Enhancement of Plumbagin Production through Elicitation in In Vitro-Regenerated Shoots of Plumbago indica L.
Abstract
1. Introduction
2. Results
2.1. Yeast Extract and Salicylic Acid Elicitation
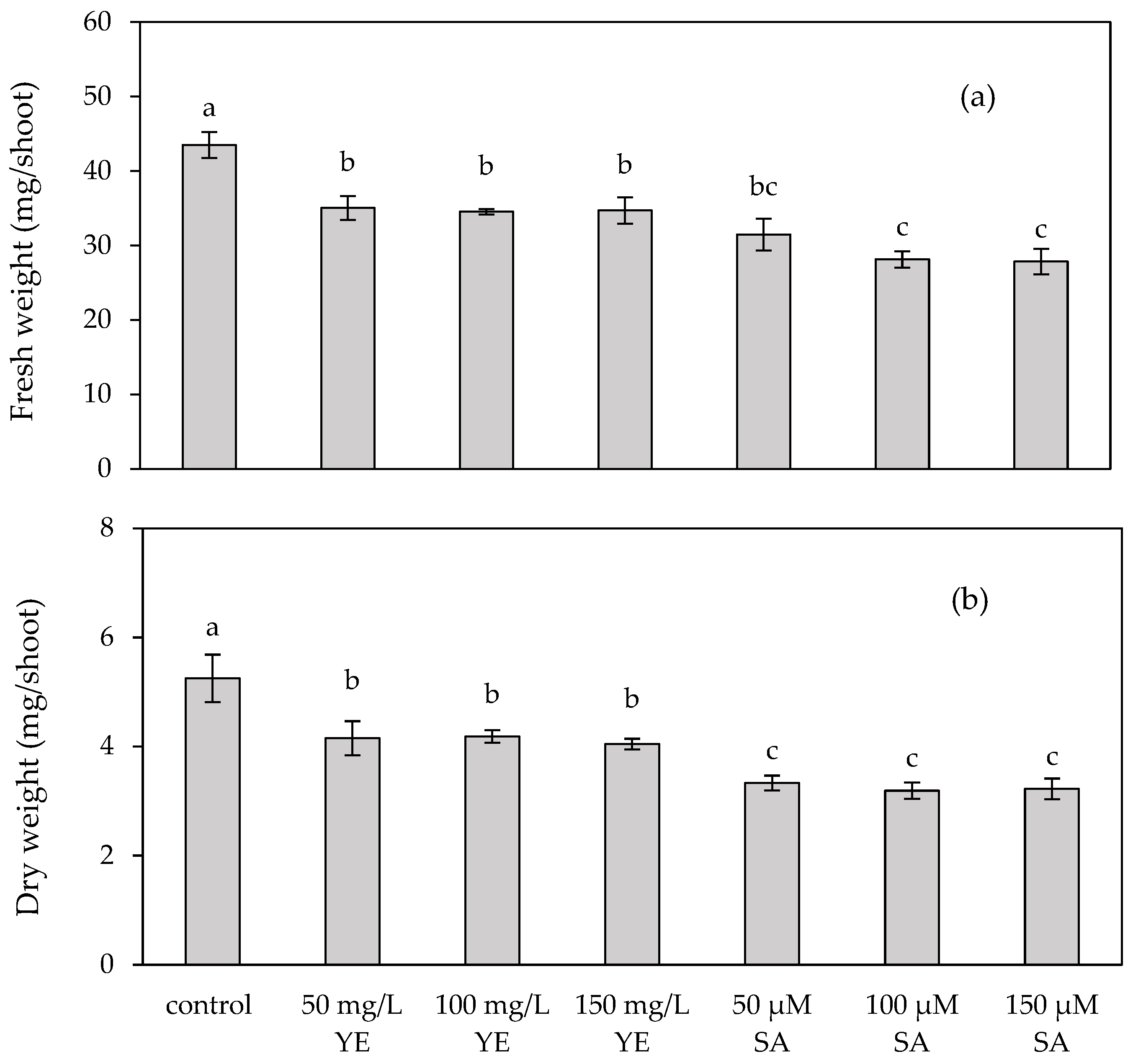
2.1.1. Fresh and Dry Weights of Regenerated Shoots
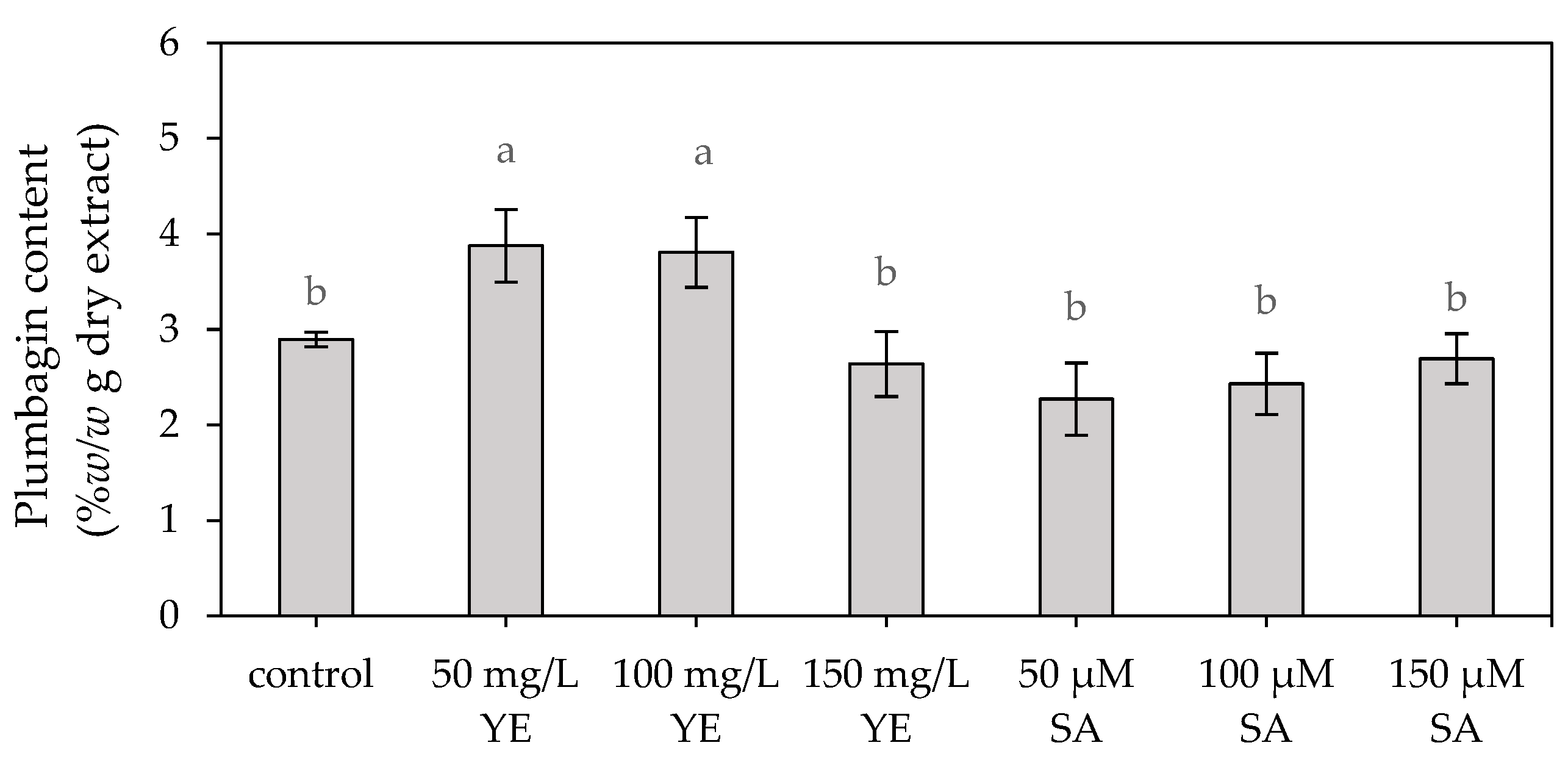
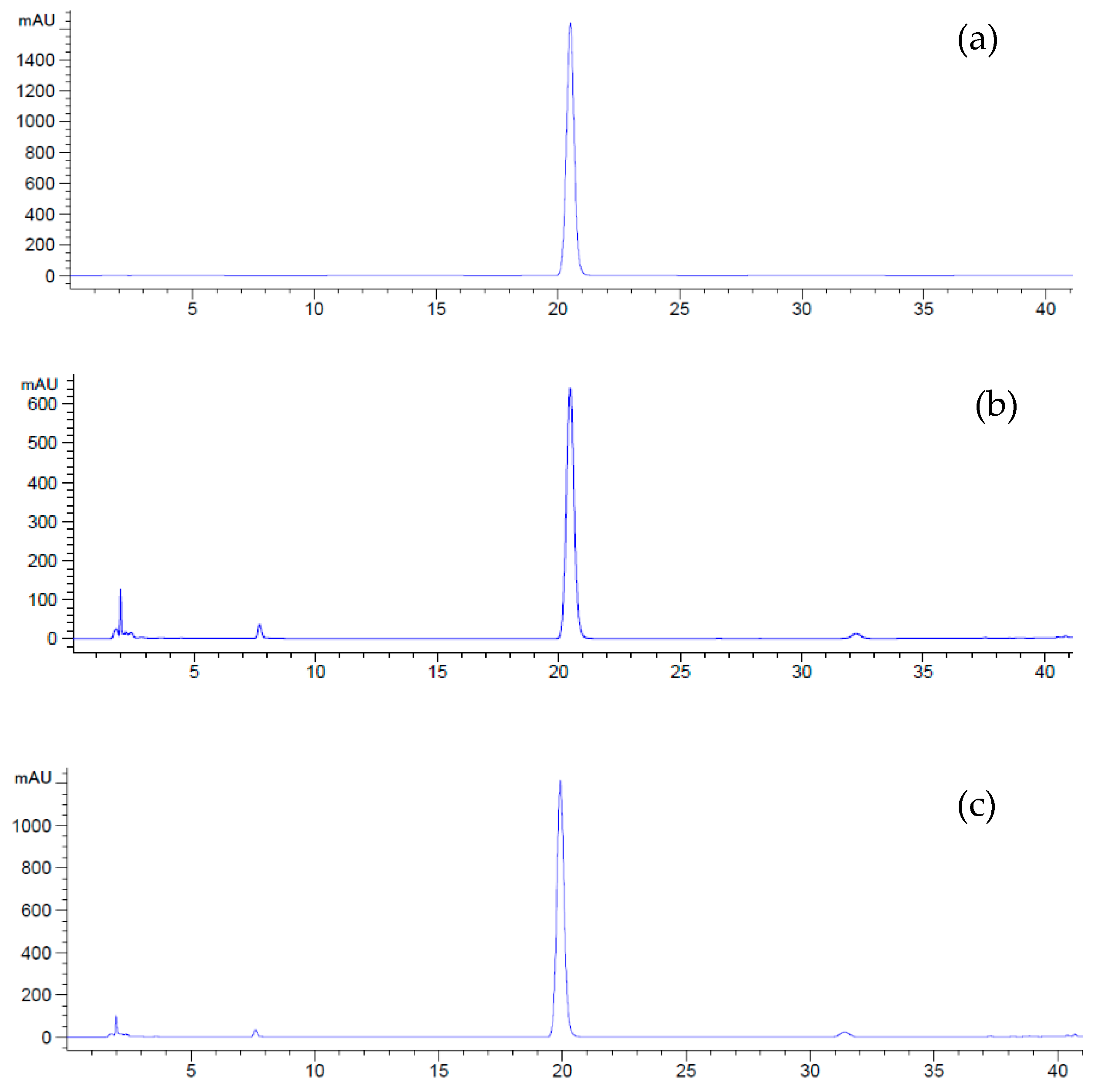
2.1.2. Plumbagin Content
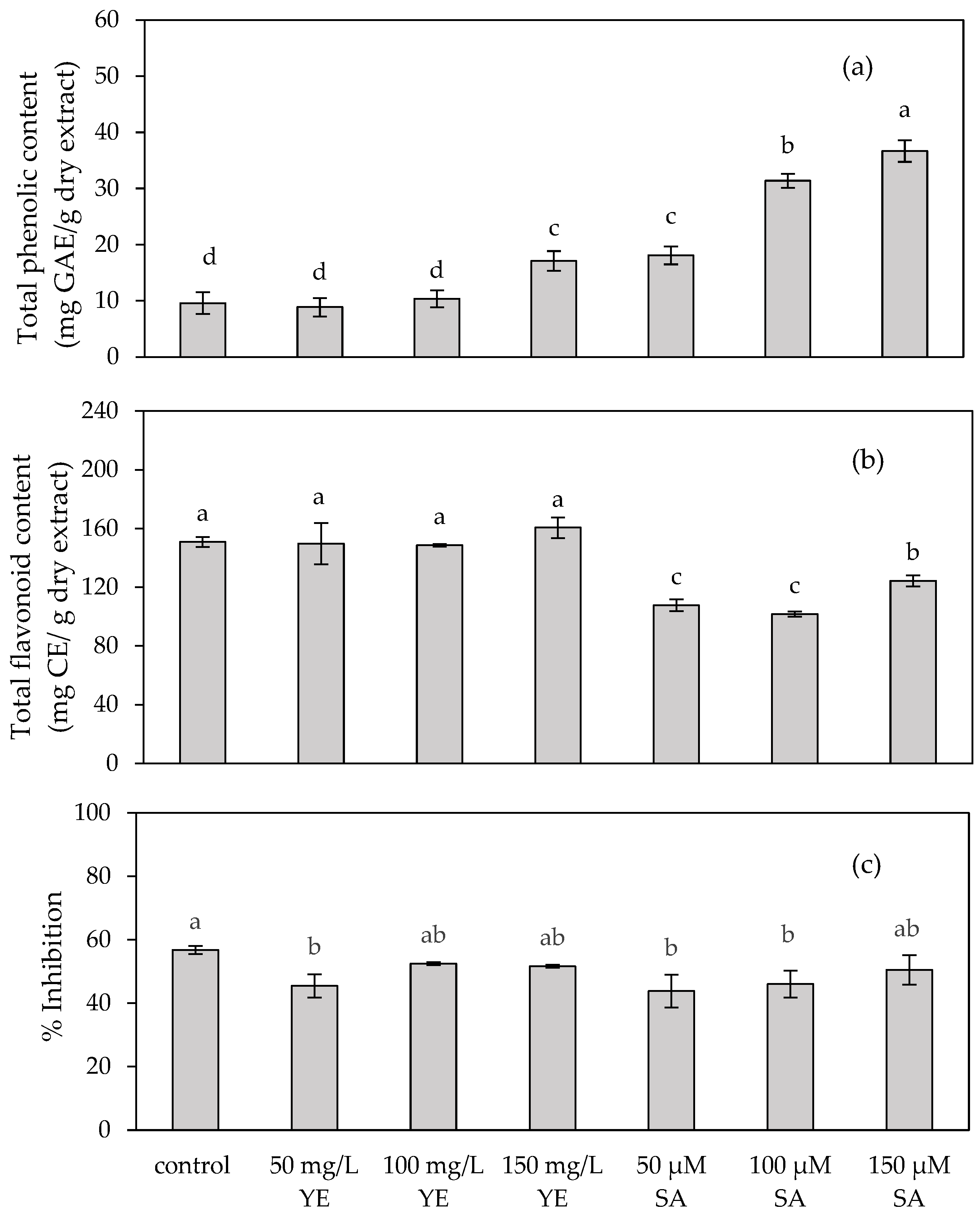
2.1.3. Total Phenolic and Flavonoid Contents, and Antioxidant Activity
2.2. Yeast Extract Elicitation Period
2.2.1. Fresh and Dry Weights of Regenerated Shoots
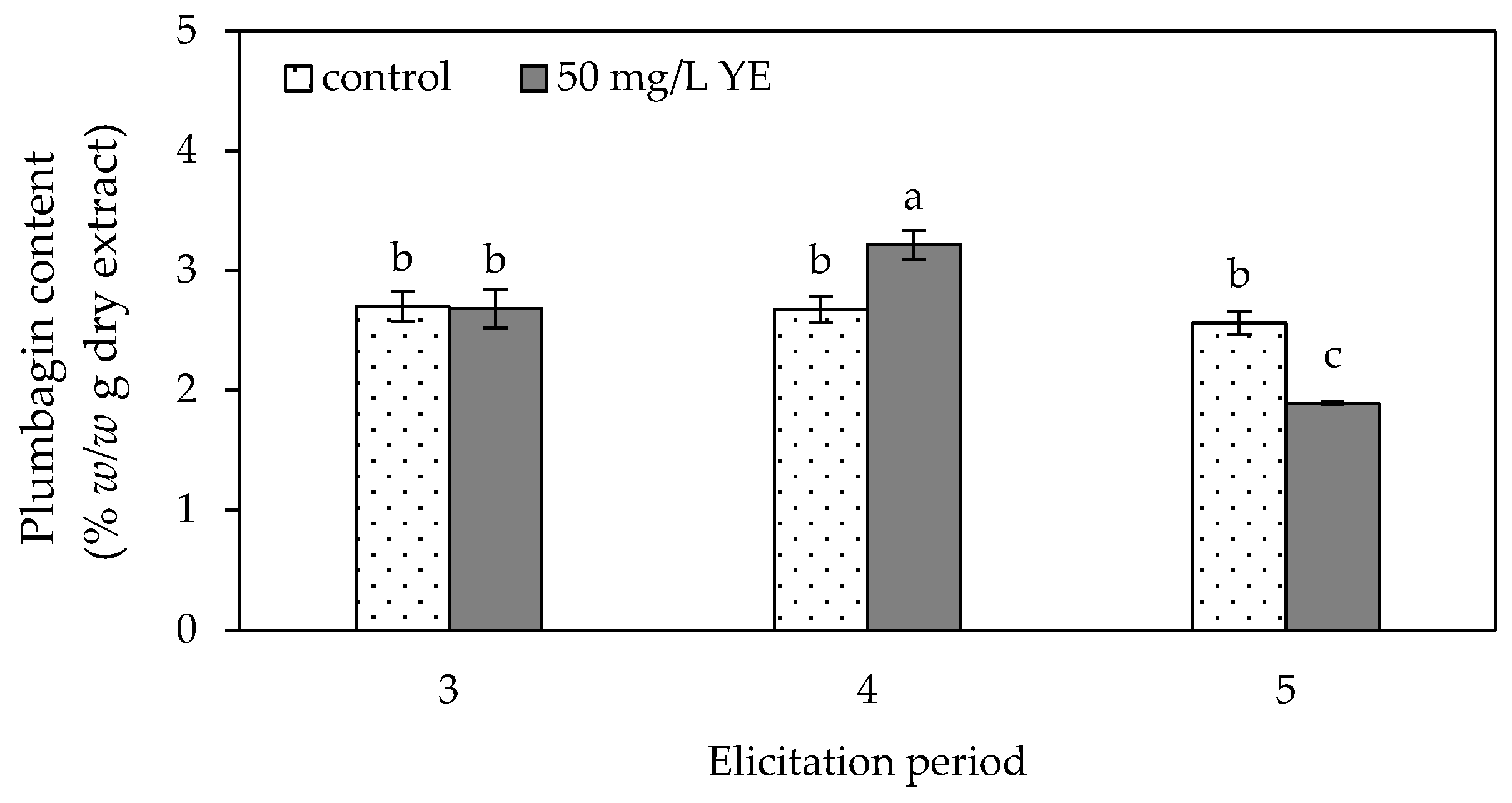
2.2.2. Plumbagin Content
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Elicitor Preparation
4.3. Elicitation with Yeast Extract and Salicylic Acid
4.4. Yeast Extract Elicitation Period
4.5. Bioactive Compound Analysis
4.5.1. Plant Extract Preparation
4.5.2. Preparation of Standard for Determination of Plumbagin Content
4.5.3. Determination of Plumbagin Content by Reversed Phase–High Performance Liquid Chromatography (RP–HPLC)
4.5.4. Determination of Total Phenolic and Flavonoid Contents, and Antioxidant Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priyanjani, H.A.S.A.; Senarath, R.M.U.S.; Senarath, W.T.P.S.K.; Munasinghe, M.L.A.M.S. Propagation, phytochemistry and pharmacology of Plumbago indica—A review. J. Pharm. Res. Int. 2021, 33, 188–202. [Google Scholar] [CrossRef]
- Jose, B.; Dhanya, B.P.; Silja, P.K.; Krishnan, P.N.; Satheeshkumar, K. Plumbago rosea L. A review on tissue culture and pharmacological research. Int. J. Pharm. Sci. Rev. Res. 2014, 25, 246–256. [Google Scholar]
- Babu, K.; Austin, A. Macro and micro-morphological studies on roots of Plumbago indica L. and Plumbago zeylanica L. J. Pharmacogn. Phytochem. 2016, 5, 351–353. [Google Scholar]
- Itharat, A.; Rattarom, R.; Hansakul, P.; Sakpakdeejaroen, I.; Ooraikul, B.; Davies, N.M. The effects of Benjakul extract and its isolated compounds on cell cycle arrest and apoptosis in human non-small cell lung cancer cell line NCI-H226. Res. Pharm. Sci. 2021, 16, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Makchuchit, S.; Rattarom, R.; Itharat, A. The anti-allergic and anti-inflammatory effects of Benjakul extract (a Thai traditional medicine), its constituent plants and its some pure constituents using in vitro experiments. Biomed. Pharmacother. 2017, 89, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.; Sahu, G.; Muralidhar, J. Screening of Plumbago species for the bio-active marker plumbagin. Pharm. Biol. 2002, 40, 508–511. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Tewtrakul, S. Plumbagin production by root cultures of Plumbago rosea. Electron. Biotechnol. 2002, 5, 11–12. [Google Scholar] [CrossRef]
- Kumar, K.S.; Bhavanandan, K.V. Micropropagation of Plumbago rosea Linn. Plant Cell Tissue Organ Cult. 1988, 15, 275–278. [Google Scholar] [CrossRef]
- Satheeshkumar, K.; Seeni, S. Production of plumbagin (5-hydroxy 2-methyl 1:4 naphthoquinone) in callus and cell suspension cultures of Plumbago indica Linn. Indian J. Biotechnol. 2002, 1, 305–308. [Google Scholar]
- Komaraiah, P.; Jogeswar, G.; Ramakrishna, S.V.; Kishor, P.B.K. Acetylsalicylic acid and ammonium induced somatic embryogenesis and enhanced plumbagin production in suspension cultures of Plumbago rosea L. In Vitro Cell. Dev. Biol. Plant 2004, 40, 230–234. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Sircar, D.; Mitra, A.; Bhattacharya, S. Hairy root culture of Plumbago indica as a potential source for plumbagin. Biol. Plant. 2008, 52, 533–537. [Google Scholar] [CrossRef]
- Vasconsuelo, A.A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plant. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Guru, A.; Dwivedi, P.; Kaur, P.; Pandey, D.K. Exploring the role of elicitors in enhancing medicinal values of plants under in vitro condition. S. Afr. J. Bot. 2022, 149, 1029–1043. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [PubMed]
- Komaraiah, P.; Amrutha, R.N.; Kishor, P.B.K.; Ramakrishna, S.V. Elicitor enhanced production of plumbagin in suspension cultures of Plumbago rosea L. Enzym. Microb. Technol. 2002, 31, 634–639. [Google Scholar] [CrossRef]
- Komaraiah, P.; Ramakrishna, S.V.; Reddanna, P.; Kishor, P.B.K. Enhanced production of plumbagin in immobilized cells of Plumbago rosea by elicitation and in situ adsorption. J. Biotechnol. 2003, 101, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Silja, P.K.; Gisha, G.P.; Satheeshkumar, K. Enhanced plumbagin accumulation in embryogenic cell suspension cultures of Plumbago rosea L. following elicitation. Plant Cell Tissue Organ Cult. 2014, 119, 469–477. [Google Scholar] [CrossRef]
- Silja, P.K.; Satheeshkumar, K. Establishment of adventitious root cultures from leaf explants of Plumbago rosea and enhanced plumbagin production through elicitation. Ind. Crops Prod. 2015, 76, 479–486. [Google Scholar] [CrossRef]
- Jaisi, A.; Panichayupakaranant, P. Enhanced plumbagin production in Plumbago indica root culture by simultaneous and sequential dual elicitations using chitosan with L-alanine and methyl-β-cyclodextrin. Bioresour. Bioprocess. 2020, 7, 10. [Google Scholar] [CrossRef]
- Jose, B.; Silja, P.K.; Pillai, D.B.; Satheeshkumar, K. In vitro cultivation of hairy roots of Plumbago rosea L. in a customized reaction kettle for the production of plumbagin-An anticancer compound. Ind. Crops Prod. 2016, 87, 89–95. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Dewanjee, S.; Bhattacharya, S. Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J. Biosci. Bioeng. 2011, 111, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Agrawal, V. In vitro shoot regeneration and enhanced synthesis of plumbagin in root callus of Plumbago zeylanica L.–an important medicinal herb. In Vitro Cell. Dev. Biol. Plant 2018, 54, 423–435. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Establishment of root suspension culture of Plumbago zeylanica and enhanced production of plumbagin. Ind. Crops Prod. 2019, 137, 419–427. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, U.; Agrawal, V. Isolation and optimization of plumbagin production in root callus of Plumbago zeylanica L. augmented with chitosan and yeast extract. Ind. Crops Prod. 2020, 151, 112446. [Google Scholar] [CrossRef]
- Katoch, K.; Gupta, S.; Gupta, A.P.; Goyal, P.; Devi, R.; Dey, A.; Pandey, D.K. Biotic elicitation for enhanced production of plumbagin in regenerated shoot cultures of Plumbago zeylanica using response surface methodology. Plant Cell Tissue Organ Cult. 2022, 151, 605–615. [Google Scholar] [CrossRef]
- Rahimi, S.; Devi, B.S.R.; Khorolragchaa, A.; Kim, Y.J.; Kim, J.H.; Jung, S.K.; Yang, D.C. Effect of salicylic acid and yeast extract on the accumulation of jasmonic acid and sesquiterpenoids in Panax ginseng adventitious roots. Russ. J. Plant Physiol. 2014, 61, 811–817. [Google Scholar] [CrossRef]
- Karalija, E.; Zeljković, S.Ć.; Parić, A. Harvest time–related changes in biomass, phenolics and antioxidant potential in Knautia sarajevensis shoot cultures after elicitation with salicylic acid and yeast. In Vitro Cell. Dev. Biol. Plant 2019, 56, 177–183. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pérez-Santín, E.; Coelho, N.; Romano, A. Elicitation improves rosmarinic acid content and antioxidant activity in Thymus lotocephalus shoot cultures. Ind. Crops Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Rithichai, P.; Songsoem, K.; Itharat, A. Elicitation of salicylic acid on secondary metabolite production and antioxidant activity of in vitro Musa acuminata L. cv. ‘Gros Michel’ shoots. Curr. Appl. Sci. Technol. 2021, 21, 569–578. [Google Scholar] [CrossRef]
- Resterucci, C.; Stallaert, V.; Milat, M.L.; Pugin, A.; Ricci, P.; Blein, J.P. Relationship between active oxygen species, lipid peroxidation, necrosis, and phytoalexin production induced by elicitins in Nicotiana. Plant Physiol. 1996, 111, 885–891. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crops Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Cousins, M.M.; Adelberg, J.; Chen, F.; Rieck, J. Secondary metabolism-inducing treatments during in vitro development of turmeric (Curcuma longa L.) rhizomes. J. Herbs Spices Med. Plants 2009, 15, 303–317. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Rithichai, P.; Boonyeun, T.; Ruangnoo, S.; Itharat, A. Improvement of dioscorealide B production by elicitation in shoot cultures of Dioscorea membranacea. Pierre ex Prain Burkill. Physiol. Mol. Biol. Plants 2020, 26, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.; Sarrou, E.; Angeli, A.; Martens, S.; Maloupa, E.; Grigoriadou, K. Developing an in vitro elicitation strategy for specialized secondary metabolites production in adventitious root cultures of Primula veris subsp. veris. Ind. Crops Prod. 2023, 197, 116618. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast extract: Characteristics, production, applications and future perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Abdul, M. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Rev. Bras. De Farmacogn. 2017, 27, 549–556. [Google Scholar] [CrossRef]
- Estrada, K.R.; Limon, H.V.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Piątczak, E.; KuŹma, Ł.; Wysokińska, H. The influence of methyl jasmonate and salicylic acid on secondary metabolite production in Rehmannia glutinosa Libosch. hairy root culture. Acta Biol. Cracov. Ser. Bot. 2016, 58, 57–65. [Google Scholar] [CrossRef]
- Pandey, D.K.; Katoch, K.; ·Das, T.; Majumder, M.; Dhama, K.; Mane, A.B.; Gopalakrishnan, A.V.; ·Dey, A. Approaches for in vitro propagation and production of plumbagin in Plumbago spp. Appl. Microbiol. Biotechnol. 2023, 107, 4119–4132. [Google Scholar] [CrossRef]
- Victório, C.P.; Cruz, d.I.P.; Kuster, R.M.; Lge, C.L.S. Terpinen-4-ol is overproduced in tissue cultures of Alpinia zerumbet (Pers.) Burtt et Smith by induction of methyl jasmonate. Lat. Am. J. Pharm. 2011, 30, 1858–1861. [Google Scholar]
- Jirakiattikul, Y.; Rithichai, P.; Prachai, R.; Itharat, A. Elicitation enhancement of bioactive compound accumulation and antioxidant activity in shoot cultures of Boesenbergia rotunda L. Agric. Nat. Resour. 2021, 55, 456–463. [Google Scholar] [CrossRef]
- Diarra, S.T.; He, J.; Wang, J.; Li, J. Ethylene treatment improves diosgenin accumulation in in vitro cultures of Dioscorea zingiberensis via up-regulation of CAS and HMGR gene expression. Electron. J. Biotechnol. 2013, 16, 6. [Google Scholar] [CrossRef]
- Itharat, A.; Sakpakdeejaroen, I. Determination of cytotoxic compounds of thai traditional medicine called benjakul using HPLC. J. Med. Assoc. Thai. 2010, 93, S198–S203. [Google Scholar]
- Kuropakornpong, P.; Itharat, A.; Ooraikul, B.; Loebenberg, R.; Davies, N.M. Development and optimization of Benjakul microemulsion formulations for enhancing topical anti-inflammatory effect and delivery. Res. Pharm. Sci. 2022, 17, 111–122. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirakiattikul, Y.; Ruangnoo, S.; Sangmukdee, K.; Chamchusri, K.; Rithichai, P. Enhancement of Plumbagin Production through Elicitation in In Vitro-Regenerated Shoots of Plumbago indica L. Plants 2024, 13, 1450. https://doi.org/10.3390/plants13111450
Jirakiattikul Y, Ruangnoo S, Sangmukdee K, Chamchusri K, Rithichai P. Enhancement of Plumbagin Production through Elicitation in In Vitro-Regenerated Shoots of Plumbago indica L. Plants. 2024; 13(11):1450. https://doi.org/10.3390/plants13111450
Chicago/Turabian StyleJirakiattikul, Yaowapha, Srisopa Ruangnoo, Kanokwan Sangmukdee, Kornkanok Chamchusri, and Panumart Rithichai. 2024. "Enhancement of Plumbagin Production through Elicitation in In Vitro-Regenerated Shoots of Plumbago indica L." Plants 13, no. 11: 1450. https://doi.org/10.3390/plants13111450
APA StyleJirakiattikul, Y., Ruangnoo, S., Sangmukdee, K., Chamchusri, K., & Rithichai, P. (2024). Enhancement of Plumbagin Production through Elicitation in In Vitro-Regenerated Shoots of Plumbago indica L. Plants, 13(11), 1450. https://doi.org/10.3390/plants13111450





