Genome-Wide Analysis of Polygalacturonase Gene Family Reveals Its Role in Strawberry Softening
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification, Phylogeny and Characterization of PG Gene Family in Strawberry Genome
2.3. RNA Extraction, cDNA Synthesis and PCR Amplification
2.4. RNA Sequencing and Transcriptome Analysis
2.5. Vector Construction and Instantaneous Silencing
2.6. Fruit Color, Hardness, and Soluble Solid Content Determination
2.7. Determination of Pectin, Cellulose and Hemicellulose Content
2.8. Measurement of PG Enzyme Activity
2.9. qRT-PCR Analysis
2.10. Statistical Analysis
3. Results
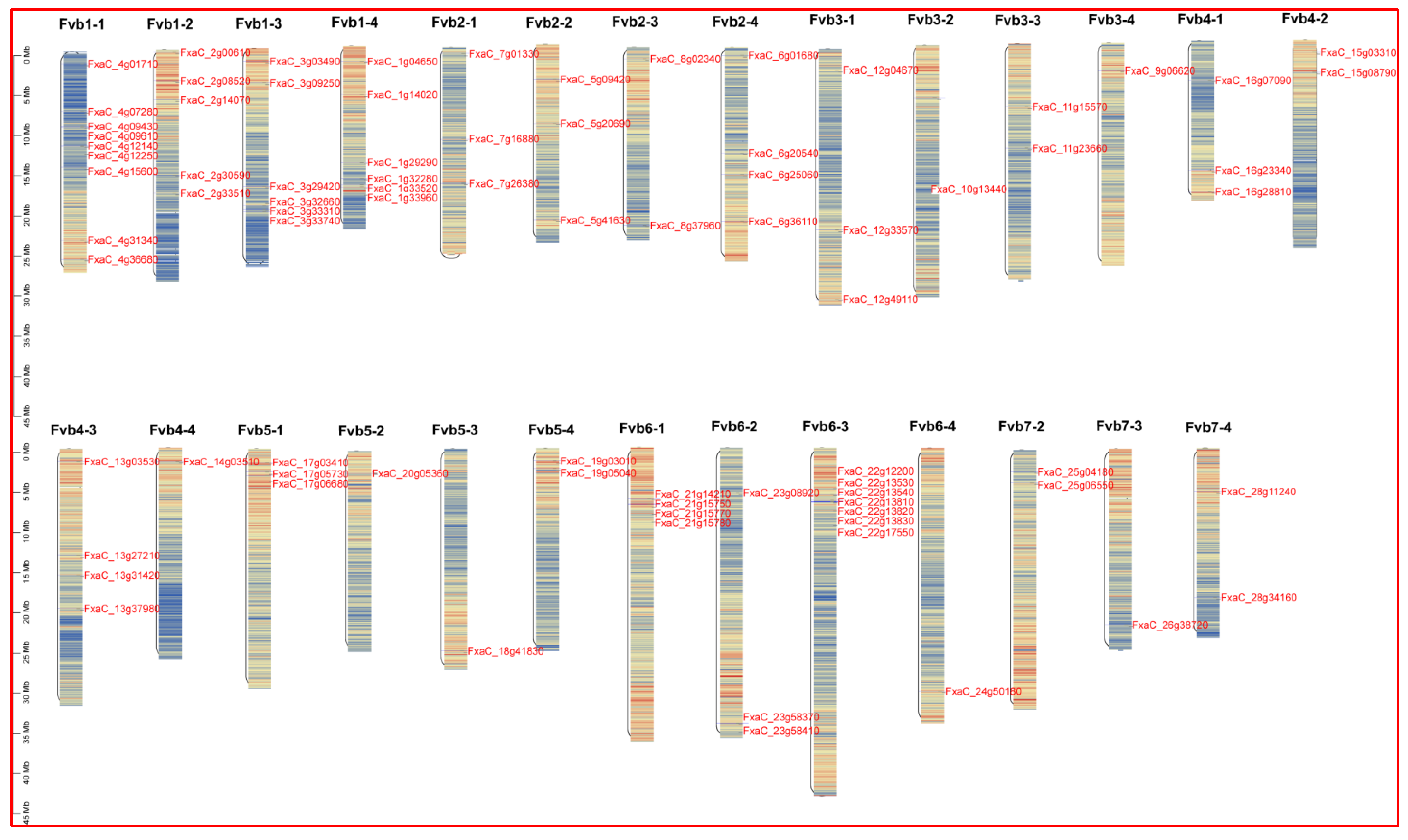
3.1. Identification of FaPGs Family in Strawberry
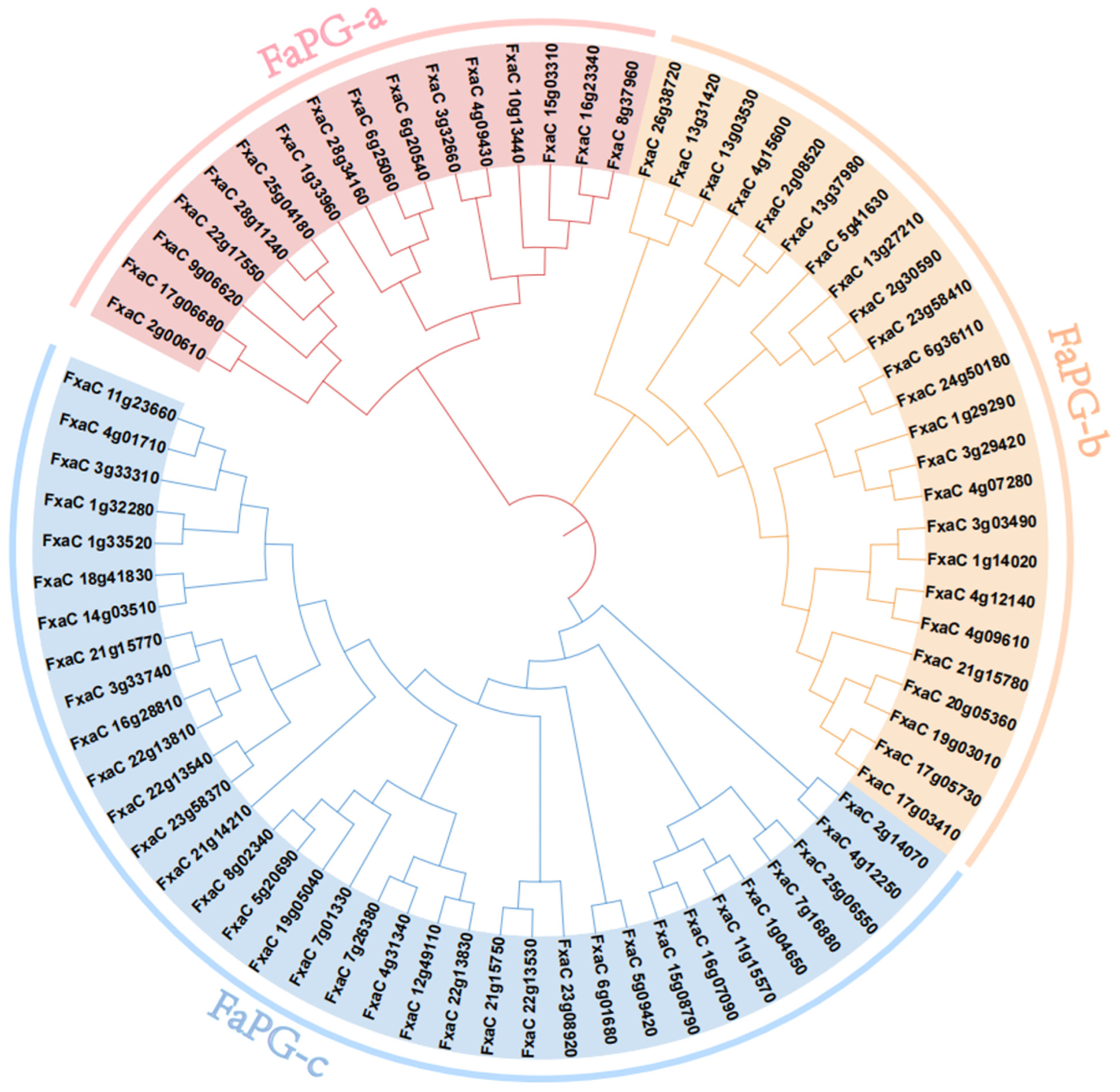
3.2. Phylogenetic Analysis
3.3. Structure and Motif Localization Analysis of FaPGs Genes
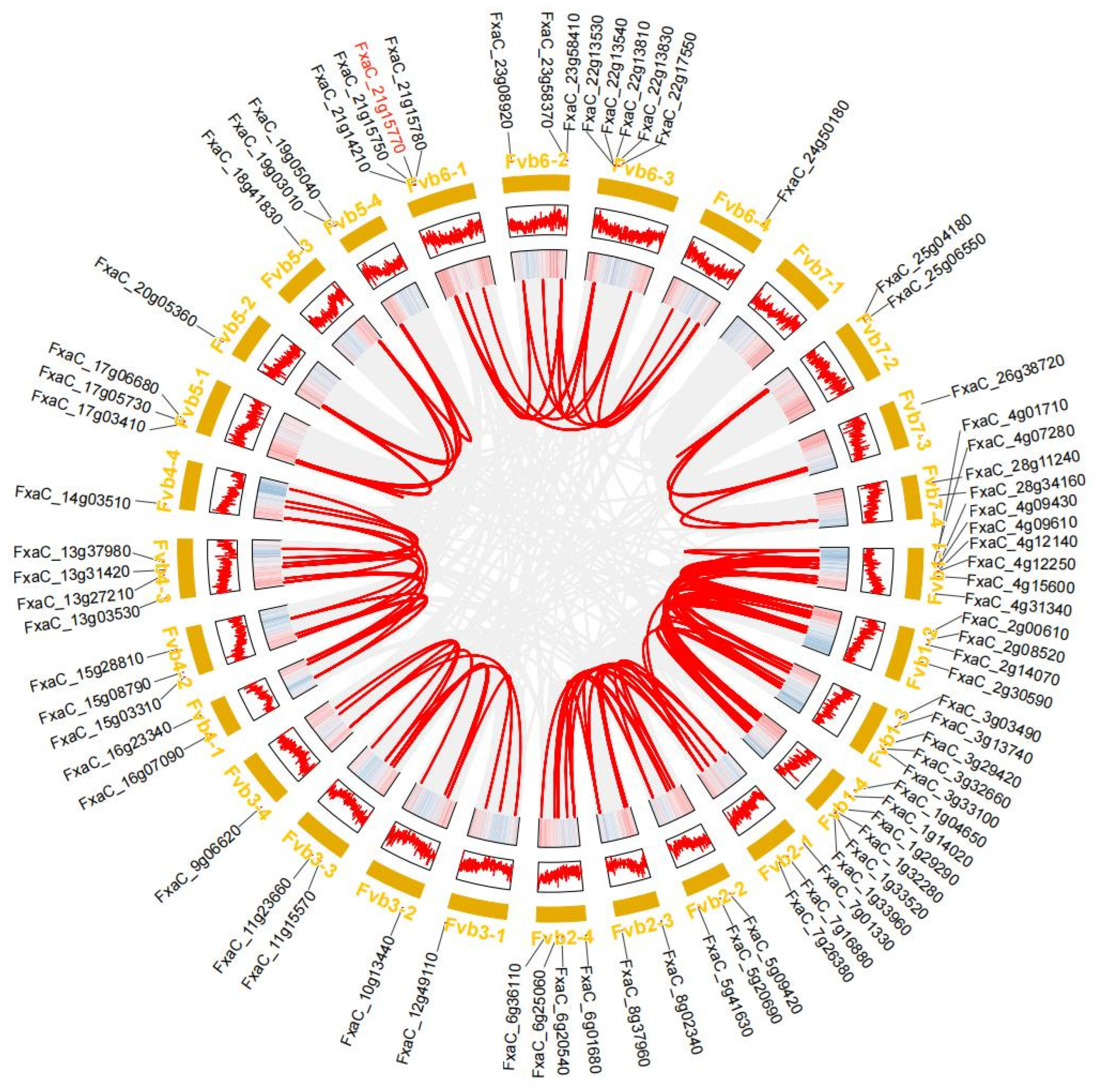
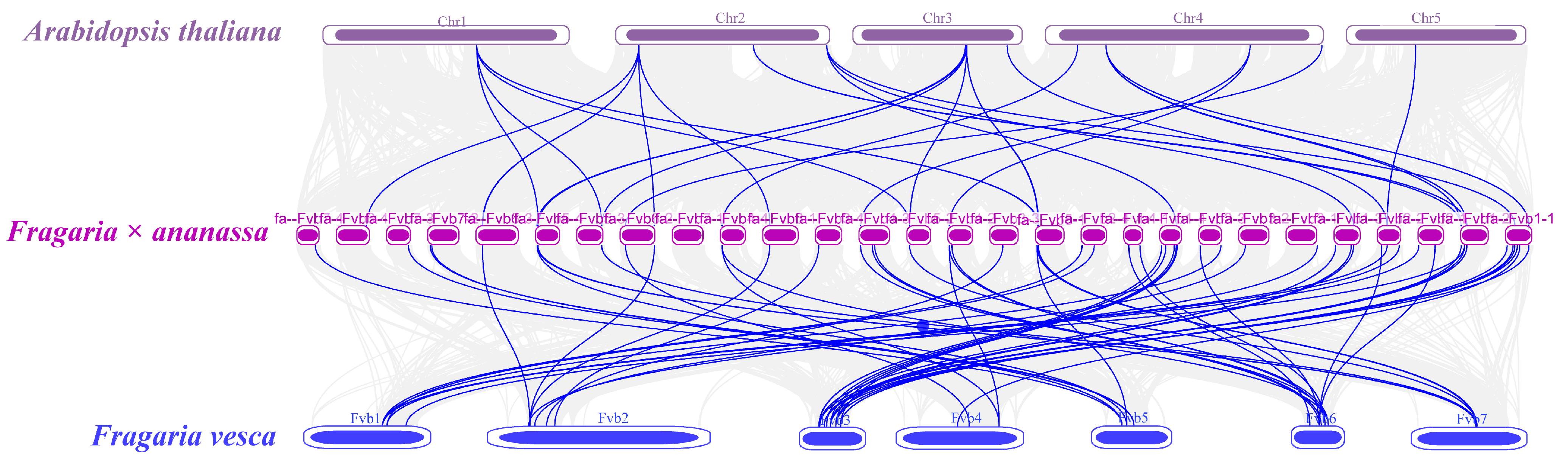
3.4. Synteny Analysis
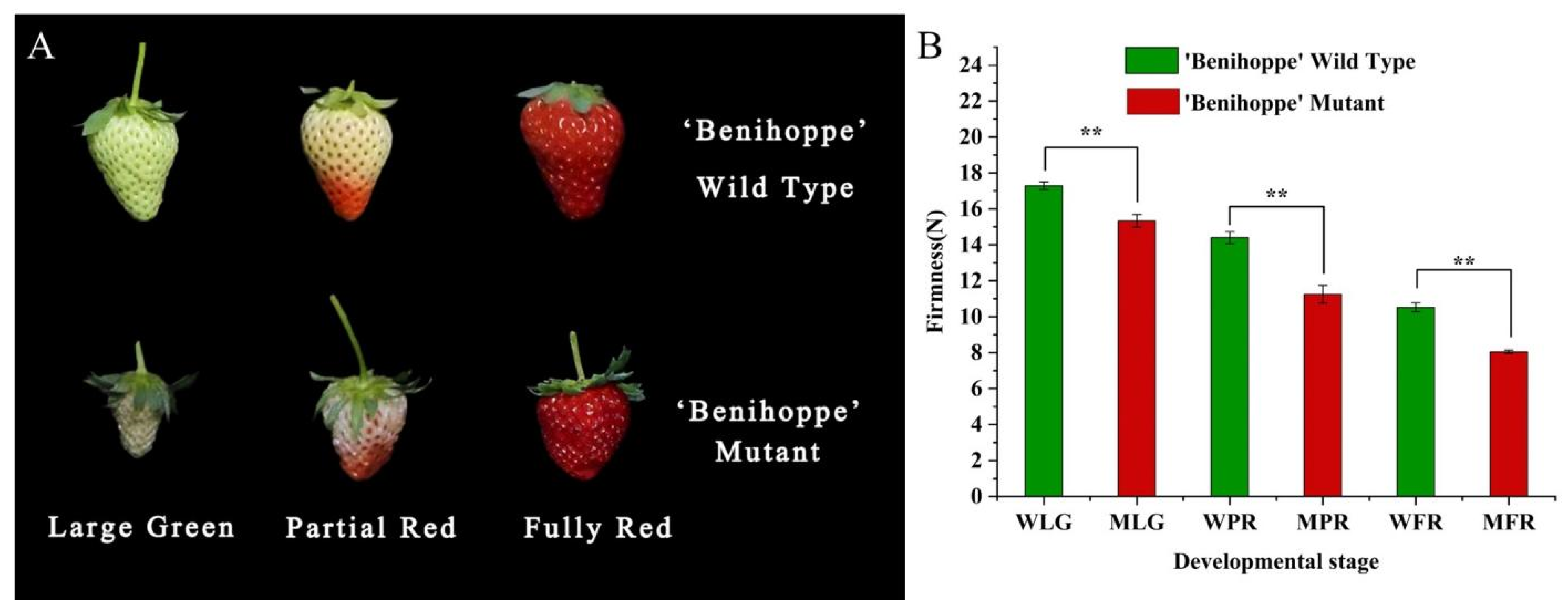
3.5. Changes in the Firmness of ‘Benihoppe’ and Its Mutant during Fruit Development
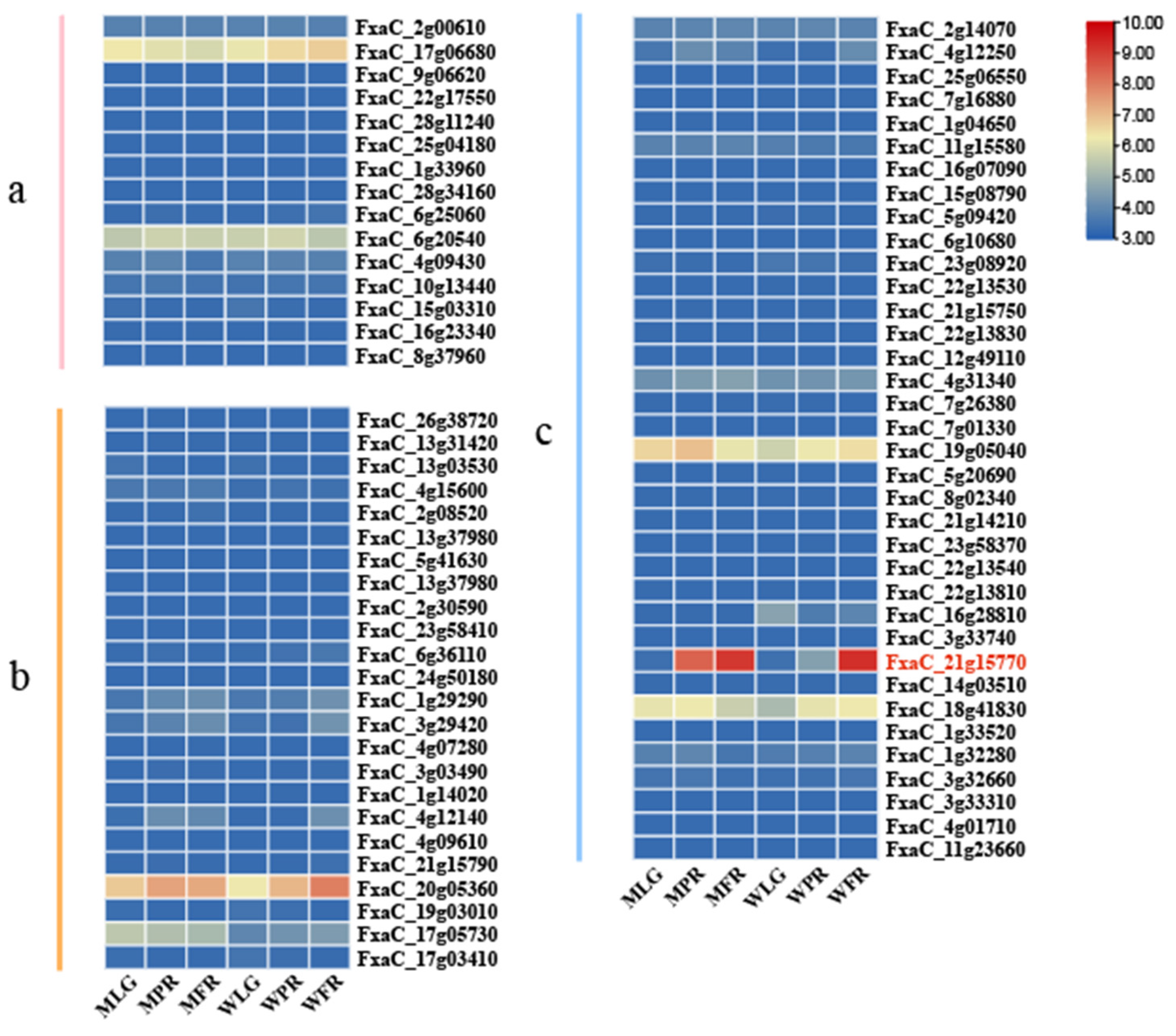
3.6. Transcriptional Differences of FaPGs Involved in Fruit Development and Ripening of ‘Benihoppe’ and Its Mutant
3.7. Silencing of FxaC_21g15770 Significantly Improved Fruit Firmness
3.8. Effects of Transient Silencing of FxaC_21g15770 on Expression of Strawberry Ripening and Softening Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leszczuk, A.; Chylinska, M.; Zdunek, A. Distribution of arabinogalactan proteins and pectins in the cells of apple (Malus × domestica) fruit during post-harvest storage. Ann. Bot. 2019, 123, 47–55. [Google Scholar] [CrossRef]
- Seymour, G.B.; Chapman, N.H.; Chew, B.L.; Rose, J.K. Regulation of ripening and opportunities for control in tomato and other fruits. Plant Biotechnol. J. 2013, 11, 269–278. [Google Scholar] [CrossRef]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyn, H.V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria × ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, G.; Li, H.; Wang, Y.; Gao, H.; Jemrić, T.; Fu, D. Molecular and Genetic Events Determining the Softening of Fleshy Fruits: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 12482. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Xia, R.; Shen, Y.; Zhu, Z.; Yu, Y.; Zang, Y. Cloning and Functional Identification of SlPG49 in Solanum lycopersicum. Appl. Sci. 2021, 11, 11450. [Google Scholar] [CrossRef]
- Poles, L.; Gentile, A.; Giuffrida, A.; Valentini, L.; Endrizzi, I.; Aprea, E.; Gasperi, F.; Distefano, G.; Artioli, G.; La Malfa, S.; et al. Role of fruit flesh cell morphology and MdPG1 allelotype in influencing juiciness and texture properties in apple. Postharvest Biol. Technol. 2020, 164, 111161. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Z.; Zhai, Y.; Huang, H.; Vainstein, A.; Ma, H. Polygalacturonase gene family analysis identifies FcPG12 as a key player in fig (Ficus carica L.) fruit softening. BMC Plant Biol. 2023, 23, 320. [Google Scholar] [CrossRef]
- Paniagua, C.; Ric-Varas, P.; García-Gago, J.A.; López-Casado, G.; Blanco-Portales, R.; Muñoz-Blanco, J.; Schückel, J.; Knox, J.P.; Matas, A.J.; Quesada, M.A.; et al. Elucidating the role of polygalacturonase genes in strawberry fruit softening. J. Exp. Bot. 2020, 71, 7103–7117. [Google Scholar] [CrossRef]
- Redondo-Nevado, J.; Moyano, E.; Medina-Escobar, N.; Caballero, J.L.; Muñoz-Blanco, J. A fruit-specific and developmentally regulated endopolygalacturonase gene from strawberry (Fragaria × ananassa cv. Chandler). J. Exp. Bot. 2001, 52, 1941–1945. [Google Scholar] [CrossRef]
- Quesada, M.A.; Blanco-Portales, R.; Posé, S.; García-Gago, J.A.; Jiménez-Bermúdez, S.; Muñoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Muñoz-Blanco, J. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef]
- Salentijn, E.; Aharoni, A.; Schaart, J.; Boone, M.; Krens, F. Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiol. Plant. 2003, 118, 571–578. [Google Scholar] [CrossRef]
- López-Casado, G.; Sánchez-Raya, C.; Ric-Varas, P.D.; Paniagua, C.; Blanco-Portales, R.; Muñoz-Blanco, J.; Pose, S.; Matas, A.J.; Mercado, J.A. CRISPR/Cas9 editing of the polygalacturonase FaPG1 gene improves strawberry fruit firmness. Hortic. Res. 2023, 10, uhad011. [Google Scholar] [CrossRef]
- García-Gago, J.A.; Posé, S.; Muñoz-Blanco, J.; Quesada, M.A.; Mercado, J.A. The polygalacturonase FaPG1 gene plays a key role in strawberry fruit softening. Plant Signal. Behav. 2009, 4, 766–768. [Google Scholar] [CrossRef]
- Sánchez-Sevilla, J.F.; Vallarino, J.G.; Osorio, S.; Bombarely, A.; Posé, D.; Merchante, C.; Botella, M.A.; Amaya, I.; Valpuesta, V. Gene expression atlas of fruit ripening and transcriptome assembly from RNA-seq data in octoploid strawberry (Fragaria × ananassa). Sci. Rep. 2017, 7, 13737. [Google Scholar] [CrossRef]
- Lin, Y.; Hou, G.; Jiang, Y.; Liu, X.; Yang, M.; Wang, L.; Long, Y.; Li, M.; Zhang, Y.; Wang, Y.; et al. Joint Transcriptomic and Metabolomic Analysis Reveals Differential Flavonoid Biosynthesis in a High-Flavonoid Strawberry Mutant. Front. Plant Sci. 2022, 13, 919619. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.W.; Wang, X.R.; Xie, X.L.; Yue, X.Y.; Tang, H.R. An alternative cetyltrimethylammonium bromide-based protocol for RNA isolation from blackberry (Rubus L.). Genet. Mol. Res. 2012, 11, 1773–1782. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Jin, J. The ultrafast and accurate mapping algorithm fanse3: Mapping a human whole-genome sequencing dataset within 30 minutes. Phenomics 2021, 1, 22–30. [Google Scholar] [CrossRef]
- Edger, P.P.; Poorten, T.J.; VanBuren, R.; Hardigan, M.A.; Colle, M.; McKain, M.R.; Smith, R.D.; Teresi, S.J.; Nelson, A.D.L.; Wai, C.M.; et al. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019, 51, 541–547. [Google Scholar] [CrossRef]
- Robinson, M.; McCarthy, D.; Smyth, G. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. nClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar]
- Drobek, M.; Frąc, M.; Zdunek, A.; Cybulska, J. The Effect of Cultivation Method of Strawberry (Fragaria × ananassa Duch.) cv. Honeoye on Structure and Degradation Dynamics of Pectin during Cold Storage. Molecules 2020, 25, 4325. [Google Scholar] [CrossRef]
- Villarreal, N.M.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biol. Technol. 2008, 47, 141–150. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiang, L.; Yue, M.; Liu, Y.; Zhang, N.; Lin, Y.; Zhang, Y.; Wang, Y.; Li, M.; Luo, Y.; Zhang, Y.; et al. A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol. J. 2023, 21, 1140–1158. [Google Scholar] [CrossRef]
- Ke, X.; Wang, H.; Li, Y.; Zhu, B.; Zang, Y.; He, Y.; Cao, J.; Zhu, Z.; Yu, Y. Genome-Wide Identification and Analysis of Polygalacturonase Genes in Solanum lycopersicum. Int. J. Mol. Sci. 2018, 19, 2290. [Google Scholar] [CrossRef]
- González-Carranza, Z.H.; Elliott, K.A.; Roberts, J.A. Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 3719–3730. [Google Scholar] [CrossRef]
- Kim, J.; Shiu, S.H.; Thoma, S.; Li, W.H.; Patterson, S.E. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol. 2006, 7, R87. [Google Scholar] [CrossRef]
- Zhai, Z.; Feng, C.; Wang, Y.; Sun, Y.; Peng, X.; Xiao, Y.; Zhang, X.; Zhou, X.; Jiao, J.; Wang, W.; et al. Genome-Wide Identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry. Int. J. Mol. Sci. 2021, 22, 12331. [Google Scholar] [CrossRef]
- Huang, W.; Chen, M.; Zhao, T.; Han, F.; Zhang, Q.; Liu, X.; Jiang, C.; Zhong, C. Genome-Wide Identification and Expression Analysis of Polygalacturonase Gene Family in Kiwifruit (Actinidia chinensis) during Fruit Softening. Plants 2020, 9, 327. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, M.; Zhang, H.; Zhang, S.; Qian, M.; Zhang, Z.; Luo, W.; Fan, J.; Liu, Z.; Wang, L. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019, 19, 587. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Zhang, Y.; Yan, X.; Han, M.; Li, J.; Li, F.; Li, F.; Zhang, D.; Zhao, C. Identification and Expression Analysis of Polygalacturonase Family Members during Peach Fruit Softening. Int. J. Mol. Sci. 2016, 17, 1933. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Fatima, F.; Haider, M.S.; Shazadee, H.; Liu, Z.; Zheng, T.; Fang, J. Genome-Wide Identification and Expression Profiling of the Polygalacturonase (PG) and Pectin Methylesterase (PME) Genes in Grapevine (Vitis vinifera L.). Int. J. Mol. Sci. 2019, 20, 3180. [Google Scholar] [CrossRef]
- Zhou, H.C.; Li, G.; Zhao, X.; Li, L.J. Comparative analysis of polygalacturonase in the fruit of strawberry cultivars. Genet. Mol. Res. 2015, 14, 12776–12787. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.A.; Govindarajulu, R.; Ashman, T.L.; Liston, A. Evolutionary origins and dynamics of octoploid strawberry subgenomes revealed by dense targeted capture linkage maps. Genome Biol. Evol. 2014, 6, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, J.; Wang, L.; Zhong, J.; Yin, H.; Wu, S.; Zhang, Z.; Yu, J. Systematic analysis of intron size and abundance parameters in diverse lineages. Sci. China Life Sci. 2013, 56, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, H.; Fan, S.; Ma, J.; Zhang, D.; Han, M. Identification and Phylogenetic Analysis of the Polygalacturonase Gene Family in Apple. Hortic. Plant J. 2016, 2, 241–252. [Google Scholar] [CrossRef]
- Mercado, J.; Pliego-Alfaro, F.; Quesada, M. Fruit Shelf Life and Potential for Its Genetic Improvement. In Breeding for Fruit Quality; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 81–104. [Google Scholar]
- Qian, M.; Xu, Z.; Zhang, Z.; Li, Q.; Yan, X.; Liu, H.; Han, M.; Li, F.; Zheng, J.; Zhang, D.; et al. The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening. Planta 2021, 254, 22. [Google Scholar] [CrossRef] [PubMed]
- Posé, S.; Paniagua, C.; Cifuentes, M.; Blanco-Portales, R.; Quesada, M.A.; Mercado, J.A. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013, 64, 3803–3815. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Chen, Y.; Li, J.; Yu, C.; Guo, Z.; Yang, G. Overexpression of the Rubus idaeus Polygalacturonases Gene RiPG2 Accelerates Fruit Softening in Solanum lycopersicum. Agronomy 2024, 14, 160. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Mattus-Araya, E.; Herrera, R. Molecular Events Occurring During Softening of Strawberry Fruit. Front. Plant Sci. 2019, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, B.J.; Grierson, D.; Chen, K.S. Insights into cell wall changes during fruit softening from transgenic and naturally occurring mutants. Plant Physiol. 2023, 192, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Bermúdez, S.; Redondo-Nevado, J.; Muñoz-Blanco, J.; Caballero, J.L.; López-Aranda, J.M.; Valpuesta, V.; Pliego-Alfaro, F.; Quesada, M.A.; Mercado, J.A. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 2002, 128, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, C.; de la Fuente, J.I.; Iannetta, P.; Botella, M.A.; Valpuesta, V. Pectin esterase gene family in strawberry fruit: Study of FaPE1, a ripening-specific isoform. J. Exp. Bot. 2004, 55, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Kim, I.-J. Modulation of fruit softening by antisense suppression of endo-β-1,4-glucanase in strawberry. Mol. Breed. 2011, 27, 375–383. [Google Scholar] [CrossRef]
- Woolley, L.C.; James, D.J.; Manning, K. Purification and properties of an endo-beta-1,4-glucanase from strawberry and down-regulation of the corresponding gene, cel1. Planta 2001, 214, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Llop-Tous, I.; Vendrell, M.; Krens, F.A.; Schaart, J.G.; Boone, M.J.; van der Valk, H.; Salentijn, E.M.J. Antisense down-regulation of strawberry endo-β-(1,4)-glucanase genes does not prevent fruit softening during ripening. Plant Sci. 2006, 171, 640–646. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Hu, R.; Lin, Y.; Yang, Y.; Chen, Q.; Li, M.; Zhang, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. Genome-Wide Analysis of Polygalacturonase Gene Family Reveals Its Role in Strawberry Softening. Plants 2024, 13, 1838. https://doi.org/10.3390/plants13131838
Zhao M, Hu R, Lin Y, Yang Y, Chen Q, Li M, Zhang Y, Zhang Y, Wang Y, He W, et al. Genome-Wide Analysis of Polygalacturonase Gene Family Reveals Its Role in Strawberry Softening. Plants. 2024; 13(13):1838. https://doi.org/10.3390/plants13131838
Chicago/Turabian StyleZhao, Mantong, Ruixin Hu, Yuanxiu Lin, Yeqiao Yang, Qing Chen, Mengyao Li, Yong Zhang, Yunting Zhang, Yan Wang, Wen He, and et al. 2024. "Genome-Wide Analysis of Polygalacturonase Gene Family Reveals Its Role in Strawberry Softening" Plants 13, no. 13: 1838. https://doi.org/10.3390/plants13131838
APA StyleZhao, M., Hu, R., Lin, Y., Yang, Y., Chen, Q., Li, M., Zhang, Y., Zhang, Y., Wang, Y., He, W., Wang, X., Tang, H., & Luo, Y. (2024). Genome-Wide Analysis of Polygalacturonase Gene Family Reveals Its Role in Strawberry Softening. Plants, 13(13), 1838. https://doi.org/10.3390/plants13131838







