polarity (i.e., chloroform to distilled water) | Shakthi Deve et al., 2014 [31] | |||||
| In vitro DPPH radical scavenging activity. | Methanol extract | Gallic acid, 3,4-dihydroxybenzoic acid, (+)-catechin, 1,2-dihydroxybenzene, syringic acid, caffeic acid, rutin, p-coumaric acid, trans-ferulic acid, resveratrol, quercetin, kaempferol, apigenin-7-glucoside, isorhamnetin, naringenin and trans-cinnamic acid (HPLC-PDA) | Al Juhaimi et al., 2018 [32] | ||||
| Protein isolates | Mazloomi et al., 2020 [33] | |||||
| Citrus unshiu (Yu.Tanaka ex Swingle) Marcow (naartjie) | Peel | Used for treating cough, cold, asthma, nausea, digestive problems and inflammation of the skin and respiratory tract, as well as improving blood circulation [19,34,35,36]. | 10 wks feeding of diet containing 0.2 and 0.5% fraction in HFD-fed male C57BL/6J mice improved glycemic control and serum lipid profile, reduced hepatic lipid accumulation and oxidative stress, serum ALT and AST, upregulated expression of hepatic Nrf-2, NQO1 and fatty acid oxidation genes and downregulated expression of hepatic inflammatory factors (IL-1β, IL-6, MCP-1 and TNF-α). | Ethyl acetate fraction of 70% ethanol extract | Isosinensetin, sinensetin, 5,7,3′,4′-tetramethoxyflavone, 5,6,7,4′-tetramethoxyflavone, nobiletin, 3,5,6,7,8,3′,4′-heptamethoxyflavone, 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone and tangeretin. (UHPLC-QTOF-MS) | The effect of the fractions was comparable and/or more potent than that of diet containing 0.1% resveratrol. | Ke et al., 2020 [19] |
| 4 wks oral adm. (18 mg) in obese patients reduced BW, BMI, waist circumference, total and LDL cholesterol, TG, ALT and AST. | Peel pellets | Narirutin, nobiletin and hesperidin (HPLC) | Kang et al., 2018 [35] | ||||
| Suppressed H2O2-induced oxidative damage in neuronal cells (HT22 cells) by reducing cytotoxicity or cell death and expression of pro-apoptotic signaling proteins (p-JNK, p-p38, caspase 3 and Bcl-2). | Boiling water extract | Nobiletin and hesperidin (HPLC) | The anti-cytotoxic and anti-apoptotic effect of the extract was comparable that of nobiletin. | Cho et al., 2015 [37] | |||
| Subcritical water extracts | Narirutin, narigenin, prunin, nobiletin, tangeretin, hesperetin-7-O-glucoside, sinensetin, hesperetin and hesperidin (HPLC) | Subcritical water extraction performed at a flow rate of 1.5 mL/min and temperature of 175 °C showed the most potent activity. | Kim and Lim, 2020 [20] | |||
| Suppressed LPS-induced inflammation in RAW 264.7 macrophages by suppressing NO production, iNOS and COX-2 expression and the secretion of inflammatory cytokines (IL-6, TNF-α and PGE2). | Bacillus subtilis-fermented water and ethanol peel extracts | Narirutin, narigenin, neoponcirin, nobiletin, tangeretin, rutin, hesperetin and hesperidin (UHPLC-QTOF-MS) | Up to 100 µg/mL of extract did not notably decrease the viability of RAW 264.7 macrophages. | Kim et al., 2019 [38] | |||
| 70% ethanol flavonoid-rich extract | Hesperidin and hesperetin (HPLC) | Huang et al., 2020 [39] | ||||
| Exerted proangiogenic effects in HUVECs by promoting cell proliferation, migration and tube formation and upregulated phosphorylation of angiogenic genes (FAK and ERK1/2). | Peel powder | Narirutin and hesperidin (HPLC and LC-MS) | Lee et al., 2016 [34] | ||||
| Acute treatment (20 mg/kg bw, p.o.) in male Wistar rats reduced aspirin-induced stomach ulceration and bleeding, as well as DNA damage in the stomach, liver and kidney. | Water (60 °C) extract | Hesperidin (HPLC) | The gastric effect of extract was comparable to that of 20 mg/kg bw hesperidin. | Shimamura et al., 2021 [36] | |||
| Citrus × paradisi Macfad (grapefruit) | Peel | Used to treat malaria [40]. |
| 70% ethanol flavonoid-rich extract | Hesperidin and hesperetin (HPLC) | Huang et al., 2020 [39] | |
| 60% ethanol extract | Díaz-Juárez et al., 2009 [41] | |||||
| 15-day supplementation of diet containing 8% of peel powder ameliorated TNBS-induced colitis in male Wistar rats by reducing colonic and serum expression of pro-inflammatory markers and improving colonic and serum antioxidant status. | Peel powder | Protocatechuic acid, isorhamnetin-3, kaempferol-3, feruloyl hexoside, caffeoyl hexoside, coumaroyl hexoside, myricetin-3, quercetin-3 and laricitrin-3 hexosides; quercetin-3 and isorhamnetin-3 glucuronides; quercetin-3-rutinoside; caftaric, coutaric, ferulic, syringic, coumaric and fertaric acids; epicatechin, catechin, resveratrol, procyanidin and viniferin | Maurer et al., 2020 [42] | ||||
| 12-day treatment (400 mg/kg bw, p.o.) in STZ-induced diabetic male Wistar rats reduced BG and improved wound healing by promoting tissue growth and collagen synthesis. | Ethanol extracts | Carotenoids and vitamin C (non-specialized in vitro spectrometric methods) | Ahmad et al., 2013 [28] | ||||
| 3-day pre-treatment (100 and 200 mg/kg bw, p.o.) of peel fraction protected against isoproterenol-induced myocardial injury in male Wistar rats by dose-dependently improving mean arterial BP, left ventricular function, cardiac biomarker enzymes and antioxidant status and myocardium histology. | Narirutin-rich fraction | Narirutin and hesperidine (HPTLC) | The myocardial protective effects of the 200 mg/kg bw fraction pre-treatment was comparable to that of 5 mg/kg bw Atenolol. | Shaikh et al., 2019 [43] | |||
| Seed | Used in the management of diabetes and obesity [44]. | 30-day treatment (100, 300 and 600 mg/kg bw, p.o.) in normal male Wistar rats dose-dependently reduced FBG, BMI, AI, CRI, LDL cholesterol and total cholesterol and increased HDL cholesterol. | Methanol extract | Adeneye, 2008 [44] | |||
| 30 min in situ intragastric pre-treatment (50–500 µL) ameliorated acute hemorrhagic pancreatitis in male Wistar rats by improving pancreatic blood flow and DNA synthesis and reducing LPO. | Commercial grapefruit seed extract (Herb-Pharma s.r.o., Velke Ludnice, Slovakia) | Dembinski et al., 2004 [45] | |||||
| 7-day pre-treatment (200, 400 and 600 mg/kg bw) protected against paracetamol-induced hepatotoxicity in male Wistar rats by reducing serum level of ALT, AST and ALP. | 70% ethanol extract | Up to 3200 mg/kg bw of extract was non-toxic or non-lethal in mice after 72 h of oral adm. | Udom et al., 2018 [46] | ||||
| Citrus limon (L.) Osbeck (lemon) | Peel | Used to treat hypertension [47]. |
| 70% ethanol flavonoid-rich extract | Hesperidin and hesperetin (HPLC) | Huang et al., 2020 [39] | |
| Ethanol extracts | Diab, 2016 [48] | |||||
| Acidified methanol (80:20% v/v of methanol and 1 N HCl solution, respectively) extract | Ademosun et al., 2019 [47] | |||||
| 12-day treatment (400 mg/kg bw, p.o.) in STZ-induced diabetic male Wistar rats reduced BG and improved wound healing by promoting tissue growth and collagen synthesis. | Ethanol extracts | Carotenoids and vitamin C (non-specialized in vitro spectrometric methods) | Ahmad et al., 2013 [28] | ||||
| Seed | 28-day treatment (100, 200 and 400 mg/kg bw, p.o.) of extract in STZ-induced diabetic rats reduced BG, HbA1c, intestinal α-glucosidase activity and serum TG, HDL cholesterol and enzyme biomarkers of renal and hepatic injury, increased serum insulin and improved erythrocyte, renal and hepatic antioxidant status. | Water extract |
| Demir and Celik, 2019 [49] | |||
| Citrus × aurantiifolia (Christm.) Swingle (Key lime) | Peel | Essential oil from peel is used for treating colds, sore throat, bronchitis, asthma and arthritis, as well as managing obesity [50]; the peel is used to treat hypertension [47]. |
| Peel essential oil | Limonene, γ-terpinene and β-pinene (GC-MS) |
| Lin et al., 2019 [51] |
| 4 wks oral adm. of peel powder in overweight and obese adolescents reduced BMI, systolic and diastolic BP and total and LDL cholesterol. | Peel powder | Hashemipour et al., 2016 [52] | |||||
| 60-day adm. of peel powder (1 g per day, p.o.) in hypercholestorolemic male New Zealand rabbits reduced fatty streaks in the coronary arteries and aorta and improved serum antioxidant status, TG and cholesterol. | Peel powder | Boshtam et al., 2013 [53] | |||||
| In vitro DPPH radical scavenging activity. | Methanol extract | Gallic acid, 3,4-dihydroxybenzoic acid, (+)-catechin, 1,2-dihydroxybenzene, syringic acid, caffeic acid, rutin, p-coumaric acid, trans-ferulic acid, resveratrol, quercetin, kaempferol, apigenin-7-glucoside, isorhamnetin, naringenin and trans-cinnamic acid (HPLC-PDA) | Al Juhaimi et al., 2018 [32] | ||||
| Acidified methanol (80:20% v/v of methanol and 1 N HCl solution, respectively) extract | Ademosun et al., 2019 [47] |
| Fruit Scientific Name (Common Name) | Parts Used | Traditional Use | Pharmacological Action | Material, Extract, Fraction, Isolate or Formulation Used | Phytochemical Profile of Material, Extract, Fraction, Isolate or Formulation Used (Method of Analysis) | Remarks | References |
|---|---|---|---|---|---|---|---|
| Prunus armeniaca L. (apricot) | Seed/kernel | Used as an expectorant, antitussive and laxative [58]. | 4 wk feeding of basal diet containing 0.5–1.5 mg/kg bw of seed flour dose-dependently ameliorated dimethylnitrosamine-induced hepatic fibrosis in male SD rats by improving hepatic histology, increasing serum antioxidant enzyme activity and reducing LPO. | Seed flour | Abdel-Rahman, 2011 [59] | ||
| 8–12 doses of 60 mg/kg bw reduced blood HDL cholesterol level in adult women after 42 days. | Seed flour | HDL cholesterol reduction was significant (p ˂ 0.05). | Kopčeková et al., 2021 [60] | ||||
| In vitro ACE inhibitory activity. | Protein or peptide isolates | González-García et al., 2018 [61] | |||||
| Acute (1 mL p.o.) treatment protected against alcohol-induced gastric intestinal injury in Wistar rats by improving epithelial and mucosal histology, improving antioxidant status and reducing inflammation. | Seed oil | Karaboğa et al., 2018 [62] | |||||
| In vitro DPPH radical scavenging activity. | 60% methanol extract of the oven-roasted seed | Gallic acid, 3,4-dihydrobenzoic acid, (+)-catechin, syringic acid, caffeic acid, rutin, p-coumaric acid, trans-ferulic acid, resveratrol, apigenin-7-glycoside, quercetin, trans-cinnamic acid, naringenin, kaempferol and isorhamnetin (HPLC-PDA). | Al Juhaimi et al., 2018 [63] | ||||
| Water extract | p-Hydroxybenzoic, syringic acid, vanillic acid, p-coumaric, caffeic acid, ferulic acid, protocatechuic acid, gallic acid and sinapinic acid (HPLC). | Mesías et al., 2013 [64] | ||||
| 40 days of feeding a basal diet containing seed flour ameliorated cholesterol-induced hypercholesterolemia in male Wistar rats by reducing serum HDL, VLDL and total cholesterols and TG and increasing HDL cholesterol. | Raw and detoxified (using 25% NaCl solution) seed flour | L-ascorbic acid and β-carotene (non-specialized in vitro spectrometric methods). | Raw and detoxified seeds reduced AI by 5- and 8-fold, respectively. | Tanwar et al., 2018 [65] | |||
| Acute (after 8 h) and 8-day i.p. treatment (2, 3 and 4 mg/kg bw) reduced BG, HbA1c and LPO and increased serum insulin and catalase activity in alloxan-induced diabetic male Swiss mice. | Detoxified (infrared-assisted) water extract | Chlorogenic acid, amygdaline and procyanidin derivatives (HPLC-PDA). | The antidiabetic and antioxidant effects of the extracts were comparable and/or more potent than those of 5 mg/kg bw Glibenclamide | Raafat et al., 2018 [58] | |||
| Protein hydrolysates | García et al., 2016 [66] | |||||
| Prunus cerasus L. (sour cherry) | Seed | Used to prepare syrup and herbal tea infusion for treating fever, liver disease and gonorrhea [67]. | 5 wk supplementation with seed powder (0.1 mg/g diet) ameliorated HFD-induced obesogenic and neuroinflammatory effects in male Wistar rats by reducing serum cholesterols and TG, systolic BP and endothelial inflammatory makers (VCAM-1 and ICAM-1) of the frontal cortex and hippocampus. | Seed powder | Micioni Di Bonaventura et al., 2020 [68] | ||
| 17 wk feeding of basal diet containing seed flour attenuated HDF-induced adipogenesis in rats by downregulating the expression of adipogenic genes in adipose tissue. | Seed flour | Cocci et al., 2021 [69] | |||||
| Oral adm. (200 mg/kg bw) of seed extract ameliorated HCl and ethanol-induced gastric lesions in Swiss albino male mice. Also reduced carrageenan-induced nociceptive pain, inflammation and oxidative stress by reducing pro-inflammatory makers (TNF-α and IL-6) and improving antioxidant status. | Ethyl acetate extract | Oleic acid, linoleic acid, linolenic acid, stearic acid and palmitic acid (GC-FID). | The gastroprotective and anti-nociceptive effects of the extract were comparable to those of 50 mg/kg bw Ranitidine and 100 mg/kg bw Ibuprofen, respectively. | Raafat et al., 2020 [70] | |||
| 50-day treatment (30 mg/kg bw p.o.) protected against ischemic diabetic retinopathy in Zucker diabetic fatty male rats by improving GT, increasing HO-1 and reducing retinal thickness. | Defatted flavonoid-rich seed extract | Varga et al., 2017 [71] | |||||
| In vitro ACE inhibitory activity. | Protein or peptide isolates | González-García et al., 2018 [61] | |||||
| Acute and 8-day adm. (100, 150 and 200 mg/kg bw, i.p.) reduced BG levels and improved antioxidant status and pancreatic histology in alloxan-induced diabetic mice. | Ethyl acetate extract | The 200 mg/kg bw extract significantly (p ˂ 0.05) outperformed 5 mg/kg bw Glibenclamide in BG lowering. | Saleh et al., 2017 [72] | ||||
| Water extract | p-Hydroxybenzoic, syringic acid, vanillic acid, p-coumaric, caffeic acid, ferulic acid, protocatechuic acid, gallic acid, sinapinic acid and gentisic acid (HPLC). | Mesías et al., 2013 [64] | ||||
| Reduced lipopolysaccharide-induced inflammatory response in leukocytes isolated from diabetic patients by inhibiting upregulation of pro-inflammatory biomarkers (TNF-α and IL-8) and increasing HO-1 expression. | Defatted flavonoid-rich seed extract (Hydromethanolic) | Mahmoud et al., 2012 [73] | |||||
| 16 wks of feeding a basal diet containing 30 mg/kg seed extract afforded cardioprotective effects in cholesterol-fed male rabbits by improving cardiac function, increasing HO-1 expression and reducing cardiac atherosclerotic plaque formation. | Defatted flavonoid-rich seed extract | Juhasz et al., 2013 [74] | |||||
| Prunus persica (L.) Batsch 1801 (peach) | Seed | Used to treat amenorrhea and rheumatoid arthritis [70]. | In vitro ACE inhibitory activity. | Protein or peptide isolates | González-García et al., 2018 [61] | ||
| Protein or peptide isolates | Hernández-Corroto et al., 2018 [75] | |||||
| Water extract | p-Hydroxybenzoic, syringic acid, vanillic acid, p-coumaric, caffeic acid, ferulic acid, protocatechuic acid, gallic acid and sinapinic acid (HPLC). | Mesías et al., 2013 [64] | ||||
| In vitro ACE inhibitory activity. | Protein or peptide isolates | Vásquez-Villanueva et al., 2019 [76] | |||||
| Protein hydrolysates | García et al., 2016 [66] | |||||
| Ethanol extract | Loizzo et al., 2015 [77] | |||||
| Hydromethanolic extract |
| Rehman et al., 2021 [78] | ||||
| Inhibited histamine release and the expression of pro-inflammatory cytokines (TNF-α and IL-6) in human mast cells. | Methanol extract and isolated phenolic glycosides (vanilloloside and lacticolorin) | Vanilloloside and lacticolorin (HPLC, NMR). | Kim et al., 2013 [79] | ||||
| Prunus domestica L. (European plum) | Seed or pit |
| Methanol extract of defatted sample | p-Hydroxybenzoic acid, vanillin, vanillic acid, 3,4-dihydroxybenzoic acid, gallic acid and syringic acid (HPLC-ESI-MS). | Khallouki et al., 2012 [80] | ||
| In vitro ACE inhibitory activity. | Protein or peptide isolates | González-García et al., 2018 [61] | |||||
| Protein hydrolysates | García et al., 2016 [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukwuma, C.I. Antioxidative, Metabolic and Vascular Medicinal Potentials of Natural Products in the Non-Edible Wastes of Fruits Belonging to the Citrus and Prunus Genera: A Review. Plants 2024, 13, 191. https://doi.org/10.3390/plants13020191
Chukwuma CI. Antioxidative, Metabolic and Vascular Medicinal Potentials of Natural Products in the Non-Edible Wastes of Fruits Belonging to the Citrus and Prunus Genera: A Review. Plants. 2024; 13(2):191. https://doi.org/10.3390/plants13020191
Chicago/Turabian StyleChukwuma, Chika I. 2024. "Antioxidative, Metabolic and Vascular Medicinal Potentials of Natural Products in the Non-Edible Wastes of Fruits Belonging to the Citrus and Prunus Genera: A Review" Plants 13, no. 2: 191. https://doi.org/10.3390/plants13020191
APA StyleChukwuma, C. I. (2024). Antioxidative, Metabolic and Vascular Medicinal Potentials of Natural Products in the Non-Edible Wastes of Fruits Belonging to the Citrus and Prunus Genera: A Review. Plants, 13(2), 191. https://doi.org/10.3390/plants13020191







