Effects of the Salt Stress Duration and Intensity on Developmental and Physiological Features of the Moss Polytrichum formosum
Abstract
1. Introduction
2. Results
2.1. In Vitro Culturing
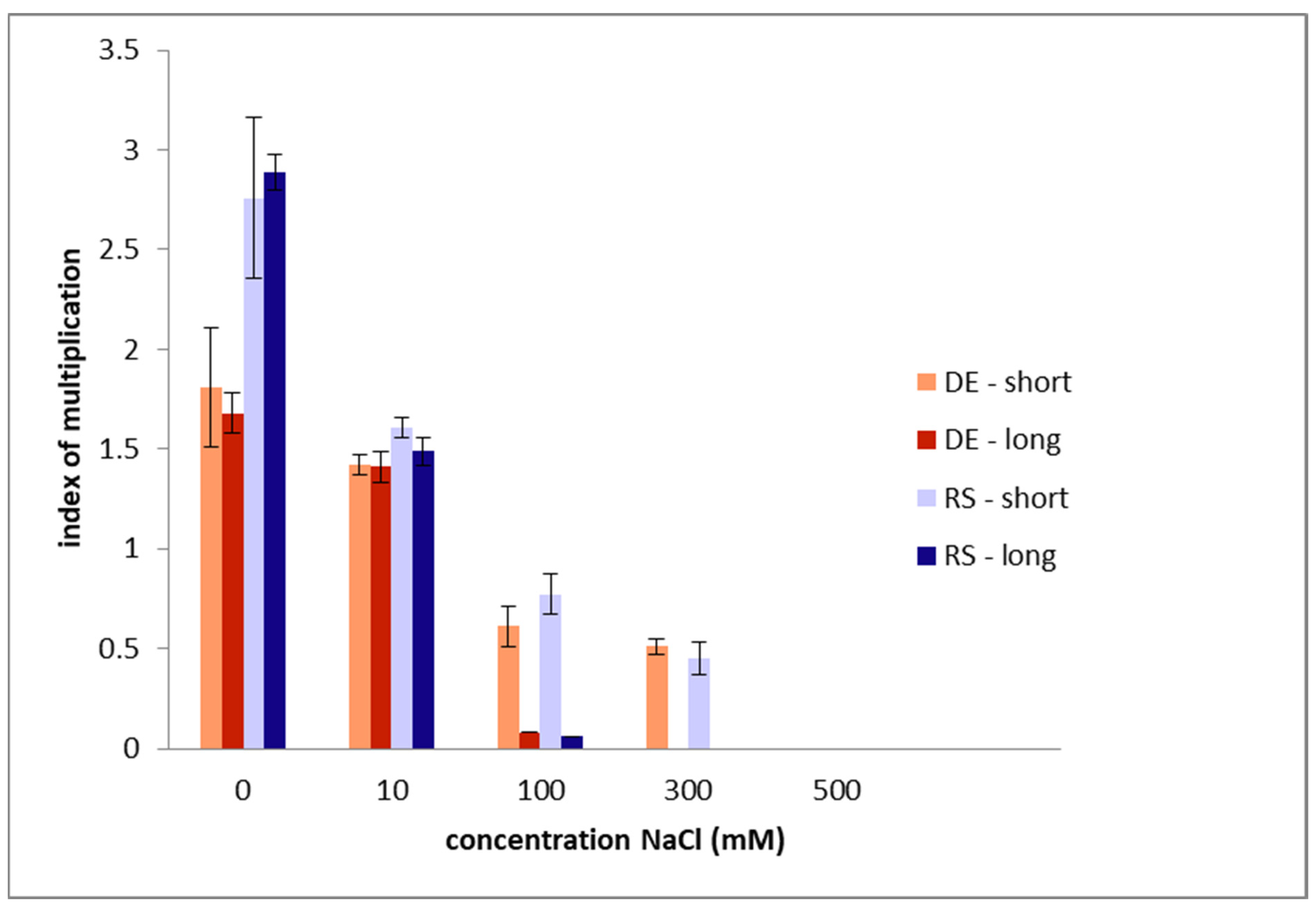
2.2. Morphogenesis
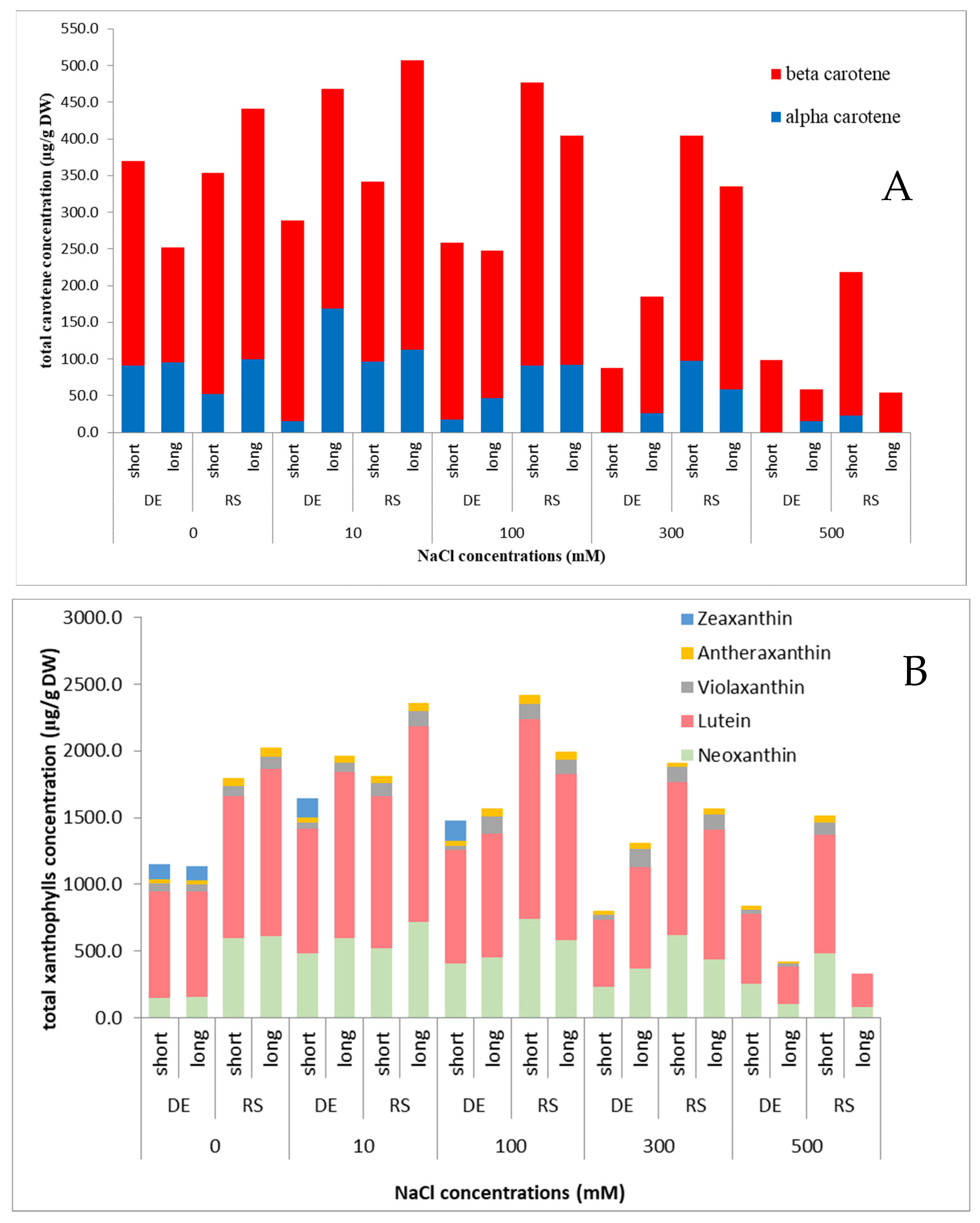
2.3. Pigments
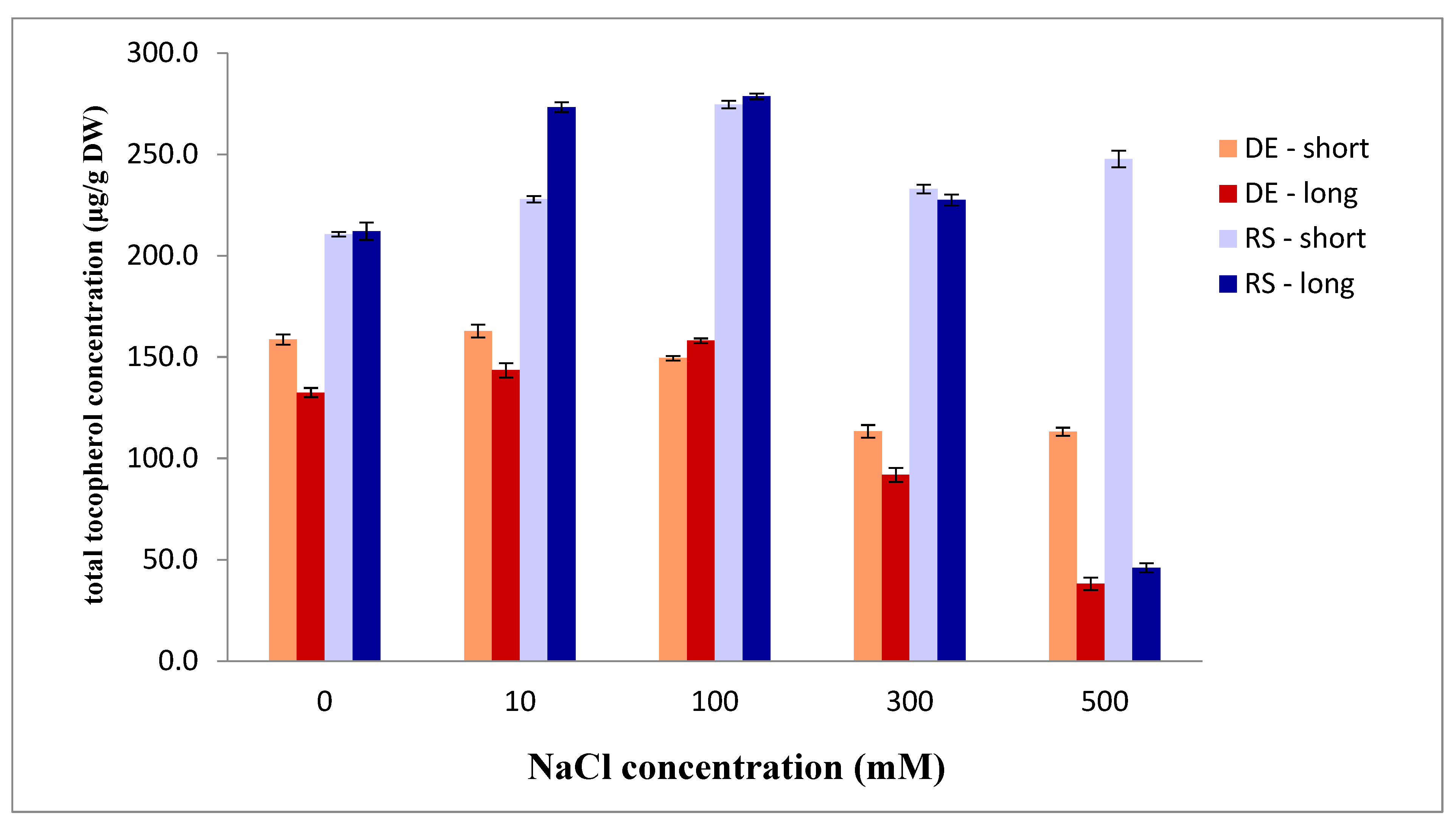
2.4. Tocopherols
2.5. Sugars
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Analysis of Chloroplast Pigments
4.4. Tocopherols
4.5. Sugars
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patino, J.; Vanderpoorten, A. Bryophyte Biogeography. Crit. Rev. Plant Sci. 2018, 37, 175–209. [Google Scholar] [CrossRef]
- Gensel, P.G. The earliest land plants. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 459–477. [Google Scholar] [CrossRef]
- Ćosić, M.; Vujičić, M.M.; Sabovljević, M.S.; Sabovljević, A.D. What do we know on salt stress in bryophytes? Plant Biosyst. 2019, 153, 478–489. [Google Scholar] [CrossRef]
- Sabovljević, M.S.; Nikolić, N.; Vujičić, M.; Sinžar-Sekulić, J.; Pantović, J.; Papp, B.; Sabovljević, A. Ecology, distribution, propagation in vitro, ex situ conservation and native population strenghtening of rare and threatened halophyte moss Entosthodon hungaricus in Serbia. Wulfenia 2018, 25, 117–130. [Google Scholar]
- Sabovljević, M.S.; Segarra-Moragues, J.G.; Puche, F.; Vujičić, M.; Cogoni, A.; Sabovljević, A. Eco-physiological and biotechnological approach to conservation of the world-wide rare and endangered aquatic liverwort Riella helicophylla (Bory et Mont.) Mont. Acta Bot. Croat. 2016, 75, 194–198. [Google Scholar] [CrossRef]
- Ćosić, M.; Vujičić, M.M.; Sabovljević, M.S.; Sabovljević, A.D. Effects of salt on selected bryophyte species tested under controlled conditions. Bot. Serbica 2020, 44, 27–35. [Google Scholar] [CrossRef]
- Ćosić, M.; Janošević, D.; Oaldje, M.; Vujičić, M.; Lang, I.; Sabovljević, M.; Sabovljević, A. Terpenoid evidences within three selected bryophyte species under salt stress as inferred by histochemical analyses. Flora 2021, 285, 151956. [Google Scholar] [CrossRef]
- Frank, W.; Ratnadewi, D.; Reski, R. Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta 2005, 220, 384–394. [Google Scholar] [CrossRef]
- Ćosić, M.V.; Tosti, T.B.; Janošević, D.A.; Vujičić, M.M.; Sabovljević, A.D.; Sabovljević, M.S. Quantification of sugar content in three bryophyte species during salt stress. Acta Biol. Plant. Agriensis 2021, 9, 73. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, Y.; Liu, Y.; Jia, R.L.; Li, X.-R. Osmotic adjustment of soil biocrust mosses in response to desiccation stress. Pedosphere 2015, 25, 459–467. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How plants tolerate salt stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Mogg, T.J.; Rilay, W.W.; Nickerson, J.G. β-Carotene oxidation products—Function and safety. Food Chem. Toxicol. 2021, 152, 112207. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Latowski, D.; Kuczynska, P.; Strzalka, K. Xantophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Mesa, T.; Munne-Bosch, S. A-Tocopherol in chloroplasts: Nothing more than an antioxidant? Curr. Opin. Plant Biol. 2023, 74, 102400. [Google Scholar] [CrossRef] [PubMed]
- Naqve, M.; Wang, X.; Shabaz, M.; Mahmood, A.; Bibi, S.; Fiaz, S. Alpha tocopherol-induced modulations in the morphophysiological attributes of okra under saline conditions. Front. Plant Sci. 2021, 12, 800251. [Google Scholar] [CrossRef] [PubMed]
- Rajčić, M.V.; Ćosić, M.V.; Tosti, T.B.; Mišić, D.M.; Sabovljević, A.D.; Sabovljević, M.S.; Vujičić, M.M. An insight into seasonal changes of carbohydrates and phenolic compounds within the moss Polytrichum formosum (Polytrichaceae). Bot. Serbica 2023, 47, 125–133. [Google Scholar] [CrossRef]
- Fernandez, O.; Bethencourt, L.; Quero, A.; Sangwan, R.S.; Clement, C. Trechalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Sabovljević, A.; Cvetić, T.; Sabovljević, M. The establishment and development of the Catherine’s moss Atrichum undulatum (Hedw.) P.Beauv. (Polytrichaceae) in in vitro conditions. Arch. Biol. Sci. 2006, 58, 87–93. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Knop, W. Quantitative Untersuchungen ueber die Ernahrungsprozesse der Pflanzen. Landwirtsch. Vers. 1965, 7, 93–107. [Google Scholar]
- Sabovljević, A.; Sabovljević, M.; Jocković, N. In vitro culture and secondary metabolite isolation in bryophytes. In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, Methods in Molecular Biology; Jain, S.M., Saxena, P.K., Eds.; Humana Press: New York, NY, USA, 2009; pp. 117–128. [Google Scholar]
- Tausz, M.; Wonisch, A.; Grill, D.; Morales, D.; Jimenez, M.S. Measuring antioxidants in tree species in natural environment: From sampling to data evaluation. J. Exp. Bot. 2003, 54, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Šircelj, H.; Mikulič-Petkovšek, M.; Batič, F. Antioxidants in spring leaves of Oxalis acetosella L. Food Chem. 2010, 123, 351–357. [Google Scholar] [CrossRef]

| Survival (%) | German Genotype | Serbian Genotype | ||
|---|---|---|---|---|
| NaCl Concentration (mM) | Short-Term Stress | Long-Term Stress | Short-Term Stress | Long-Term Stress |
| 0 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 10 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 100 | 100 ± 0.00 | 76.67 ± 1.80 | 100 ± 0.00 | 100 ± 0.00 |
| 300 | 98.33 ± 0.50 | 70 ± 2.20 | 98.33 ± 0.60 | 88.33 ± 0.50 |
| 500 | 80 ± 0.80 | 50 ± 0.90 | 78.33 ± 0.75 | 73.33 ± 0.80 |
| (A) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DE | SHORT-TERM STRESS | LONG-TERM STRESS | |||||||||
| NaCl (mM) | 0 | 10 | 100 | 300 | 500 | 0 | 10 | 100 | 300 | 500 | |
| Monosaccharides | Arabinose | 7.910 | 6.954 | 8.026 | 3.981 | 5.531 | 6.379 | 6.951 | 7.811 | 9.367 | 1.567 |
| Galactose | 13.481 | 11.851 | 13.678 | 6.785 | 10.952 | 10.871 | 11.000 | 12.362 | 7.917 | 10.263 | |
| Glucose | 82.542 | 60.010 | 48.560 | 49.938 | 33.207 | 65.290 | 56.371 | 82.681 | 90.225 | 117.227 | |
| Fructose | 35.600 | 23.375 | 16.408 | 15.597 | 13.590 | 27.071 | 23.771 | 38.467 | 45.296 | 61.979 | |
| Rhamnose | 0.787 | 0.657 | 0.708 | 0.685 | 0.680 | 0.869 | 0.858 | 0.850 | 0.869 | 0.862 | |
| Ribose | 5.824 | 8.380 | 7.562 | 5.075 | 6.496 | 2.526 | 3.781 | 4.396 | 5.489 | 4.048 | |
| Xylose | 4.483 | 7.530 | 7.435 | 7.455 | 7.484 | 4.511 | 4.546 | 4.531 | 4.511 | 4.525 | |
| Disaccharides | Sucrose | 42.767 | 40.912 | 64.224 | 37.488 | 47.080 | 34.717 | 58.682 | 40.146 | 56.695 | 71.342 |
| Isomaltose | 1.982 | 0.362 | 1.651 | 0.341 | 1.011 | 1.486 | 0.217 | 0.991 | 1.284 | 0.995 | |
| Maltose | 7.183 | 4.235 | 1.232 | 8.110 | 6.058 | 1.203 | 2.965 | 4.606 | 4.666 | 3.580 | |
| Melibiose | 2.512 | 3.263 | 3.050 | 1.347 | 0.639 | 0.419 | 0.563 | 2.535 | 2.983 | 7.553 | |
| Trehalose | 3.080 | 3.126 | 6.960 | 8.515 | 6.146 | 8.244 | 6.820 | 2.356 | 5.336 | 8.189 | |
| Turanose | 37.404 | 29.327 | 40.174 | 27.207 | 25.503 | 24.863 | 36.927 | 38.573 | 38.573 | 28.834 | |
| Trisaccharides | Isomaltotriose | 0.701 | 0.779 | 0.596 | 0.608 | 0.645 | 0.487 | 0.818 | 1.920 | 0.541 | 0.942 |
| Maltotriose | 0.884 | 2.134 | 1.151 | 0.925 | 1.241 | 1.390 | 1.174 | 1.639 | 0.756 | 1.240 | |
| Panose | 1.065 | 0.901 | 0.899 | 0.911 | 0.905 | 1.742 | 1.661 | 1.716 | 1.742 | 1.715 | |
| Raffinose | 1.317 | 1.151 | 1.175 | 1.159 | 1.152 | 1.184 | 1.131 | 1.177 | 1.184 | 1.169 | |
| Tetrasaccharides | Stachyose | 0.623 | 0.558 | 0.598 | 0.573 | 0.565 | 0.665 | 0.652 | 0.635 | 0.665 | 0.654 |
| Sugar alcohols | Galactitol | 0.910 | 2.977 | 0.141 | 0.375 | 1.038 | 1.279 | 1.960 | 3.475 | 0.193 | 1.477 |
| Glycerol | 4.362 | 7.589 | 4.203 | 4.204 | 7.044 | 5.813 | 18.982 | 3.531 | 4.080 | 8.101 | |
| Mannitol | 1.437 | 1.247 | 1.229 | 1.304 | 1.251 | 1.480 | 1.548 | 1.354 | 1.480 | 1.466 | |
| Sorbitol | 12.595 | 12.760 | 13.198 | 13.648 | 14.586 | 11.051 | 14.981 | 12.225 | 12.346 | 12.051 | |
 | |||||||||||
| (B) | |||||||||||
| RS | SHORT-TERM STRESS | LONG-TERM STRESS | |||||||||
| NaCl (mM) | 0 | 10 | 100 | 300 | 500 | 0 | 10 | 100 | 300 | 500 | |
| Monosaccharides | Arabinose | 6.455 | 3.940 | 8.035 | 2.184 | 9.188 | 6.849 | 5.403 | 8.660 | 6.649 | 0.300 |
| Galactose | 12.377 | 6.715 | 13.693 | 12.847 | 15.431 | 12.909 | 9.208 | 15.989 | 5.116 | 15.418 | |
| Glucose | 72.779 | 31.111 | 64.167 | 74.465 | 91.944 | 58.276 | 91.636 | 65.023 | 58.054 | 31.319 | |
| Fructose | 21.786 | 29.936 | 24.995 | 30.416 | 32.004 | 18.714 | 30.095 | 36.638 | 35.830 | 22.574 | |
| Rhamnose | 1.973 | 1.209 | 1.139 | 1.167 | 1.372 | 2.043 | 1.183 | 2.074 | 2.013 | 1.828 | |
| Ribose | 3.437 | 9.015 | 2.855 | 3.188 | 4.624 | 2.631 | 2.453 | 8.721 | 1.992 | 3.949 | |
| Xylose | 6.195 | 2.951 | 3.002 | 2.971 | 3.780 | 6.203 | 2.947 | 6.235 | 6.248 | 5.409 | |
| Disaccharides | Sucrose | 45.591 | 11.190 | 78.936 | 82.235 | 88.118 | 43.059 | 71.888 | 72.947 | 26.991 | 13.824 |
| Isomaltose | 1.546 | 0.230 | 0.190 | 0.821 | 0.697 | 0.930 | 0.552 | 0.271 | 0.202 | 0.489 | |
| Maltose | 2.520 | 2.370 | 1.845 | 2.084 | 2.146 | 2.417 | 3.168 | 2.319 | 2.196 | 1.880 | |
| Melibiose | 4.231 | 5.044 | 3.011 | 5.051 | 2.999 | 3.215 | 1.986 | 2.102 | 0.986 | 0.542 | |
| Trehalose | 3.944 | 6.922 | 7.926 | 2.570 | 12.840 | 0.958 | 6.674 | 8.119 | 9.293 | 9.661 | |
| Turanose | 24.891 | 12.885 | 22.058 | 34.683 | 24.404 | 25.843 | 32.560 | 26.663 | 14.593 | 5.328 | |
| Trisaccharides | Isomaltotriose | 0.591 | 0.572 | 0.690 | 2.197 | 1.012 | 1.854 | 0.634 | 0.651 | 0.768 | 0.977 |
| Maltotriose | 1.041 | 0.954 | 0.820 | 1.070 | 0.971 | 0.953 | 0.869 | 1.207 | 0.944 | 0.993 | |
| Panose | 7.118 | 4.887 | 4.993 | 4.808 | 5.451 | 3.588 | 4.859 | 3.608 | 3.619 | 3.316 | |
| Raffinose | 1.278 | 1.237 | 1.234 | 1.266 | 1.254 | 1.304 | 1.258 | 1.319 | 1.268 | 1.288 | |
| Tetrasaccharides | Stachyose | 0.871 | 0.737 | 0.777 | 0.789 | 0.794 | 0.806 | 0.719 | 0.827 | 0.813 | 0.791 |
| Sugar alcohols | Galactitol | 0.619 | 0.701 | 0.907 | 2.117 | 0.724 | 1.591 | 0.721 | 2.035 | 2.269 | 2.654 |
| Glycerol | 4.335 | 13.385 | 13.514 | 3.444 | 8.670 | 2.255 | 3.837 | 4.570 | 8.026 | 8.257 | |
| Mannitol | 2.258 | 1.515 | 1.440 | 1.520 | 1.683 | 1.961 | 1.456 | 2.170 | 2.154 | 6.830 | |
| Sorbitol | 12.691 | 12.376 | 8.289 | 9.097 | 10.613 | 7.396 | 13.028 | 13.135 | 13.697 | 11.939 | |
 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajčić, M.V.; Šircelj, H.; Matić, N.A.; Pavkov, S.D.; Poponessi, S.; Tosti, T.B.; Sabovljević, A.D.; Sabovljević, M.S.; Vujičić, M.M. Effects of the Salt Stress Duration and Intensity on Developmental and Physiological Features of the Moss Polytrichum formosum. Plants 2024, 13, 1438. https://doi.org/10.3390/plants13111438
Rajčić MV, Šircelj H, Matić NA, Pavkov SD, Poponessi S, Tosti TB, Sabovljević AD, Sabovljević MS, Vujičić MM. Effects of the Salt Stress Duration and Intensity on Developmental and Physiological Features of the Moss Polytrichum formosum. Plants. 2024; 13(11):1438. https://doi.org/10.3390/plants13111438
Chicago/Turabian StyleRajčić, Marija V., Helena Šircelj, Nikolina A. Matić, Sara D. Pavkov, Silvia Poponessi, Tomislav B. Tosti, Aneta D. Sabovljević, Marko S. Sabovljević, and Milorad M. Vujičić. 2024. "Effects of the Salt Stress Duration and Intensity on Developmental and Physiological Features of the Moss Polytrichum formosum" Plants 13, no. 11: 1438. https://doi.org/10.3390/plants13111438
APA StyleRajčić, M. V., Šircelj, H., Matić, N. A., Pavkov, S. D., Poponessi, S., Tosti, T. B., Sabovljević, A. D., Sabovljević, M. S., & Vujičić, M. M. (2024). Effects of the Salt Stress Duration and Intensity on Developmental and Physiological Features of the Moss Polytrichum formosum. Plants, 13(11), 1438. https://doi.org/10.3390/plants13111438









