Effect of Origin, Seed Coat Color, and Maturity Group on Seed Isoflavones in Diverse Soybean Germplasm
Abstract
:1. Introduction
2. Results and Discussion
2.1. Natural Variation of Isoflavones in Diverse Soybean Germplasm
2.2. Effect of Origin on Soybean Seed Isoflavones
2.3. Seed Coat Color Significantly Influenced the Soybean Seed Isoflavones
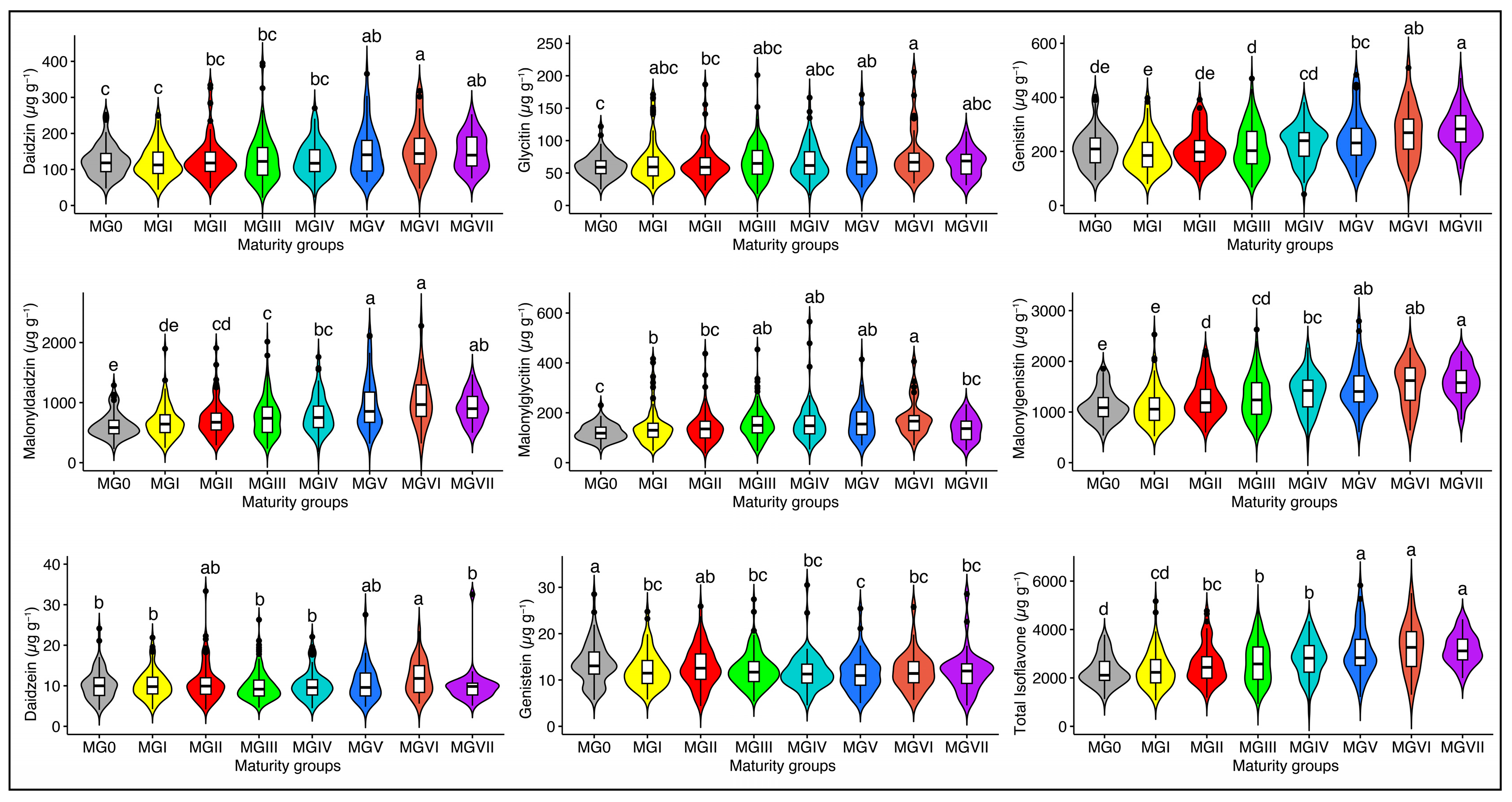
2.4. Effect of Maturity Group on Soybean Seed Isoflavones
2.5. Correlation, Heatmap, and Stability Analysis of Isoflavones
3. Materials and Methods
3.1. Planting Materials
3.2. Field Experiments
3.3. Extraction and Determination of Isoflavones
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, C.; Qi, X.; Li, M.; Wong, F.; Lam, H. Recent developments of genomic research in soybean. J. Genet. Genom. 2012, 39, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Yadav, D.; Vij, S. Soybean bioactive molecules: Current trend and future prospective. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2017; pp. 267–294. [Google Scholar]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.J.; Zhao, Y.; Glasier, J.; Cullen, D.; Barnes, S.; Turner, C.H.; Wastney, M.; Weaver, C.M. Comparative effect of soy protein, soy isoflavones, and 17β-estradiol on bone metabolism in adult ovariectomized rats. J. Bone Miner. Res. 2004, 20, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Appleby, P.N.; Key, T.J. Fruit, vegetable, and fiber intake in relation to cancer risk: Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2014, 100, 394S–398S. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. New Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Khan Afridi, A.A.; Wasay, M.; Iqbal, R. Isoflavones and alzheimer’s disease: The effects of soy in diet. Pak. J. Neurol. Sci. (PJNS) 2014, 9, 40–45. [Google Scholar]
- Azam, M.; Zhang, S.; Abdelghany, A.M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Seed isoflavone profiling of 1168 soybean accessions from major growing ecoregions in China. Food Res. Int. 2020, 130, 108957. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, E.H.; Park, I.; Yu, B.R.; Lim, J.D.; Lee, Y.S.; Lee, J.H.; Kim, S.H.; Chung, I.M. Isoflavones profiling of soybean [Glycine max (L.) Merrill] germplasms and their correlations with metabolic pathways. Food Chem. 2014, 153, 258–264. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Han, F.; Li, B.; Yan, S.; Sun, J.; Wang, L. Isoflavone content of soybean cultivars from maturity group 0 to VI grown in northern and southern China. J. Am. Oil Chem. Soc. 2014, 91, 1019–1028. [Google Scholar] [CrossRef]
- Qiu, L.; Li, Y.; Guan, R.; Liu, Z.; Wang, L.; Chang, R. Establishment, representative testing and research progress of soybean core collection and mini core collection. Acta Agron. Sin. 2009, 35, 571–579. [Google Scholar] [CrossRef]
- Sun, J.M.; Han, F.X.; Yan, S.R.; Yang, H.; Li, B. Development of a novel soybean cultivar Zhonghuang 68 with high isoflavone Content and low off-flavor. Soybean Sci. 2015, 34, 900–905. [Google Scholar]
- Lee, S.J.; Yan, W.; Ahn, J.K.; Chung, I.M. Effects of year, site, genotype and their interactions on various soybean isoflavones. Field Crops Res. 2003, 81, 181–192. [Google Scholar] [CrossRef]
- Rasolohery, C.A.; Berger, M.; Lygin, A.V.; Lozovaya, V.V.; Nelson, R.L.; Daydé, J. Effect of temperature and water availability during late maturation of the soybean seed on germ and cotyledon isoflavone content and composition. J. Sci. Food Agric. 2008, 88, 218–228. [Google Scholar] [CrossRef]
- Tsai, H.S.; Huang, L.J.; Lai, Y.H.; Chang, J.C.; Lee, R.S.; Chiou, R.Y. Solvent effects on extraction and HPLC analysis of soybean isoflavones and variations of isoflavone compositions as affected by crop season. J. Agric. Food Chem. 2007, 55, 7712–7715. [Google Scholar] [CrossRef]
- Azam, M.; Zhang, S.; Qi, J.; Abdelghany, A.M.; Shaibu, A.S.; Ghosh, S.; Feng, Y.; Huai, Y.; Gebregziabher, B.S.; Li, J. Profiling and associations of seed nutritional characteristics in Chinese and USA soybean cultivars. J. Food Compos. Anal. 2021, 98, 103803. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, Y.S.; Kim, S.T.; Yoon, W.B.; Han, W.Y.; Kang, I.K.; Choung, M.G. Seed coat color and seed weight contribute differential responses of targeted metabolites in soybean seeds. Food Chem. 2017, 214, 248–258. [Google Scholar] [CrossRef]
- Choi, Y.M.; Yoon, H.; Shin, M.J.; Lee, Y.; Hur, O.S.; Lee, B.C.; Ha, B.K.; Wang, X.; Desta, K.T. Metabolite contents and antioxidant activities of soybean (Glycine max (L.) Merrill) seeds of different seed coat colors. Antioxidants 2021, 10, 1210. [Google Scholar] [CrossRef]
- Desta, K.T.; Hur, O.S.; Lee, S.; Yoon, H.; Shin, M.J.; Yi, J.; Lee, Y.; Ro, N.Y.; Wang, X.; Choi, Y.M. Origin and seed coat color differently affect the concentrations of metabolites and antioxidant activities in soybean (Glycine max (L.) Merrill) seeds. Food Chem. 2022, 381, 132249. [Google Scholar] [CrossRef]
- Song, J.; Liu, Z.; Hong, H.; Ma, Y.; Tian, L.; Li, X.; Li, Y.H.; Guan, R.; Guo, Y.; Qiu, L.J. Identification and validation of loci governing seed coat color by combining association mapping and bulk segregation analysis in soybean. PLoS ONE 2016, 11, e0159064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, G.; Zhao, H.; Yuan, C.; Liu, X.; Li, Y.; Wang, Y.; Dong, Y. Genetic diversity of soybean landraces with different seed coat color. Legume Genom. Genet. 2023, 22, 14. [Google Scholar] [CrossRef]
- Sun, W.; Meng, X.; Liang, L.; Jiang, W.; Huang, Y.; He, J.; Hu, H.; Almqvist, J.; Gao, X.; Wang, L. Molecular and biochemical analysis of chalcone synthase from Freesia hybrid in flavonoid biosynthetic pathway. PLoS ONE 2015, 10, e0119054. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, J.-S.; Lee, C.-Y.; Jeong, M.-G.; Xu, J.L.; Choi, Y.; Jung, H.-W.; Choi, H.-K. Dissecting seed pigmentation-associated genomic loci and genes by employing dual approaches of reference-based and k-mer-based GWAS with 438 Glycine accessions. PLoS ONE 2020, 15, e0243085. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yuan, C.; Wang, Y.; Liu, X.; Qi, G.; Wang, Y.; Dong, L.; Zhao, H.; Li, Y.; Dong, Y. Identification of genetic loci conferring seed coat color based on a high-density map in soybean. Front. Plant Sci. 2022, 13, 968618. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, S.K.; Seo, J.H.; Kang, B.K.; Park, J.H.; Kim, J.H.; Sung, J.S.; Baek, I.Y.; Shin, S.O.; Jung, C.S. Protein, amino acid, oil, fatty acid, sugar, anthocyanin, isoflavone, lutein, and antioxidant variations in colored seed-coated soybeans. Plants 2021, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Lee, E.A.; Woodrow, L.; Seguin, P.; Kumar, J.; Rajcan, I.; Ablett, G.R. Genotype × Environment interaction and stability for isoflavone content in soybean. Crop Sci. 2009, 49, 1313–1321. [Google Scholar] [CrossRef]
- Carrera, C.; Martínez, M.J.; Dardanelli, J.; Balzarini, M. Environmental variation and correlation of seed components in nontransgenic soybeans: Protein, oil, unsaturated fatty acids, tocopherols, and isoflavones. Crop Sci. 2011, 51, 800–809. [Google Scholar] [CrossRef]
- Lee, J.H.; Choung, M.G. Comparison of nutritional components in soybean varieties with different geographical origins. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 254–263. [Google Scholar] [CrossRef]
- Kim, E.H.; Ro, H.M.; Kim, S.L.; Kim, H.S.; Chung, I.M. Analysis of isoflavone, phenolic, soyasapogenol, and tocopherol compounds in soybean [ Glycine max (L.) Merrill] germplasms of different seed weights and origins. J. Agric. Food Chem. 2012, 60, 6045–6055. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Sherrand, M.; Pagadala, S.; Wixon, R.; Scott, R.A. Isoflavone content among maturity group 0 to II soybeans. J. Am. Oil Chem. Soc. 2000, 77, 483–487. [Google Scholar] [CrossRef]
- Song, W.; Yang, R.; Wu, T.; Wu, C.; Sun, S.; Zhang, S.; Jiang, B.; Tian, S.; Liu, X.; Han, T. Analyzing the effects of climate factors on soybean protein, oil contents, and composition by extensive and high-density sampling in China. J. Agric. Food Chem. 2016, 64, 4121–4130. [Google Scholar] [CrossRef]
- Tsukamoto, C.; Shimada, S.; Igita, K.; Kudou, S.; Kokubun, M.; Okubo, K.; Kitamura, K. Factors affecting isoflavone content in soybean seeds—Changes in isoflavones, saponins, and composition of fatty-acids at different temperatures during seed development. J. Agric. Food Chem. 1995, 43, 1184–1192. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, Y.S.; Chang, W.S.; Moon, J.K.; Choung, M.G. Seed maturity differentially mediates metabolic responses in black soybean. Food Chem. 2013, 141, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; McGonigle, B. Metabolic engineering of isoflavone biosynthesis. Adv. Agron. 2005, 86, 147–190. [Google Scholar]
- Choi, Y.-M.; Yoon, H.; Lee, S.; Ko, H.-C.; Shin, M.-J.; Lee, M.C.; Hur, O.S.; Ro, N.Y.; Desta, K.T. Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Sci. Rep. 2020, 10, 19960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Zhang, F.X.; Zhang, M.W.; Wei, Z.C.; Yang, C.Y.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Chi, J.W. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J. Agric. Food Chem. 2011, 59, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.b.; Liu, Z.x.; Yang, C.y.; Xu, R.; Lu, W.G.; Zhang, L.F.; Wang, Q.; Wei, S.H.; Yang, C.M.; Wang, H.C.; et al. Stability of growth periods traits for soybean cultivars across multiple locations. J. Integr. Agric. 2016, 15, 963–972. [Google Scholar] [CrossRef]
- Abdulai, M.; Sallah, P.; Safo-Kantanka, O. Maize grain yield stability analysis in full season lowland maize in Ghana. Int. J. Agric. Biol. 2007, 9, 41–45. [Google Scholar]
- Temesgen, T.; Keneni, G.; Sefera, T.; Jarso, M. Yield stability and relationships among stability parameters in faba bean (Vicia faba L.) genotypes. Crop J. 2015, 3, 258–268. [Google Scholar] [CrossRef]
- You, F.M.; Jia, G.; Xiao, J.; Duguid, S.D.; Rashid, K.Y.; Booker, H.M.; Cloutier, S. Genetic variability of 27 traits in a core collection of flax (Linum usitatissimum L.). Front. Plant Sci. 2017, 8, 1636. [Google Scholar] [CrossRef]
- Yang, R.; Song, W.; Sun, S.; Wu, C.; Wang, H.; Han, T. Comparison of soybean yield and yield-related traits of agri-technology demonstration counties in different regions of China. Soybean Sci. 2012, 31, 557–567. [Google Scholar]
- Song, W.; Sun, S.; Ibrahim, S.E.; Xu, Z.; Wu, H.; Hu, X.; Jia, H.; Cheng, Y.; Yang, Z.; Jiang, S.; et al. Standard Cultivar Selection and Digital Quantification for Precise Classification of Maturity Groups in Soybean. Crop Sci. 2019, 59, 1997–2006. [Google Scholar] [CrossRef]
- Nelson, R.L.; Amdor, P.J.; Orf, J.H.; Lambert, J.W.; Cavins, J.F.; Kleiman, R.; Laviolette, F.A.; Athow, K.L. Evaluation of the USDA Soybean Germplasm Collection: Maturity Groups 000 to IV; Technical Bulletins 157020; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 1987; 267p. [Google Scholar]
- Sun, J.; Sun, B.L.; Han, F.X.; Yan, S.R.; Yang, H.; Akio, K. Rapid HPLC method for determination of 12 isoflavone components in soybean seeds. Agric. Sci. China 2011, 10, 70–77. [Google Scholar] [CrossRef]




| Isoflavones | Range | Minimum | Maximum | Mean | Std. | CV |
|---|---|---|---|---|---|---|
| (μg g−1) | (μg g−1) | (μg g−1) | Deviation | (%) | ||
| Daidzin | 373.36 | 21.57 | 394.94 | 131.09 | 56.00 | 42.72 |
| Glycitin | 190.45 | 15.37 | 205.83 | 67.07 | 28.05 | 41.82 |
| Genistin | 468.00 | 41.48 | 509.49 | 221.91 | 78.69 | 35.46 |
| Malonyldaidzin | 2098.51 | 179.07 | 2277.58 | 771.49 | 326.18 | 42.28 |
| Malonylglycitin | 528.61 | 36.71 | 565.32 | 149.70 | 65.88 | 44.01 |
| Malonylgenistin | 2428.54 | 364.72 | 2793.27 | 1296.36 | 410.69 | 31.68 |
| Daidzein | 29.30 | 4.05 | 33.36 | 10.40 | 3.94 | 37.88 |
| Genistein | 26.11 | 4.38 | 30.49 | 12.26 | 3.98 | 32.46 |
| Total Isoflavone | 5146.04 | 677.25 | 5823.29 | 2670.70 | 857.32 | 32.10 |
| ID | TIF | CV (%) | Coat Color | Country | MG |
|---|---|---|---|---|---|
| WDD01618 | 5823.29 | 5.96 | Yellow | USA | V |
| WDD01632 | 5503.4 | 4.57 | Yellow | USA | VI |
| WDD00698 | 5263.62 | 2.65 | Yellow | USA | V |
| WDD03039 | 5177.78 | 1.76 | Yellow | USA | V |
| ZDD02450 | 5170.84 | 3.05 | Black | China | I |
| Zhonghuang 68 | 4985.98 | 1.99 | Yellow | China | III |
| WDD01619 | 4735.96 | 2.71 | Yellow | USA | V |
| ZDD10734 | 4708.28 | 3.65 | Black | China | VI |
| WDD02107 | 4705.91 | 3.63 | Yellow | USA | I |
| ZDD02864 | 4671.88 | 3.85 | Yellow | China | V |
| WDD03084 | 4649.65 | 2.56 | Yellow | USA | II |
| WDD03006 | 4516.07 | 1.95 | Yellow | USA | III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, M.; Zhang, S.; Qi, J.; Abdelghany, A.M.; Shaibu, A.S.; Feng, Y.; Ghosh, S.; Agyenim-Boateng, K.G.; Liu, Y.; Yao, L.; et al. Effect of Origin, Seed Coat Color, and Maturity Group on Seed Isoflavones in Diverse Soybean Germplasm. Plants 2024, 13, 1774. https://doi.org/10.3390/plants13131774
Azam M, Zhang S, Qi J, Abdelghany AM, Shaibu AS, Feng Y, Ghosh S, Agyenim-Boateng KG, Liu Y, Yao L, et al. Effect of Origin, Seed Coat Color, and Maturity Group on Seed Isoflavones in Diverse Soybean Germplasm. Plants. 2024; 13(13):1774. https://doi.org/10.3390/plants13131774
Chicago/Turabian StyleAzam, Muhammad, Shengrui Zhang, Jie Qi, Ahmed M. Abdelghany, Abdulwahab Saliu Shaibu, Yue Feng, Suprio Ghosh, Kwadwo Gyapong Agyenim-Boateng, Yitian Liu, Luming Yao, and et al. 2024. "Effect of Origin, Seed Coat Color, and Maturity Group on Seed Isoflavones in Diverse Soybean Germplasm" Plants 13, no. 13: 1774. https://doi.org/10.3390/plants13131774
APA StyleAzam, M., Zhang, S., Qi, J., Abdelghany, A. M., Shaibu, A. S., Feng, Y., Ghosh, S., Agyenim-Boateng, K. G., Liu, Y., Yao, L., Li, J., Li, B., Wang, B., & Sun, J. (2024). Effect of Origin, Seed Coat Color, and Maturity Group on Seed Isoflavones in Diverse Soybean Germplasm. Plants, 13(13), 1774. https://doi.org/10.3390/plants13131774











