Transferring an Adult-Plant Stripe-Rust Resistance Gene Yr7VS from Chromosome 7V of Dasypyrum villosum (L.) to Bread Wheat
Abstract
1. Introduction
2. Results
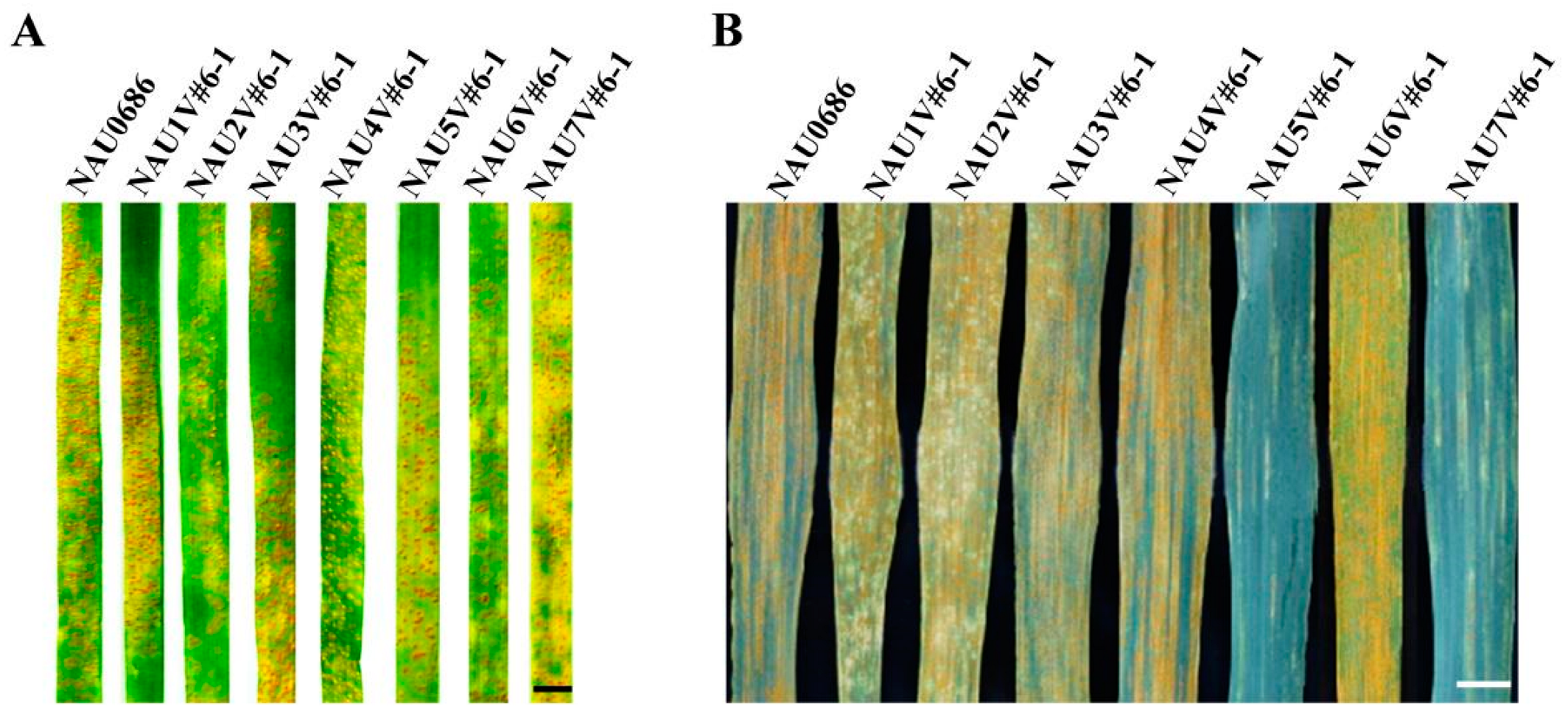
2.1. Stripe-Rust Responses of 1V#6 to 7V#6 Disomic Lines
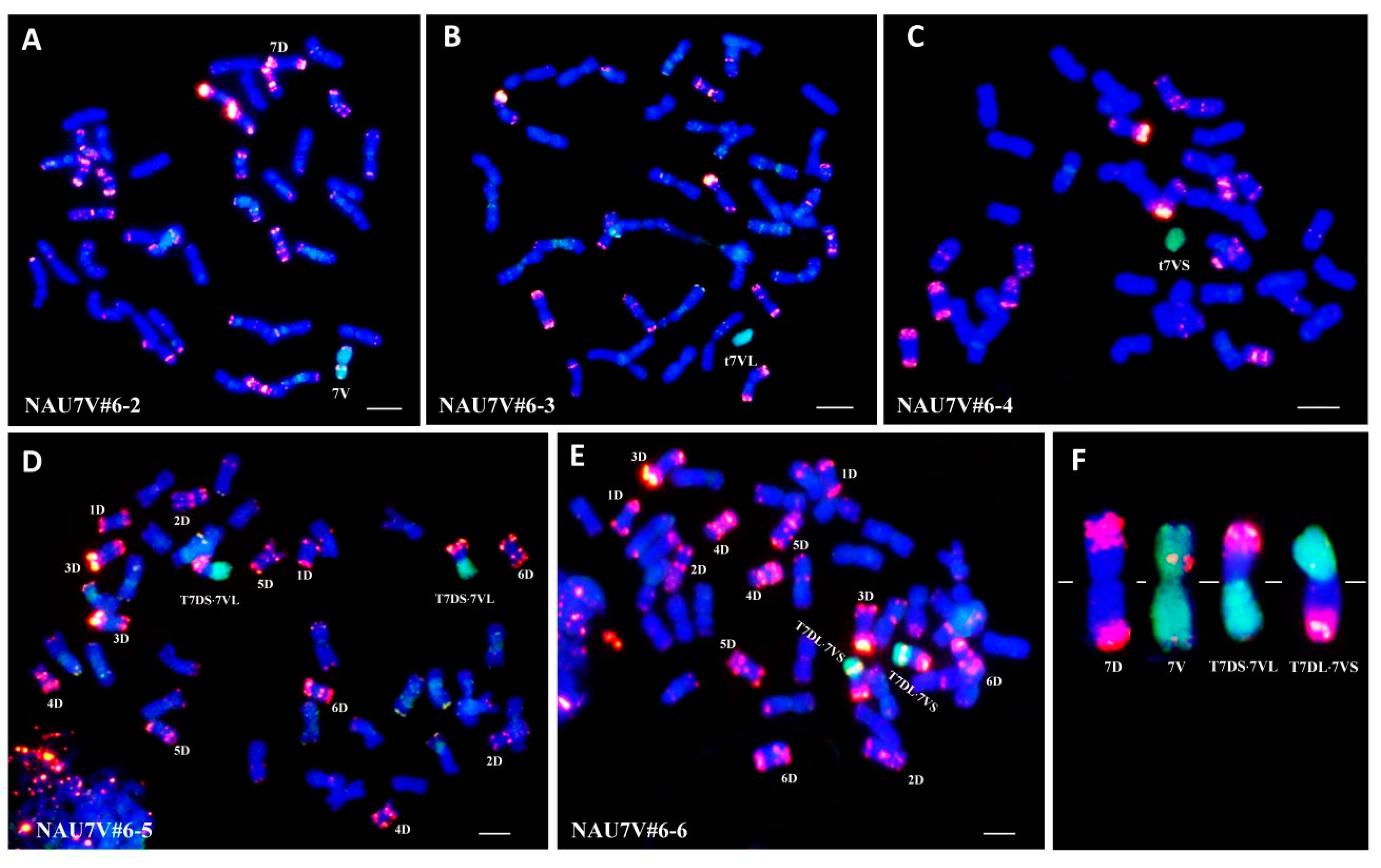
2.2. Development of a Double Monosomic Line of Chromosomes 7D and 7V#6
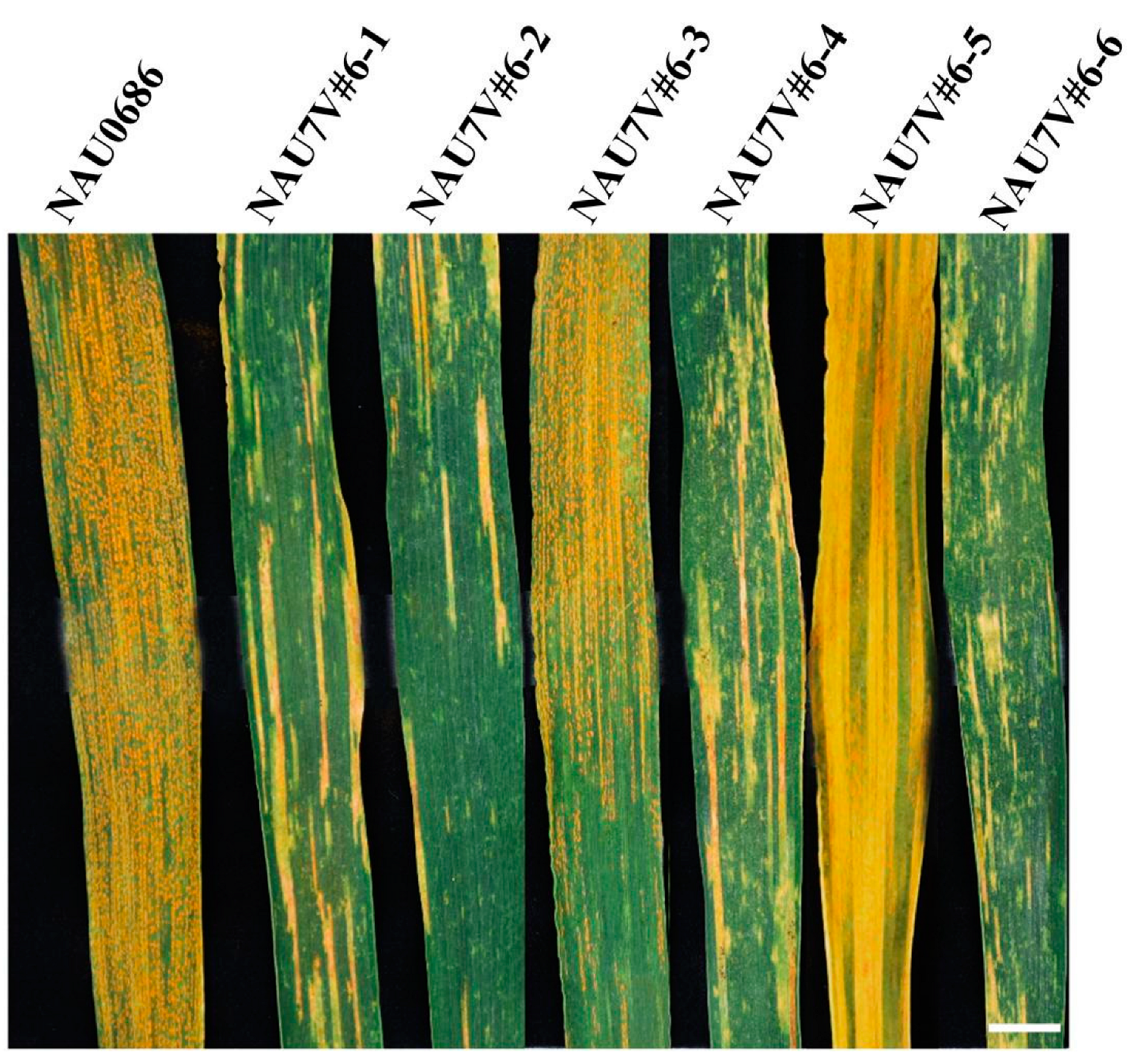
2.3. Development of Chromosome 7V#6 Alterations
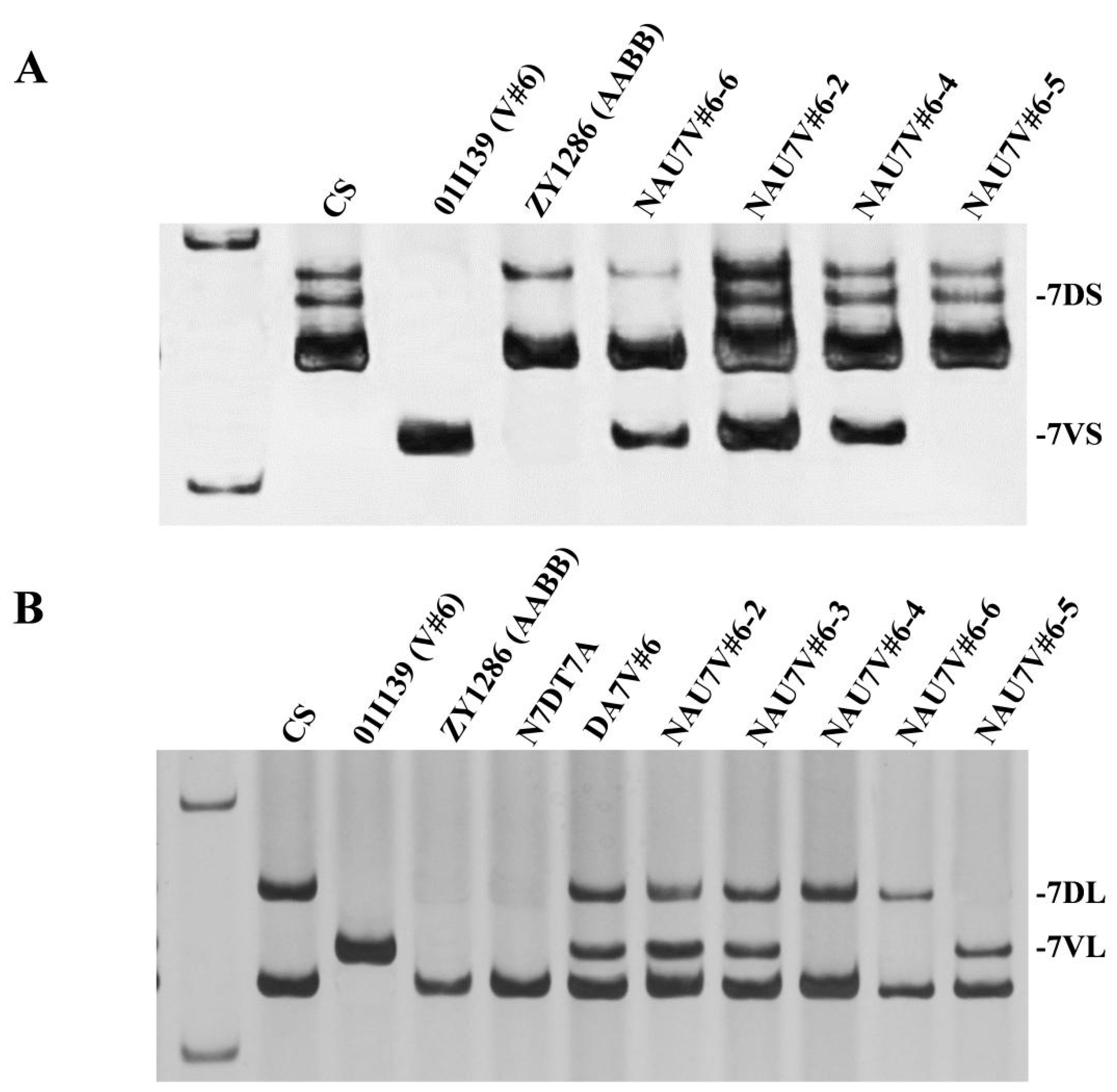
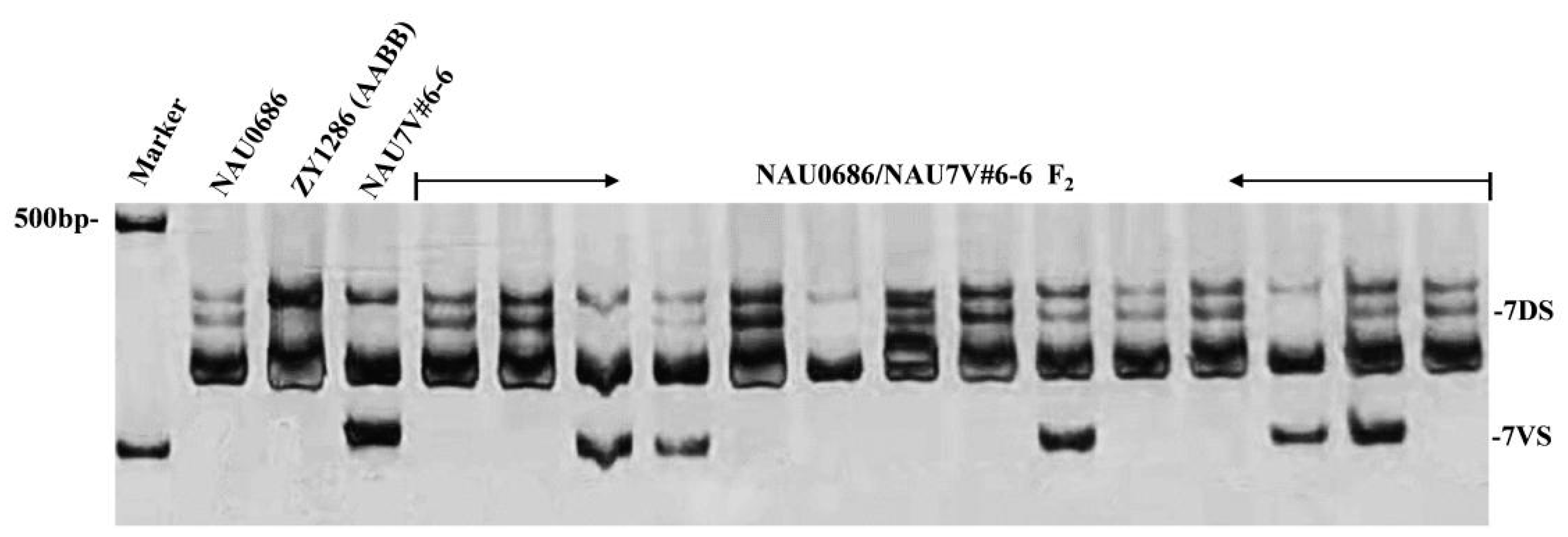
2.4. Chromosome Location of the Stripe-Rust Resistance Conferred by 7V#6
2.5. Evaluation of Major Agronomic Traits
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Cytogenetic Analysis
4.3. Molecular Marker Analysis
4.4. Stripe-Rust Evaluation
4.5. Evaluation of Agronomic Traits
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X. Pathogens which threaten food security: Puccinia striiformis, the wheat stripe rust pathogen. Food Sec. 2020, 12, 239–251. [Google Scholar] [CrossRef]
- Develey-Rivière, M.P.; Galiana, E. Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Du, J.; Chen, H.; Gong, S.; Jin, Y.; Meng, X.; Zhang, T.; Fu, B.; Molnár, I.; Holušová, K.; et al. Wheat Pm55 alleles exhibit distinct interactions with an inhibitor to cause different powdery mildew resistance. Nat. Commun. 2024, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.C. Host defense in a developmental context. Mol. Plant Pathol. 2005, 6, 347–360. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Zhang, H.; Hao, Q.; Lyu, B.; Wang, M.; Epstein, L.; Liu, M.; Kou, C.; Qi, J.; et al. An ancestral NB-LRR with duplicated 3′UTRs confers stripe rust resistance in wheat and barley. Nat. Commun. 2019, 10, 4023. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Zheng, Y.; Liu, X.; Yu, Y.; Zhang, G.; Epstein, L.; Mao, X.; Wu, J.; Yuan, C.; Lv, B.; et al. Sequencing trait-associated mutations to clone wheat rust-resistance gene YrNAM. Nat. Commun. 2023, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Kang, J.; Bräunlich, S.; Boni, R.; Chauhan, H.; Selter, L.L.; Robinson, M.D.; Schmid, M.W.; Wiederhold, E.; Hensel, G.; et al. Abscisic acid is a substrate of the ABC transporter encoded by the durable wheat disease resistance gene Lr34. New Phytol. 2019, 223, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S.; et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef]
- Tekin, M.; Emiralioğlu, O.; Yeken, M.Z.; Nadeem, M.A.; Çiftçi, V.; Baloch, F.S. Wild relatives and their contributions to wheat breeding. In Ancient Wheats; Zencirci, N., Ulukan, H., Baloch, F.S., Mansoor, S., Rasheed, A., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Shi, Z.X.; Chen, X.M.; Line, R.F.; Leung, H.; Wellings, C.R. Development of resistance gene analog polymorphism markers for the Yr9 gene resistance to wheat stripe rust. Genome 2001, 44, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dundas, I.; Dong, C.; Li, G.; Trethowan, R.; Yang, Z.; Hoxha, S.; Zhang, P. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 2020, 133, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, Z.; Zhang, X.; Yang, Z.; Li, X.; Jia, J.; Zhan, H.; Guo, H.; Wang, J. Putative Thinopyrum intermedium-derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor. Appl. Genet. 2013, 126, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, Y.; Wang, J.; Fu, S.; Wang, C.; Wang, M.; Zhou, C.; Hu, X.; Wang, T.; Yang, W.; et al. Development and cytological characterization of wheat-Thinopyrum intermedium translocation lines with novel stripe rust resistance gene. Front. Plant Sci. 2023, 14, 1135321. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Jia, J.; Zhang, X.; Li, X.; Yang, Z.; Ma, J.; Guo, H.; Zhan, H.; Qiao, L.; Chang, Z. Molecular mapping of the stripe rust resistance gene Yr69 on wheat chromosome 2AS. Plant Dis. 2016, 100, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- De Pace, C.; Vaccino, P.; Cionini, G.; Pasquini, M.; Bizzarri, M.; Qualset, C.O. Wild crop relatives: Genomic and breeding resources, cereals, vol 1, chapter 4. Springer, Heidelberg. Exp. Agric. 2011, 47, 736. [Google Scholar]
- Grądzielewska, A. The genus Dasypyrum—Part 2. Dasypyrum villosum—A wild species used in wheat improvement. Euphytica 2006, 152, 441–454. [Google Scholar]
- Hou, F.; Chen, H.; Zhang, T.; Jin, Y.; Kong, L.; Liu, X.; Xing, L.; Cao, A.; Zhang, R. Introgression of an all-stage and broad-spectrum powdery mildew resistance gene Pm3VS from Dasypyrum villosum chromosome 3V into wheat. Plant Dis. 2024; Epub ahead of print. [Google Scholar]
- Xing, L.; Hu, P.; Liu, J.; Witek, K.; Zhou, S.; Xu, J.; Zhou, W.; Gao, L.; Huang, Z.; Zhang, R.; et al. Pm21 from Haynaldia villosa encodes a CC-NBS-LRR protein conferring powdery mildew resistance in wheat. Mol. Plant 2018, 11, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Lu, C.T.; Meng, X.R.; Fan, Y.L.; Du, J.; Liu, R.R.; Feng, Y.; Xing, L.; Cápal, P.; Holušová, K.; et al. Fine mapping of powdery mildew and stripe rust resistance genes Pm5V/Yr5V transferred from Dasypyrum villosum into wheat without yield penalty. Theor. Appl. Genet. 2022, 135, 3629–3642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Fan, Y.L.; Kong, L.N.; Wang, Z.J.; Wu, J.Z.; Xing, L.P.; Cao, A.Z.; Feng, Y.G. Pm62, an adult-plant powdery mildew resistance gene introgressed from Dasypyrum villosum chromosome arm 2VL into wheat. Theor. Appl. Genet. 2018, 131, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Xiong, C.X.; Mu, H.Q.; Yao, R.N.; Meng, X.R.; Kong, L.N.; Xing, L.P.; Wu, J.Z.; Feng, Y.G.; Cao, A.Z. Pm67, a new powdery mildew resistance gene transferred from Dasypyrum villosum chromosome 1V to common wheat (Triticum aestivum L.). Crop J. 2021, 9, 882–888. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Sun, B.X.; Chen, J.; Cao, A.Z.; Xing, L.P.; Feng, Y.G.; Lan, C.; Chen, P. Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor. Appl. Genet. 2016, 129, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, T.; Li, W.; Kong, L.N.; Liu, X.Y.; Xing, L.P.; Cao, A.Z.; Zhang, R.Q. Introgression of an adult-plant powdery mildew resistance gene Pm4VL from Dasypyrum villosum chromosome 4V into bread wheat. Front. Plant Sci. 2024, 15, 1401525. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Jones, S.S.; Murray, T.D.; Line, R.F. Evaluation of Dasypyrum villosum populations for resistance to cereal eyespot and stripe rust pathogens. Plant Dis. 2000, 84, 40–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Tang, S.; Lang, T.; Wang, Y.; Long, H.; Deng, G.; Chen, Q.; Guo, Y.; Xuan, P.; Xiao, J.; et al. Molecular cytogenetic identification of the wheat-Dasypyrum villosum T3DL·3V#3S translocation line with resistance against stripe rust. Plants 2022, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Sears, E.R. Misdivision of univalents in common wheat. Chromosoma 1952, 4, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Zhang, P.; Linc, G.; Gill, B.S. Robertsonian translocations in wheat arise by centric misdivision of univalents at anaphase I and rejoining of broken centromeres during interkinesis of meiosis II. Cytogenet Genome Res. 2005, 109, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewski, A.J. Further manipulation by centric misdivision of the 1RS.1BL translocation in wheat. Euphytica 1997, 94, 257–261. [Google Scholar] [CrossRef]
- Hurni, S.; Brunner, S.; Buchmann, G.; Herren, G.; Jordan, T.; Krukowski, P.; Wicker, T.; Yahiaoui, N.; Mago, R.; Keller, B. Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 2013, 76, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Y.; Liu, C.; Gong, S.; Chen, Z.; Chen, R.; Liu, T.; Liu, R.; Du, H.; Guo, R.; Li, G.; et al. Orthologous genes Pm12 and Pm21 from two wild relatives of wheat show evolutionary conservation but divergent powdery mildew resistance. Plant Commun. 2022, 4, 100472. [Google Scholar] [CrossRef] [PubMed]
- Tomkowiak, A.; Skowrońska, R.; Kwiatek, M.; Spychała, J.; Weigt, D.; Kurasiak-Popowska, D. Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR. Open Life Sci. 2021, 16, 172–183. [Google Scholar] [PubMed]
- Klymiuk, V.; Haile, T.; Ens, J.; Wiebe, K.; Fatiukha, A.; Krugman, T.; Ben-David, R.; Hübner, S.; Cloutier, S.; Pozniak, C.J. Genetic architecture of rust resistance in a wheat (Triticum turgidum) diversity panel. Front. Plant Sci. 2023, 14, 1145371. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, Y.C.; Chen, X.M. Mapping a stripe rust resistance gene YrC591 in wheat variety C591 with SSR and AFLP markers. Theor. Appl. Genet. 2009, 118, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Wang, L.; Zhu, T.; Jorgensen, C.M.; Luo, M.; Deal, K.R.; Gu, Y.; Gill, B.S.; Distelfeld, A.; Devos, K.M.; et al. Reassessment of the evolution of wheat chromosomes 4A, 5A, and 7B. Theor. Appl. Genet. 2018, 131, 2451–2462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Sun, H.; Li, Y.; Feng, Y.; Jiao, C.; Li, M.; Song, X.; Wang, T.; Wang, Z.; et al. A chromosome-scale genome assembly of Dasypyrum villosum provides insights into its application as a broad-spectrum disease resistance resource for wheat improvement. Mol. Plant 2023, 16, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, M.; Hu, Y.; Gan, M.; Jiang, B.; Hao, M.; Ning, S.; Yuan, Z.; Chen, X.; Chen, X.; et al. Pyramiding of adult-plant resistance genes enhances all-stage resistance to wheat stripe rust. Plant Dis. 2023, 107, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Frick, M.; Huel, R.; Nykiforuk, C.L.; Wang, X.; Gaudet, D.A.; Eudes, F.; Conner, R.L.; Kuzyk, A.; Chen, Q.; et al. The stripe rust resistance gene Yr10 encodes an evolutionary-conserved and unique CC-NBS-LRR sequence in wheat. Mol. Plant 2014, 7, 1740–1755. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.; Sela, H.; Fahima, T.; Dubcovsky, J. Kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef]
- Kato, A.; Lamb, J.C.; Birchler, J.A. Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc. Natl. Acad. Sci. USA 2004, 101, 13554–13559. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zhuang, L.F.; Wang, Y.Z.; Li, Y.; Wang, Q.; Wang, D.R.; Tan, L.; Shen, J.; Xu, H.; Zhao, H.; et al. Development of oligonucleotides and multiplex probes for quick and accurate identification of wheat and Thinopyrum bessarabicum chromosomes. Genome 2017, 60, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wei, X.; Xiao, J.; Yuan, C.X.; Wu, Y.F.; Cao, A.Z.; Xing, L.P.; Chen, P.D.; Zhang, S.Z.; Wang, X.E. Whole genome development of intron targeting (IT) markers specific for Dasypyrum villosum chromosomes based on next-generation sequencing technology. Mol. Breed. 2017, 37, 115. [Google Scholar] [CrossRef]
- Han, D.; Wang, Q.; Chen, X.; Zeng, Q.; Wu, J.; Xue, W.; Zhan, G.; Huang, L.L.; Kang, Z. Emerging Yr26-virulent races of Puccinia striiformis f. tritici are threatening wheat production in the Sichuan basin, China. Plant Dis 2015, 99, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Line, R.F.; Qayoum, A. Virulence, Aggressiveness, Evolution, and Distribution of Races of Puccinia striiformis (the Cause of Stripe Rust of Wheat) in North America 1968–1987; US Department of Agriculture Technical Bulletin: Canberra, Australia, 1992; p. 74.
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic scale for estimating rust intensity of leaves and stems of cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]


| Chromosome Status | (7VS/7VS) | (7VS/7DS) | (7DS/7DS) | Expected Ratio | χ2 |
|---|---|---|---|---|---|
| Number of F2 individuals | 72 | 161 | 69 | 1:2:1 | 1.3 |
| Strip-rust severity (%) of individual F2 plants | 20 (10–30) | 28.5 (20–50) | 76.5 (60–100) | - | - |
| Response of F2:3 lines | Homozygous resistant | Segregating | Homozygous susceptible | - |
| Line | Chromosome Structure | Description |
|---|---|---|
| NAU0686 | AABBDD (2n = 42) | High-yield wheat cultivar highly susceptible to stripe rust; used as the recurrent parent for all alien introgression lines |
| N7DT7A | Null 7D (2n = 42) | Nulli-tetrasomic line of Chinese Spring |
| 01I139 | V#6V#6 (2n = 14) | Donor Dasypyrum villosum collected from Greece |
| ZY1286 | AABB (2n = 28) | Spring durum wheat used to cross with D. villosum 01I139 |
| NAU7V#6-1 | DA7V#6 (2n = 44) | NAU0686, with an added pair of chromosome 7V#6 of 01I139 |
| NAU7V#6-2 | MA7V#6 (2n = 42) | 7D and 7V#6, double monosomic line |
| NAU7V#6-3 | t7V#6L (2n = 43) | 7V#6L telosomic addition line |
| NAU7V#6-4 | t7V#6L (2n = 43) | 7V#6S telosomic addition line |
| NAU7V#6-5 | T7DS·7V#6L (2n = 42) | Compensation translocation line in which chromosome arm 7V#6L is substituted for 7DL |
| NAU7V#6-6 | T7DL·7V#6S (2n = 42) | Compensation translocation line in which chromosome arm 7V#6S is substituted for 7DS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, F.; Jin, Y.; Hu, J.; Kong, L.; Liu, X.; Xing, L.; Cao, A.; Zhang, R. Transferring an Adult-Plant Stripe-Rust Resistance Gene Yr7VS from Chromosome 7V of Dasypyrum villosum (L.) to Bread Wheat. Plants 2024, 13, 1875. https://doi.org/10.3390/plants13131875
Hou F, Jin Y, Hu J, Kong L, Liu X, Xing L, Cao A, Zhang R. Transferring an Adult-Plant Stripe-Rust Resistance Gene Yr7VS from Chromosome 7V of Dasypyrum villosum (L.) to Bread Wheat. Plants. 2024; 13(13):1875. https://doi.org/10.3390/plants13131875
Chicago/Turabian StyleHou, Fu, Yinyu Jin, Jin Hu, Lingna Kong, Xiaoxue Liu, Liping Xing, Aizhong Cao, and Ruiqi Zhang. 2024. "Transferring an Adult-Plant Stripe-Rust Resistance Gene Yr7VS from Chromosome 7V of Dasypyrum villosum (L.) to Bread Wheat" Plants 13, no. 13: 1875. https://doi.org/10.3390/plants13131875
APA StyleHou, F., Jin, Y., Hu, J., Kong, L., Liu, X., Xing, L., Cao, A., & Zhang, R. (2024). Transferring an Adult-Plant Stripe-Rust Resistance Gene Yr7VS from Chromosome 7V of Dasypyrum villosum (L.) to Bread Wheat. Plants, 13(13), 1875. https://doi.org/10.3390/plants13131875






