Foliar Application of Chitosan (CTS), γ-Aminobutyric Acid (GABA), or Sodium Chloride (NaCl) Mitigates Summer Bentgrass Decline in the Subtropical Zone
Abstract
1. Introduction
2. Results
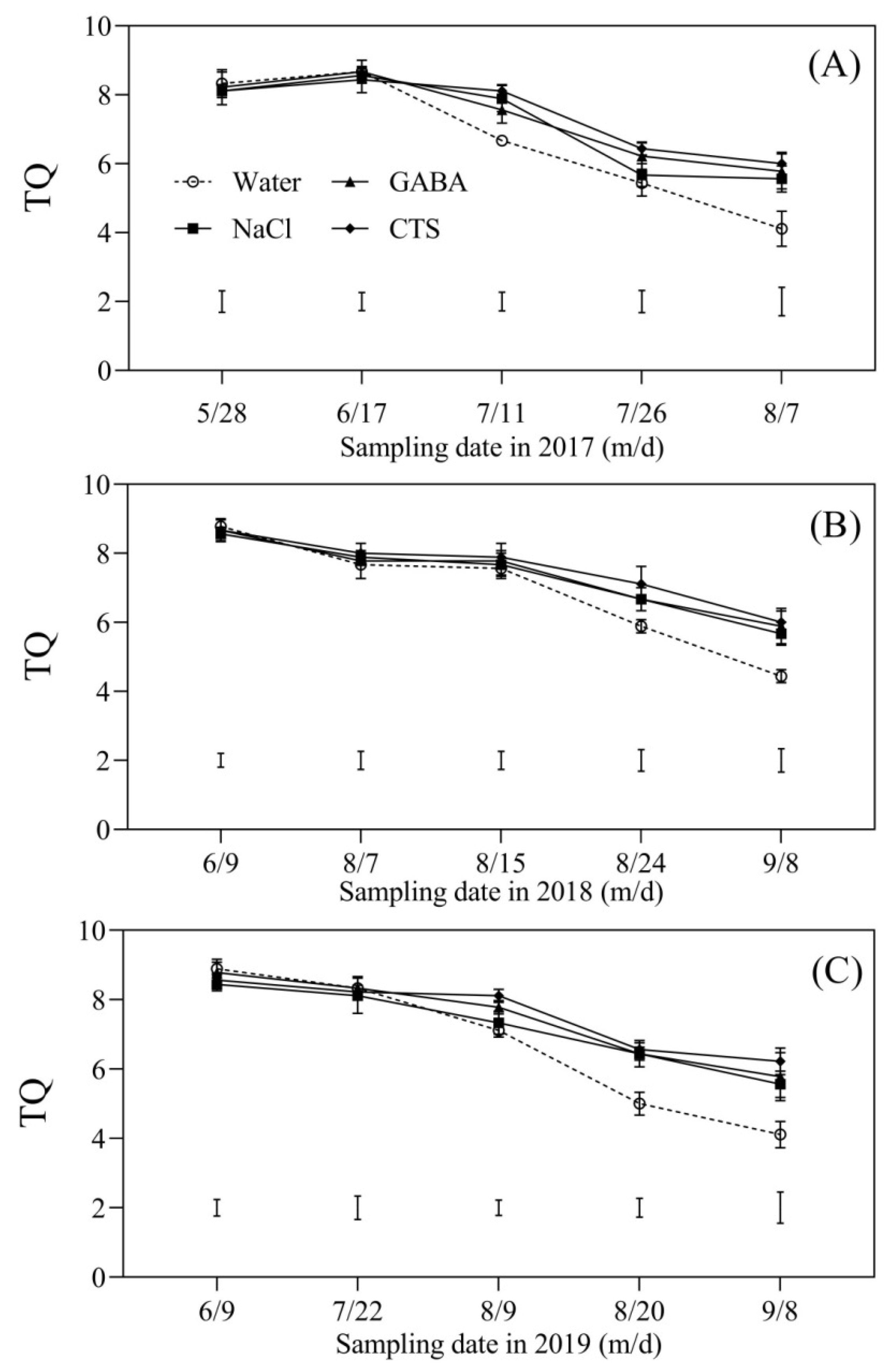
2.1. Turf Quality Affected by the Foliar Application of GABA, NaCl, or CTS during Hot Summer
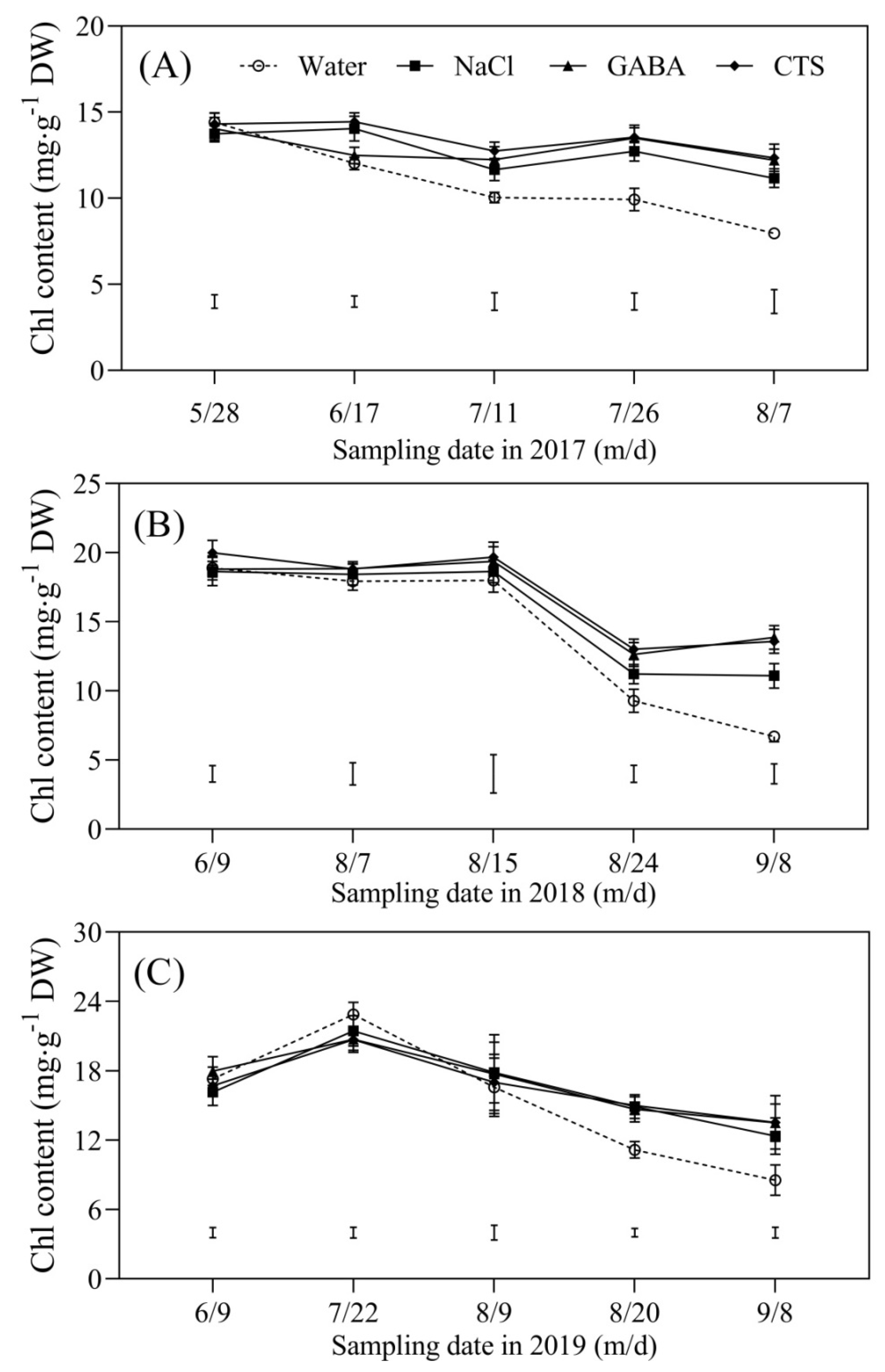
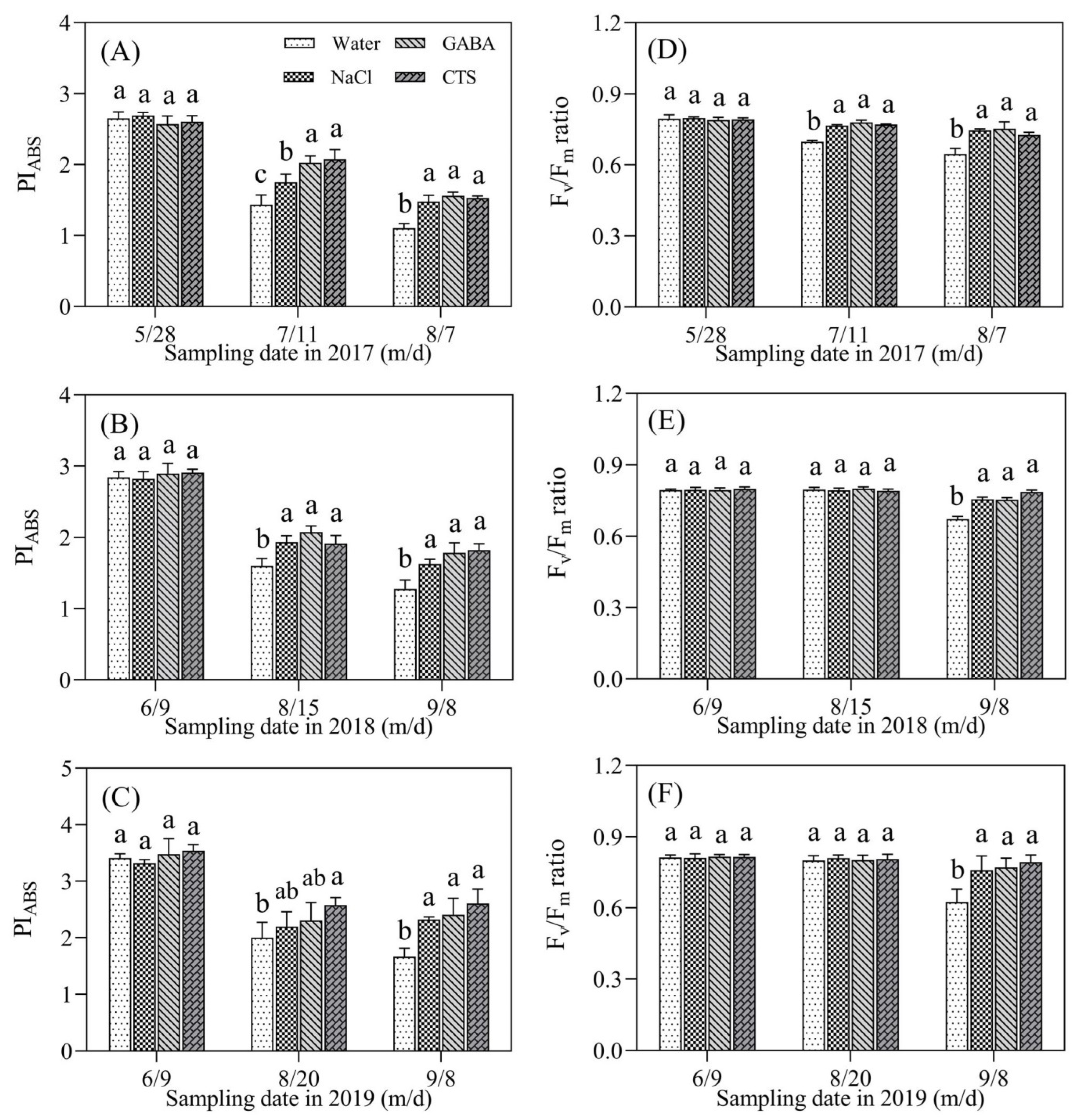
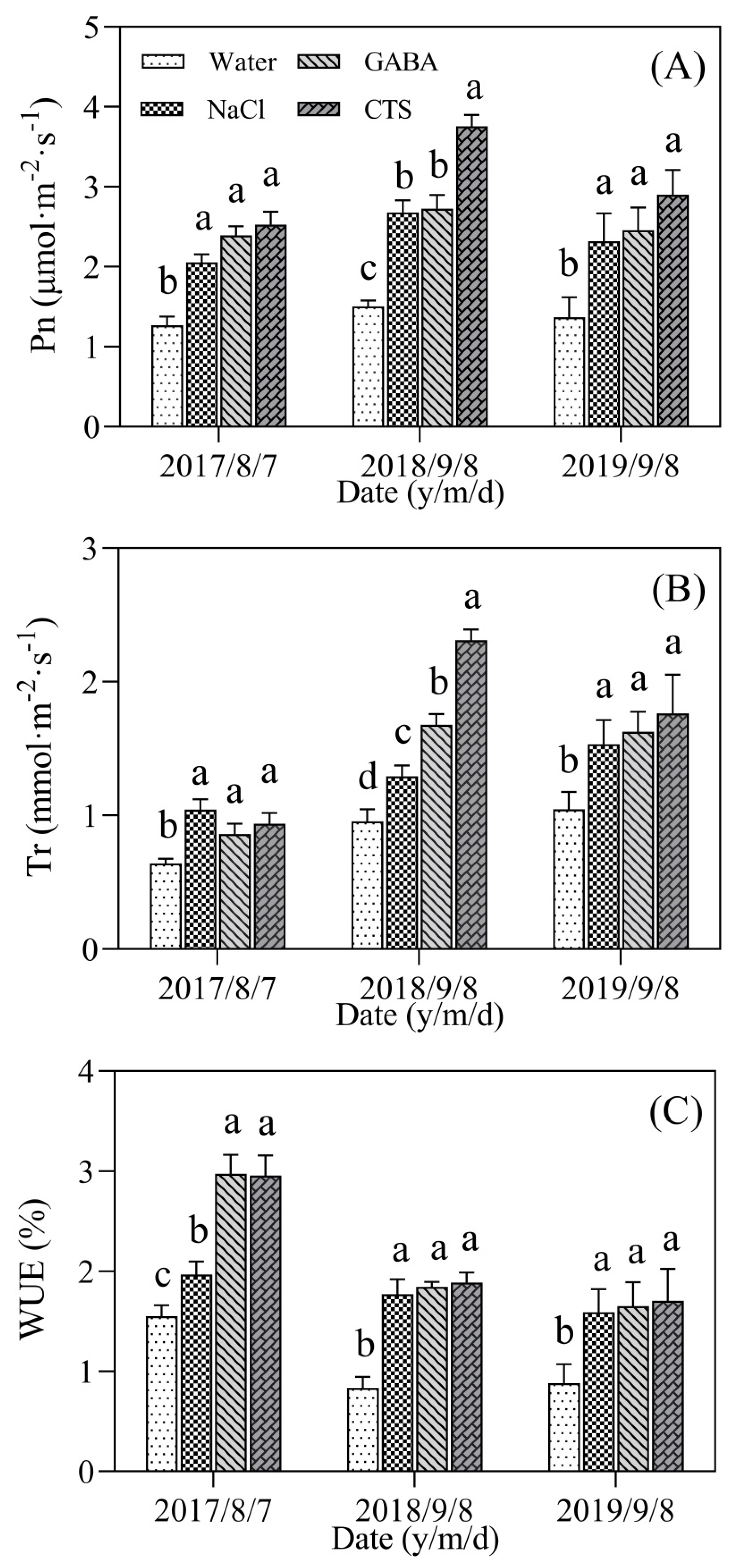
2.2. Chlorophyll Content and Photosynthesis Affected by GABA, NaCl, or CTS during Hot Summer
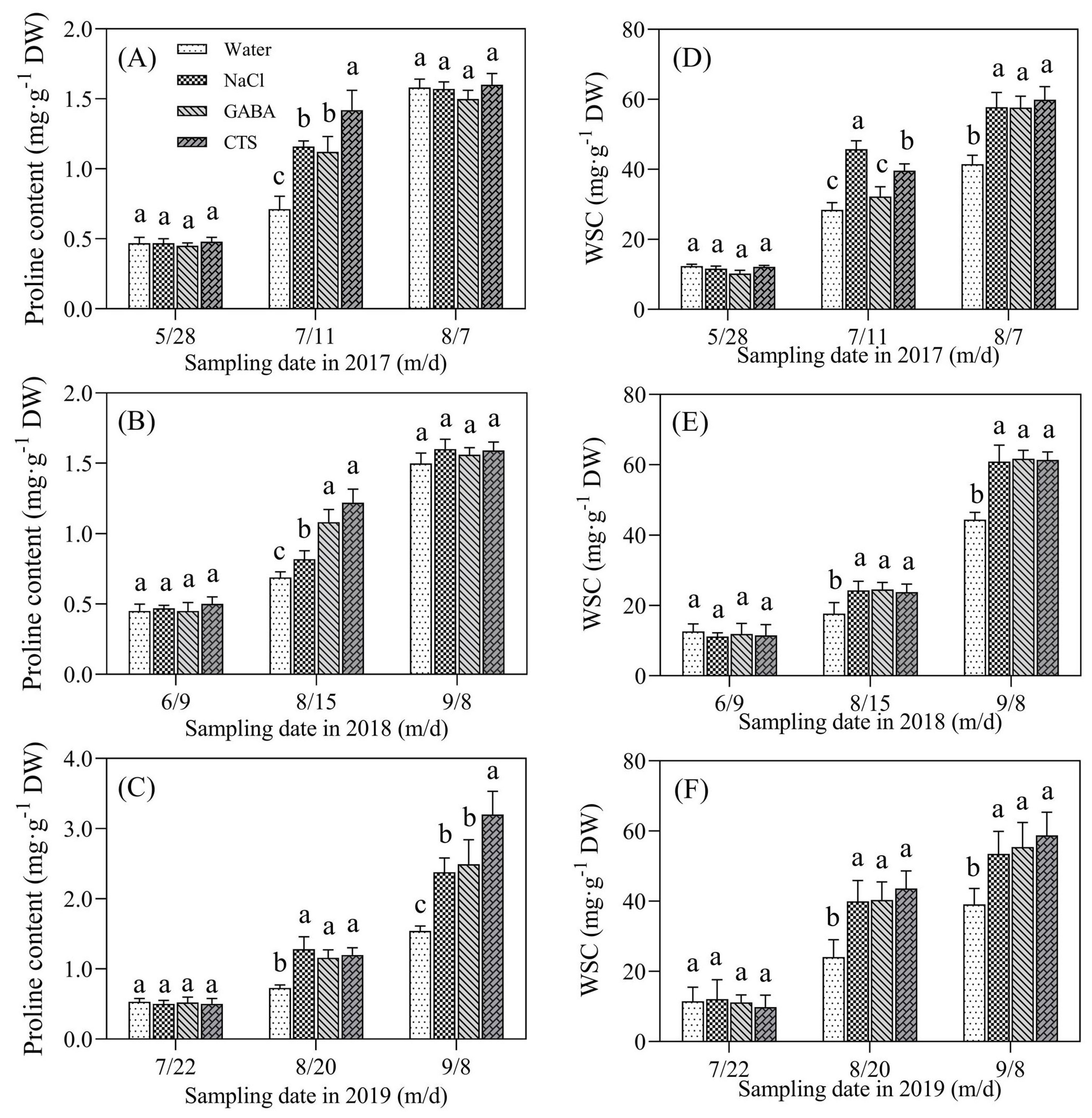
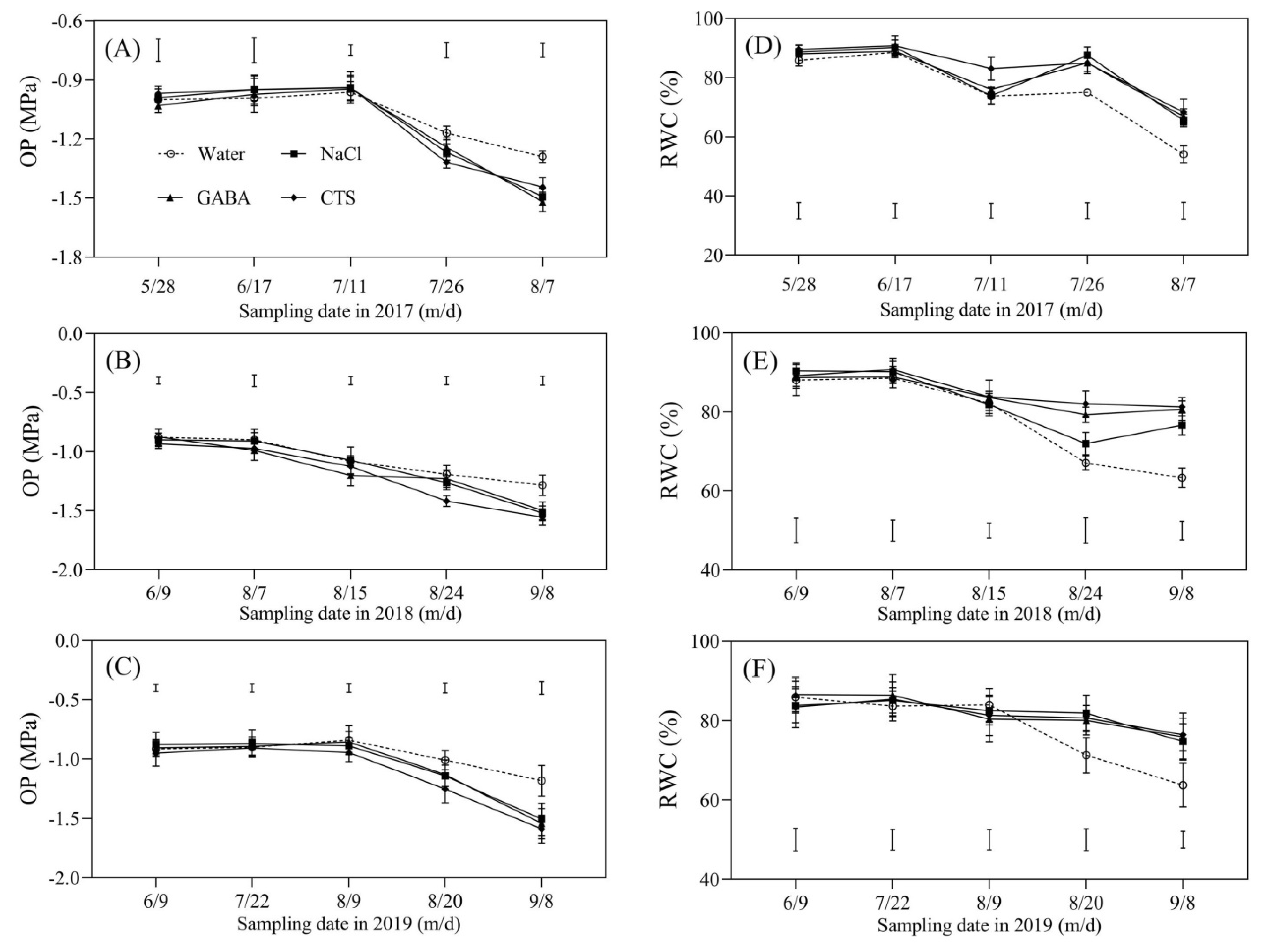
2.3. Water Status and Osmolytes Affected by the Foliar Application of GABA, NaCl, or CTS during Hot Summer
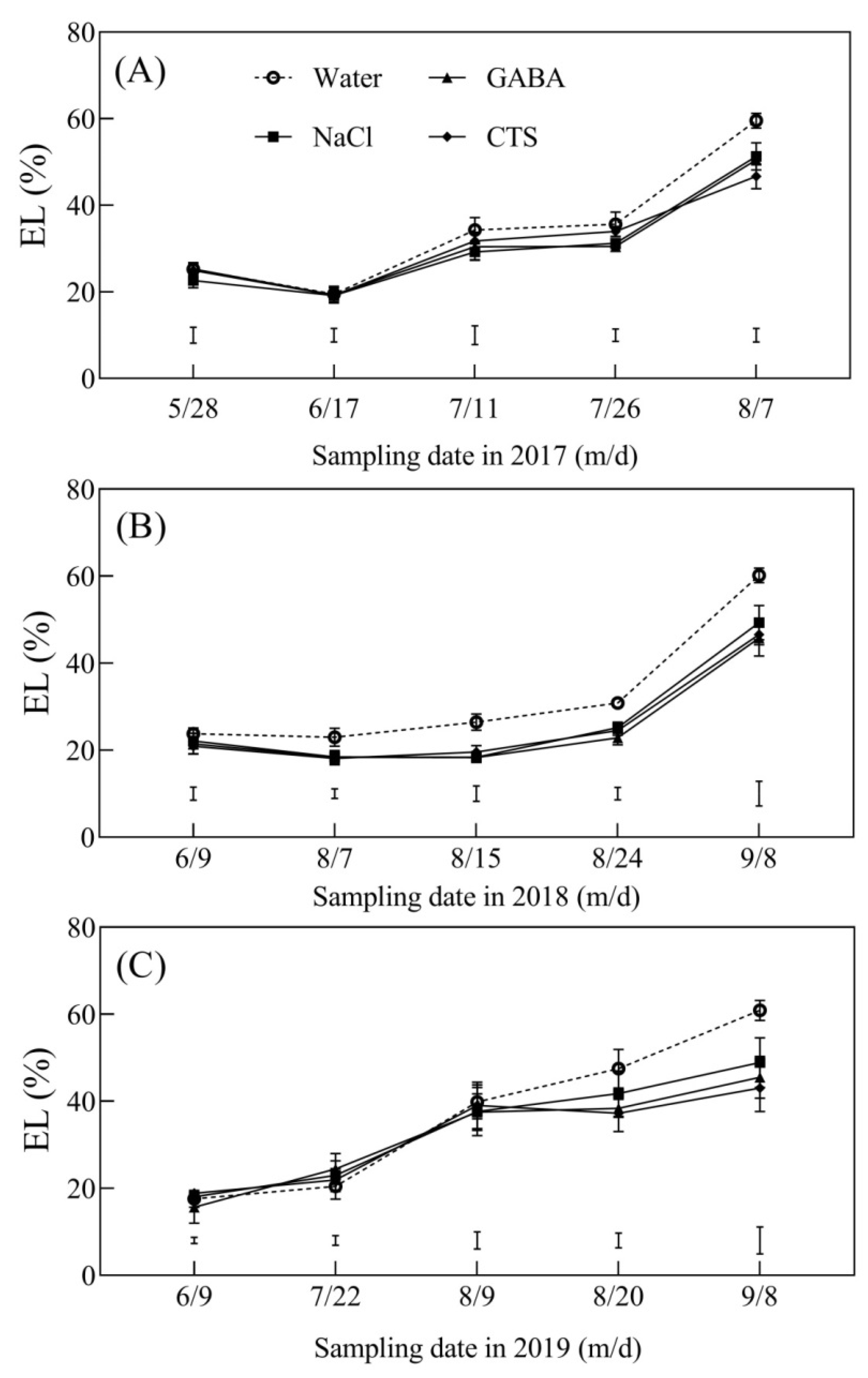
2.4. Oxidative Damage and Cell Membrane Stability Affected by the Foliar Application of GABA, NaCl, or CTS during Hot Summer
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurements of Turf Quality, Chlorophyll Content, and Photosynthetic Characteristics
4.3. Measurements of Water Status and Osmolyte Content
4.4. Measurement of Oxidative Damage and Cell Membrane Stability
4.5. Statistics and Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Zeng, W.; Cheng, B.; Xu, J.; Han, L.; Peng, Y. Turf quality and physiological responses to summer stress in four creeping bentgrass cultivars in a subtropical zone. Plants 2022, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Fry, J.; Huang, B. Applied Turfgrass Science and Physiology; John Wiley Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Chakraborty, N.; Bae, J.; Warnke, S.; Chang, T.; Jung, G. Linkage map construction in allotetraploid creeping bentgrass (Agrostis stolonifera L.). Theor. Appl. Genet. 2005, 111, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tian, Y.; Zhang, B.; Hassan, M.J.; Li, Z.; Zhu, Y. Chitosan (CTS) alleviates heat-induced leaf senescence in creeping bentgrass by regulating chlorophyll metabolism, antioxidant defense, and the heat shock pathway. Molecules 2021, 26, 5337. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ma, Y.; Merewitz, E. Leaf trimming and high temperature regulation of phytohormones and polyamines in creeping bentgrass leaves. J. Am. Soc. Hortic. Sci. 2016, 141, 66–75. [Google Scholar] [CrossRef]
- Miller, G.L.; Brotherton, M.A. Creeping bentgrass summer decline as influenced by climatic conditions and cultural practices. Agron. J. 2020, 112, 3500–3512. [Google Scholar] [CrossRef]
- Fu, J.; Dernoeden, P.H. Creeping bentgrass putting green turf responses to two summer irrigation practices: Rooting and soil temperature. Crop Sci. 2009, 49, 1063–1070. [Google Scholar] [CrossRef]
- Totten, F.W.; Liu, H.; McCarty, L.B.; Baldwin, C.M.; Bielenberg, D.G.; Toler, J.E. Efficiency of foliar versus granular fertilization: A field study of creeping bentgrass performance. J. Plant Nutr. 2008, 31, 972–982. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Qi, H.; Cheng, B.; Hassan, M.J. Foliar application of diethyl aminoethyl hexanoate (DA-6) alleviated summer bentgrass decline and heat damage to creeping bentgrass. Crop Sci. 2024, 64, 1039–1050. [Google Scholar] [CrossRef]
- Li, K.; Xu, E. The role and the mechanism of γ-aminobutyric acid during central nervous system development. Neurosci. Bull. 2008, 24, 195–200. [Google Scholar] [CrossRef]
- Ren, T.; Zheng, P.; Zhang, K.; Liao, J.; Xiong, F.; Shen, Q.; Ma, Y.; Fang, W.; Zhu, X.J. Effects of Effects of gaba on the polyphenol accumulation and antioxidant activities in tea plants (Camellia sinensis L.) under heat-stress conditions. Plant Physiol. Bioch. 2021, 159, 363–371. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Peng, C.; Shi, H.; Yang, J.; He, M.; Zhang, M.; Zhou, Y.; Duan, L. Exogenous gamma-aminobutyric acid coordinates active oxygen and amino acid homeostasis to enhance heat tolerance in wheat seedlings. J. Plant Growth Regul. 2022, 41, 2787–2797. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.H.M.; Nayyar, H. GABA (γ-Aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 2019, 9, 7788. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razik, E.S.; Alharbi, B.M.; Bilal Pirzadah, T.; Alnusairi, G.S.H.; Soliman, M.H.; Hakeem, K.R. γ-Aminobutyric acid (GABA) mitigates drought and heat stress in sunflower (Helianthus annuus L.) by regulating its physiological, biochemical and molecular pathways. Physiol. Plant. 2020, 172, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Huang, B. Alteration of transcripts of stress-protective genes and transcriptional factors by γ-aminobutyric acid (GABA) associated with improved heat and drought tolerance in creeping bentgrass (Agrostis stolonifera). Int. J. Mol. Sci. 2018, 19, 1623. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Z.; Li, Z.; Peng, Y.; Zhang, X.; Ma, X.; Huang, L.; Liu, W.; Nie, G.; He, L.W. Regulation of heat shock factor pathways by γ-aminobutyric acid (GABA) associated with thermotolerance of creeping bentgrass. Int. J. Mol. Sci. 2019, 20, 4713. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, L.; Zhou, M.; Yang, Z.; Meng, Y.; Zhang, X. Comparative physiological and transcriptomic analyses reveal mechanisms of improved osmotic stress tolerance in annual ryegrass by exogenous chitosan. Genes 2019, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Liang, L.; Li, Z.; Hassan, M.J.; Peng, Y.; Wang, D. Enhanced photosynthesis, carbohydrates, and energy metabolism associated with chitosan-induced drought tolerance in creeping bentgrass. Crop Sci. 2020, 60, 1064–1076. [Google Scholar] [CrossRef]
- Geng, W.; Li, Z.; Hassan, M.J.; Peng, Y. Chitosan regulates metabolic balance, polyamine accumulation, and Na+ transport contributing to salt tolerance in creeping bentgrass. BMC Plant Biol. 2020, 20, 506. [Google Scholar] [CrossRef]
- Li, Q.; Bian, Y.; Li, R.; Yang, Z.; Liu, N.; Yu, J. Chitosan-enhanced heat tolerance associated with alterations in antioxidant defense system and gene expression in creeping bentgrass. Grass Res. 2023, 3, 7. [Google Scholar] [CrossRef]
- Li, Q.; Li, R.; He, F.; Yang, Z.; Yu, J. Growth and physiological effects of chitosan on heat tolerance in creeping bentgrass (Agrostis stolonifera). Grass Res. 2022, 2, 41–47. [Google Scholar] [CrossRef]
- Geilfus, C. Chloride in soil: From nutrient to soil pollutant. Environ. Exp. Bot. 2019, 157, 299–309. [Google Scholar] [CrossRef]
- Sakadevan, K.; Nguyen, M.L. Rational utilization of salt affected soils and saline waters for crop production and the protection of soil and water in agricultural catchments. Cheminform 2011, 23, 180–192. [Google Scholar]
- Umezawa, T.; Shimizu, K.; Kato, M.; Ueda, T. Enhancement of salt tolerance in soybean with NaCl pretreatment. Physiol. Plant. 2010, 110, 59–63. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Liang, L.L.; Cheng, B.Z.; Dong, Y.; Wei, J.Q.; Tian, X.L.; Peng, Y.; Li, Z. Pretreatment with nacl promotes the seed germination of white clover by affecting endogenous phytohormones, metabolic regulation, and dehydrin-encoded genes expression under water stress. Int. J. Mol. Sci. 2018, 19, 3570. [Google Scholar] [CrossRef]
- Li, Z.; Peng, D.; Zhang, X.; Peng, Y.; Chen, M.; Ma, X.; Huang, L.; Yan, Y. Na+ induces the tolerance to water stress in white clover associated with osmotic adjustment and aquaporins-mediated water transport and balance in root and leaf. Environ. Exp. Bot. 2017, 144, 11–24. [Google Scholar] [CrossRef]
- Wijewardene, I.; Mishra, N.; Sun, L.; Smith, J.; Zhu, X.; Payton, P.; Shen, G.; Zhang, H. Improving drought-, salinity-, and heat-tolerance in transgenic plants by co-overexpressing Arabidopsis vacuolar pyrophosphatase gene AVP1 and Larrea Rubisco activase gene RCA. Plant Sci. 2020, 296, 110499. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, B. Responses of creeping bentgrass to trinexapac-ethyl and biostimulants under summer stress. Hortsci. A Publ. Am. Soc. Hortic. Sci. 2010, 45, 125–131. [Google Scholar] [CrossRef]
- Li, Z.; Burgess, P.; Peng, Y.; Huang, B. Regulation of nutrient accumulation by γ-aminobutyric acid associated with GABA priming-enhanced heat tolerance in creeping bentgrass. Grass Res. 2022, 2, 5. [Google Scholar] [CrossRef]
- Huang, B.; Liu, X.; Fry, J.D. Effects of high temperature and poor soil aeration on root growth and viability of creeping bentgrass. Crop Sci. 1998, 38, 1618–1622. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Ervin, E. Effects of nitrate and cytokinin on creeping bentgrass under supraoptimal temperatures. J. Plant Nutr. 2013, 36, 1549–1564. [Google Scholar] [CrossRef]
- Rossi, S.; Chapman, C.; Huang, B. Suppression of heat-induced leaf senescence by γ-aminobutyric acid, proline, and ammonium nitrate through regulation of chlorophyll degradation in creeping bentgrass. Environ. Exp. Bot. 2020, 177, 104116. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H. Differential accumulation of proteins in leaves and roots associated with heat tolerance in two Kentucky bluegrass genotypes differing in heat tolerance. Acta Physiol. Plant 2016, 38, 213. [Google Scholar] [CrossRef]
- Cheng, B.; Zhou, M.; Tang, T.; Hassan, M.J.; Zhou, J.; Tan, M.; Li, Z.; Peng, Y. A Trifolium repens flavodoxin-like quinone reductase 1 (TrFQR1) improves plant adaptability to high temperature associated with oxidative homeostasis and lipids remodeling. Plant J. 2023, 115, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Hassan, M.J.; Kang, D.; Peng, Y.; Li, Z. Photosynthetic maintenance and heat shock protein accumulation relating to γ-aminobutyric acid (GABA)-regulated heat tolerance in creeping bentgrass (Agrostis stolonifera). S. Afr. J. Bot. 2021, 141, 405–413. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Osmotic adjustment and root growth associated with drought preconditioning-enhanced heat tolerance in Kentucky bluegrass. Crop Sci. 2001, 41, 1168–1173. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Mol. Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-um, S.; Takabe, T.; Kirdmanee, C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Signorelli, S.; Coitino, E.L.; Borsani, O.; Monza, J. Molecular mechanisms for the reaction between (•)OH radicals and proline: Insights on the role as ros scavenger in plant stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Huang, B. Physiological effects of γ-aminobutyric acid application on improving heat and drought tolerance in creeping bentgrass. J. Am. Soc. Hortic. Sci. 2016, 141, 76–84. [Google Scholar] [CrossRef]
- Beard, J.B. Turf Management for Golf Courses, 2nd ed.; Ann Arbor Press: Chelsea, MI, USA, 2002. [Google Scholar]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 1989, 29, 230–233. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Pamela, P.D.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 152, 59–66. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat1. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Li, Z.; Tang, M.; Hassan, M.; Zhang, Y.; Han, L.; Peng, Y. Adaptability to high temperature and stay-green genotypes associated with variations in antioxidant, chlorophyll metabolism, and γ-aminobutyric acid accumulation in creeping bentgrass species. Front. Plant Sci. 2021, 12, 2341. [Google Scholar] [CrossRef] [PubMed]

| Treatment | 2017 | 2018 | 2019 | |||
|---|---|---|---|---|---|---|
| SFV | Order | SFV | Order | SFV | Order | |
| Water | 0.32 | 4 | 0.27 | 4 | 0.25 | 4 |
| NaCl | 0.56 | 3 | 0.52 | 3 | 0.57 | 3 |
| GABA | 0.60 | 2 | 0.56 | 2 | 0.58 | 2 |
| CTS | 0.62 | 1 | 0.62 | 1 | 0.70 | 1 |
| Name | Source | Purity | Price | Cost |
|---|---|---|---|---|
| (USD·g−1) | (USD·m−2·time−1) | |||
| GABA | Sigma-Aldrich Trading Co., Ltd. (Shanghai, China) | 99% | 1.9822 | 0.0256 |
| CTS | Weifang Dongxing Chitosan Factory (Weifang, China) | 90% | 0.0445 | 0.0050 |
| NaCl | Chengdu Kelong Chemical Co., Ltd. (Chengdu, China) | 99% | 0.0018 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Zhou, Q.; Li, L.; Hassan, M.J.; Zeng, W.; Peng, Y.; Li, Z. Foliar Application of Chitosan (CTS), γ-Aminobutyric Acid (GABA), or Sodium Chloride (NaCl) Mitigates Summer Bentgrass Decline in the Subtropical Zone. Plants 2024, 13, 1773. https://doi.org/10.3390/plants13131773
Cheng B, Zhou Q, Li L, Hassan MJ, Zeng W, Peng Y, Li Z. Foliar Application of Chitosan (CTS), γ-Aminobutyric Acid (GABA), or Sodium Chloride (NaCl) Mitigates Summer Bentgrass Decline in the Subtropical Zone. Plants. 2024; 13(13):1773. https://doi.org/10.3390/plants13131773
Chicago/Turabian StyleCheng, Bizhen, Qinyu Zhou, Linju Li, Muhammad Jawad Hassan, Weihang Zeng, Yan Peng, and Zhou Li. 2024. "Foliar Application of Chitosan (CTS), γ-Aminobutyric Acid (GABA), or Sodium Chloride (NaCl) Mitigates Summer Bentgrass Decline in the Subtropical Zone" Plants 13, no. 13: 1773. https://doi.org/10.3390/plants13131773
APA StyleCheng, B., Zhou, Q., Li, L., Hassan, M. J., Zeng, W., Peng, Y., & Li, Z. (2024). Foliar Application of Chitosan (CTS), γ-Aminobutyric Acid (GABA), or Sodium Chloride (NaCl) Mitigates Summer Bentgrass Decline in the Subtropical Zone. Plants, 13(13), 1773. https://doi.org/10.3390/plants13131773






