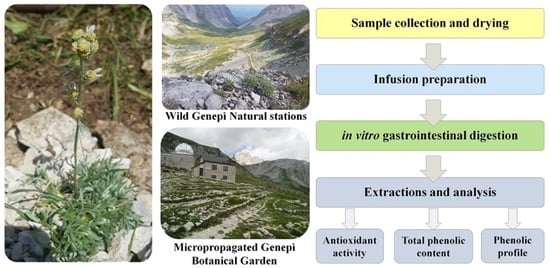
Wild and Micropropagated Artemisia eriantha Infusions: In Vitro Digestion Effects on Phenolic Pattern and Antioxidant Activity
Abstract
:1. Introduction
2. Results
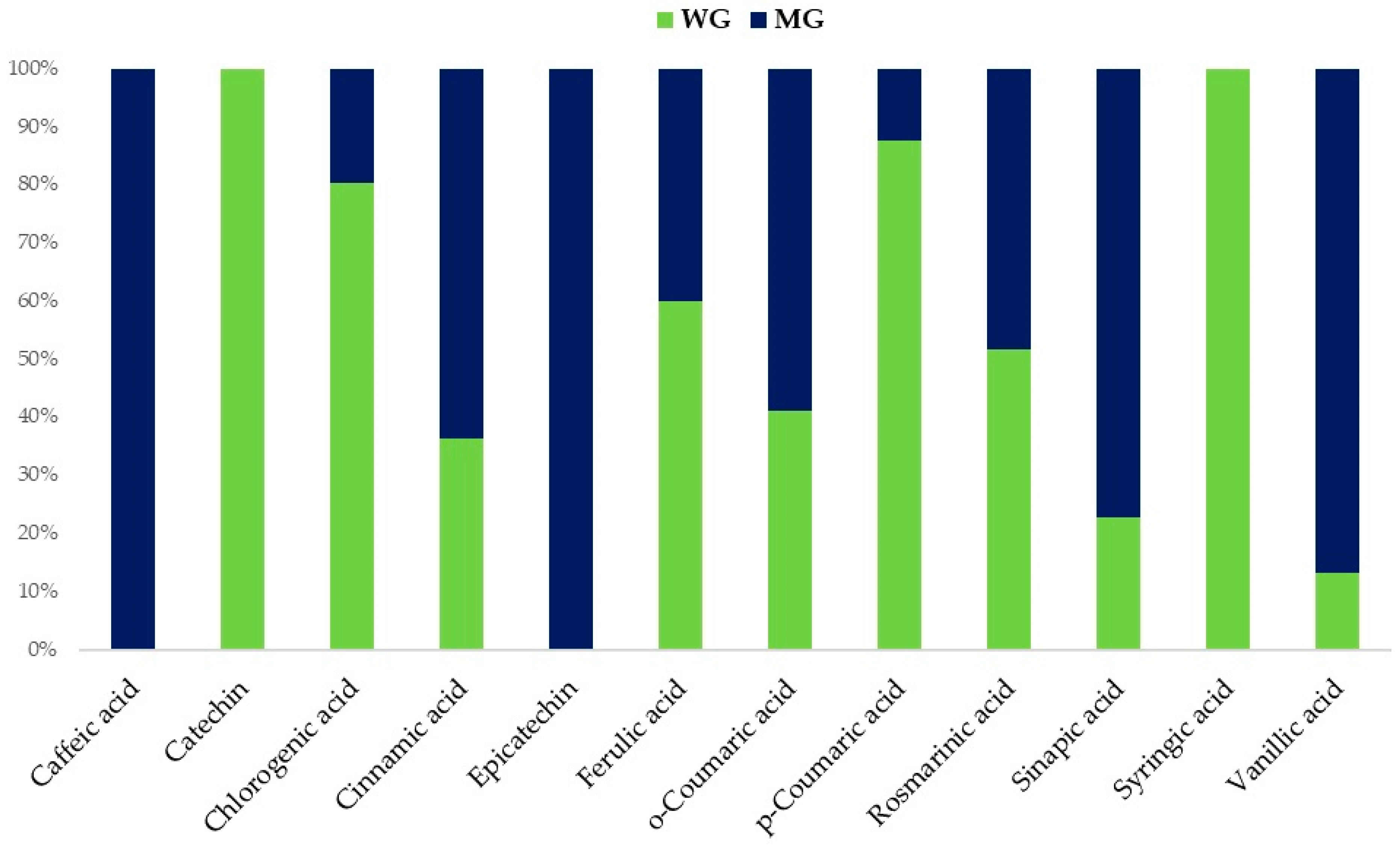
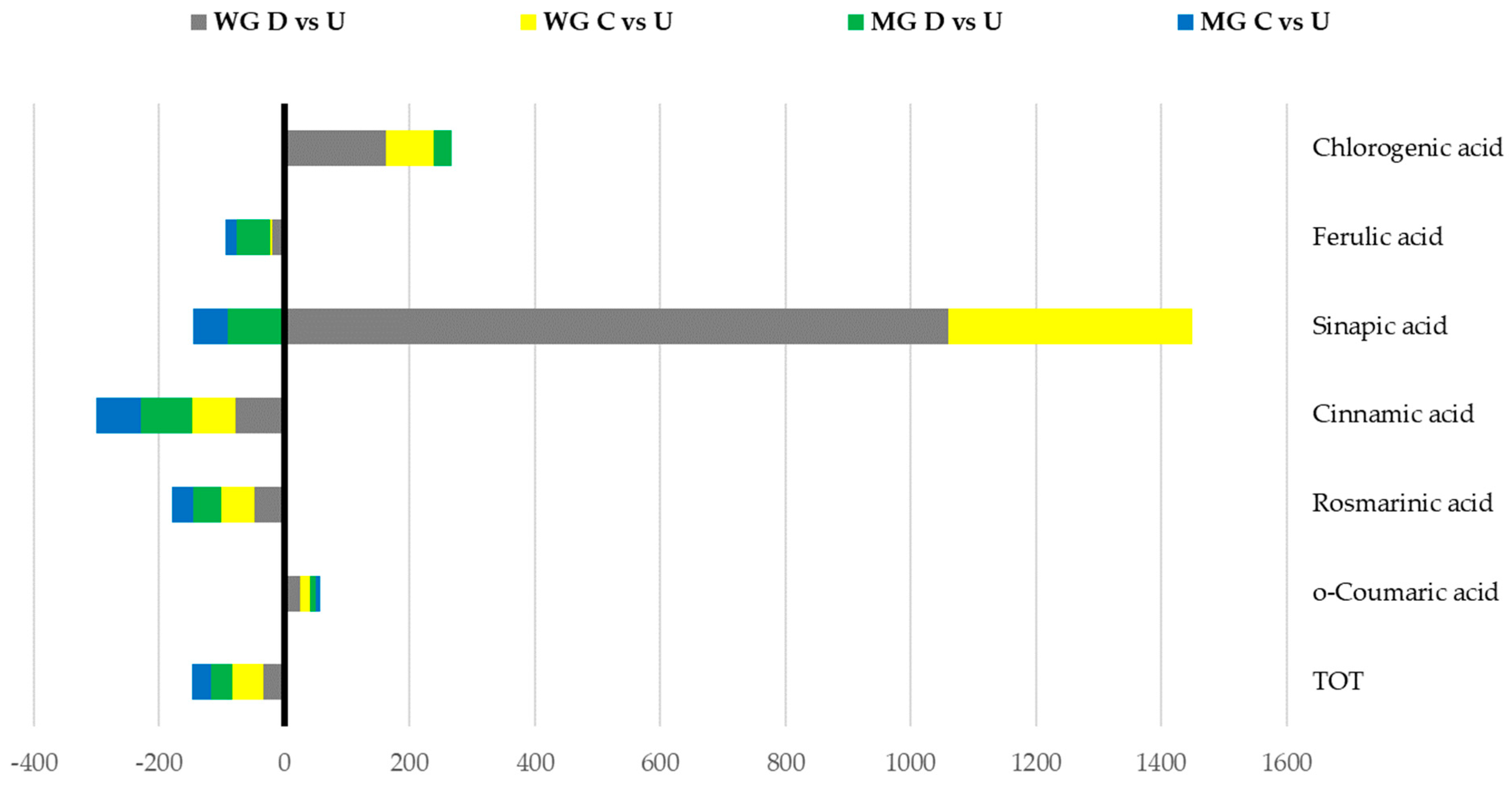
2.1. Polyphenolic Profiles
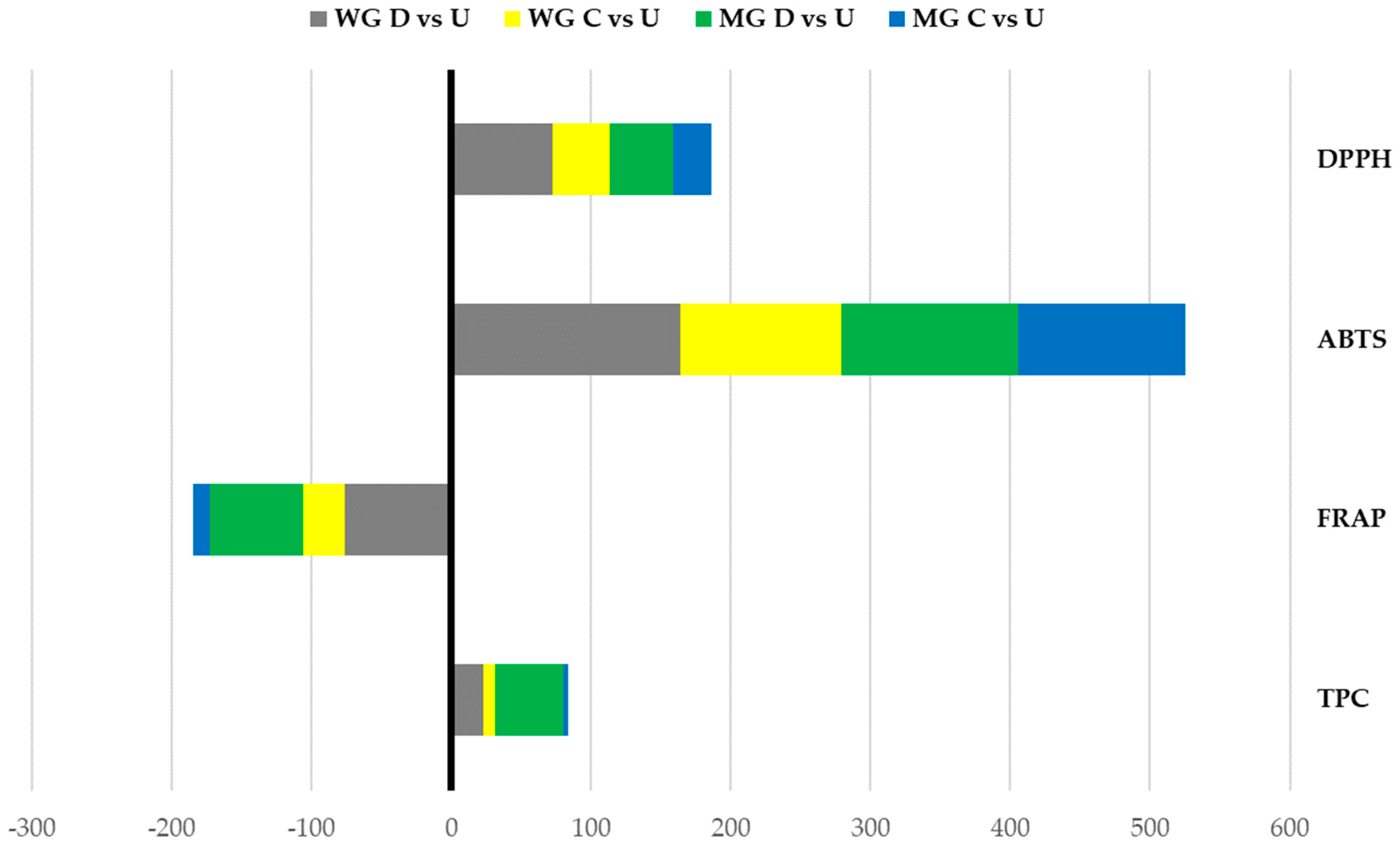
2.2. Antioxidant Activity
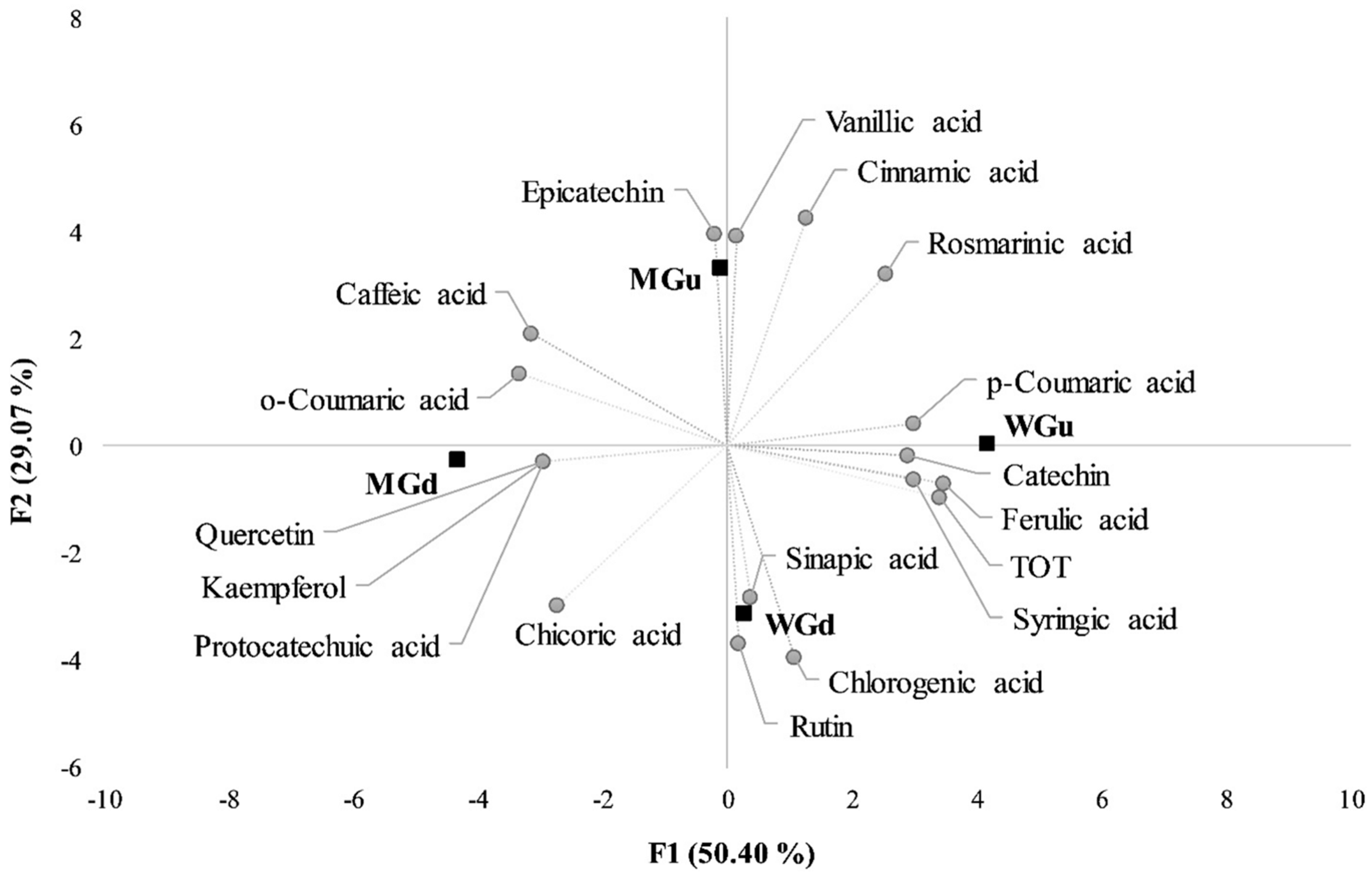
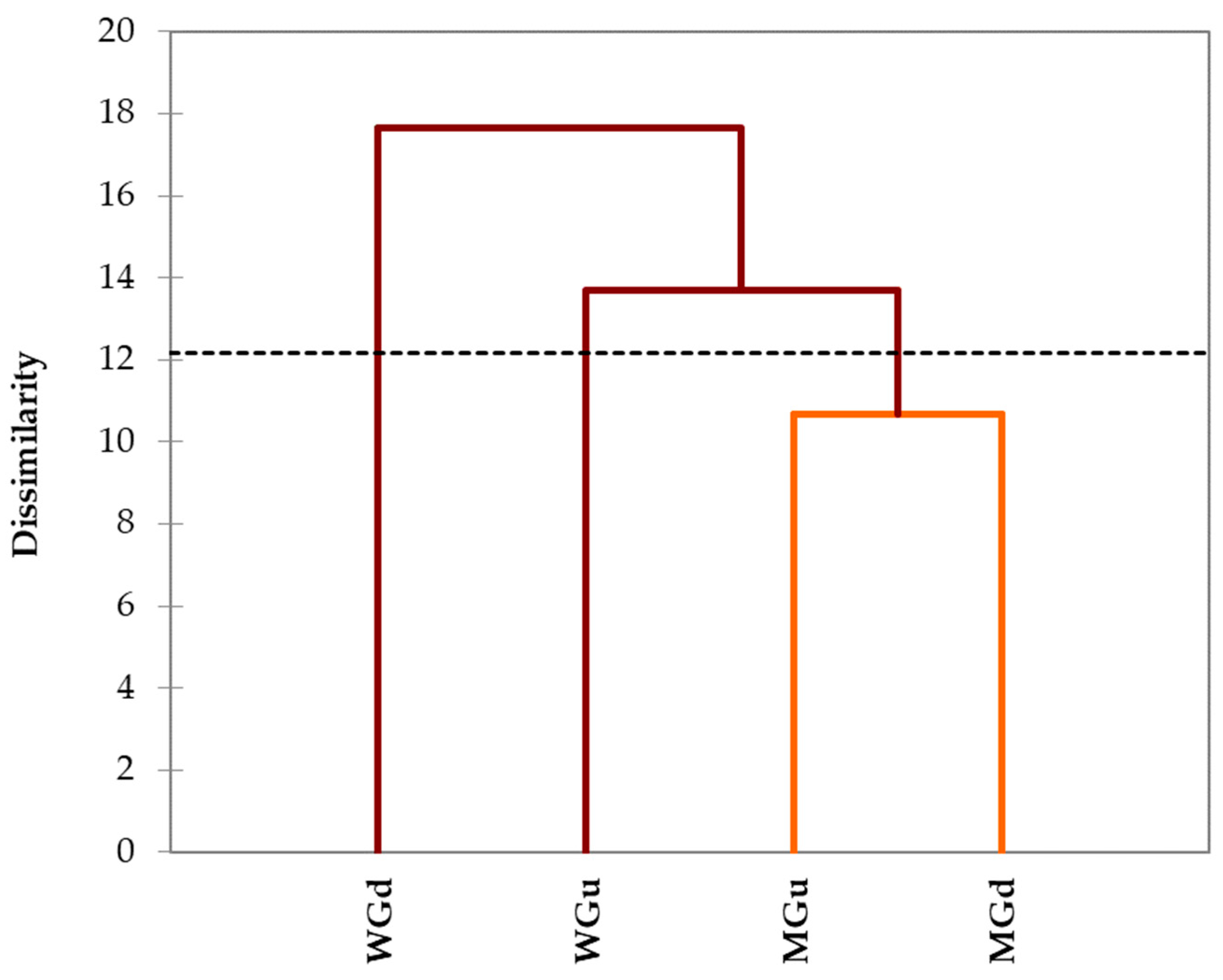
2.3. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Genepì Infusions Preparation
4.3. In Vitro Gastrointestinal Digestion
4.4. Total Phenolic Content and Antioxidant Activity
4.5. HPLC-DAD Polyphenolic Profile Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Chapter 14—Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M.K., Kon, K.V., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 205–221. ISBN 978-0-12-398539-2. [Google Scholar]
- Vallès, J.; Garcia, S.; Hidalgo, O.; Martín, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, Genome Evolution, Biotechnological Issues and Research Including Applied Perspectives in Artemisia (Asteraceae); Elsevier: Amsterdam, The Netherlands, 2011; Volume 60, ISBN 9780123858511. [Google Scholar]
- Hussain, M.; Thakur, R.K.; Khazir, J.; Ahmed, S.; Khan, M.I.; Rahi, P.; Peer, L.A.; Pragadheesh, V.; Kaur, S.; Raina, S.N.; et al. Traditional Uses, Phytochemistry, Pharmacology, and Toxicology of the Genus Artemisia L. (Asteraceae): A High-Value Medicinal Plant. Curr. Top. Med. Chem. 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Czerwińska, M.E.; Mardari, C.; Zengin, G.; Sinan, K.I.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Luca, S.V. Exploring the Artemisia Genus: An Insight into the Phytochemical and Multi-Biological Potential of A. campestris subsp. Lednicensis (Spreng.) Greuter & Raab-Straube. Plants 2022, 11, 2874. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A.; et al. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxid. Med. Cell. Longev. 2022, 2022, 5628601. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Pace, L.; Pellegrini, M.; Palmieri, S.; Rocchi, R.; Lippa, L.; Del Gallo, M. Plant Growth-Promoting Rhizobacteria for in Vitro and Ex Vitro Performance Enhancement of Apennines’ Genepì (Artemisia umbelliformis subsp. eriantha), an Endangered Phytotherapeutic Plant. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 134–142. [Google Scholar] [CrossRef]
- Rubiolo, P.; Matteodo, M.; Bicchi, C.; Appendino, G.; Gnavi, G.; Bertea, C.; Maffei, M. Chemical and Biomolecular Characterization of Artemisia umbelliformis Lam., an Important Ingredient of the Alpine Liqueur “Genepì”. J. Agric. Food Chem. 2009, 57, 3436–3443. [Google Scholar] [CrossRef]
- Vouillamoz, J.F.; Carlen, C.; Taglialatela-Scafati, O.; Pollastro, F.; Appendino, G. The Génépi Artemisia Species. Ethnopharmacology, Cultivation, Phytochemistry, and Bioactivity. Fitoterapia 2015, 106, 231–241. [Google Scholar] [CrossRef]
- Bicchi, C.; D’Amato, A.; Nano, G.M.; Frattini, C. Capillary GLC Controls of Some Alpine Artemisiae and of the Related Liqueurs. Chromatographia 1984, 18, 560–566. [Google Scholar] [CrossRef]
- Fasciani, P.; Marcozzi, G.; Reale, S.; Pace, L. Volatile Compounds of Ex Vitro and Wild Plantlets of Artemisia umbelliformis subsp. eriantha (Apennines’ Genepì). Acta Hortic. 2017, 1155, 565–572. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Nguyen, H.T.; Islam, M.Z.; Obi, T.; Pothinuch, P.; Zar, P.P.K.; Hou, D.X.; Van Nguyen, T.; Nguyen, T.M.; Van Dao, C.; et al. Pharmacological Characteristics of Artemisia vulgaris L. in Isolated Porcine Basilar Artery. J. Ethnopharmacol. 2016, 182, 16–26. [Google Scholar] [CrossRef]
- Martins, A.; Mignon, R.; Bastos, M.; Batista, D.; Neng, N.R.; Nogueira, J.M.F.; Vizetto-Duarte, C.; Custódio, L.; Varela, J.; Rauter, A.P. In Vitro Antitumoral Activity of Compounds Isolated from Artemisia gorgonum Webb. Phytother. Res. 2014, 28, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Afsar, S.K.; Rajesh Kumar, K.; Venu Gopal, J.; Raveesha, P. Assessment of Anti-Inflammatory Activity of Artemisia vulgaris Leaves by Cotton Pellet Granuloma Method in Wistar Albino Rats. J. Pharm. Res. 2013, 7, 463–467. [Google Scholar] [CrossRef]
- Corrêa-Ferreira, M.L.; Verdan, M.H.; dos Reis Lívero, F.A.; Galuppo, L.F.; Telles, J.E.Q.; Alves Stefanello, M.É.; Acco, A.; de Oliveira Petkowicz, C.L. Inulin-Type Fructan and Infusion of Artemisia vulgaris Protect the Liver against Carbon Tetrachloride-Induced Liver Injury. Phytomedicine 2017, 24, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sefi, M.; Fetoui, H.; Makni, M.; Zeghal, N. Mitigating Effects of Antioxidant Properties of Artemisia Campestris Leaf Extract on Hyperlipidemia, Advanced Glycation End Products and Oxidative Stress in Alloxan-Induced Diabetic Rats. Food Chem. Toxicol. 2010, 48, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, W.H. Biochemical Effects, Hypolipidemic and Anti-Inflammatory Activities of Artemisia vulgaris Extract in Hypercholesterolemic Rats. J. Clin. Biochem. Nutr. 2015, 57, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, M.; Gallego, M.; Segovia, F.; Almajano, M. Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions. Antioxidants 2014, 3, 116–128. [Google Scholar] [CrossRef]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The Potential of Artemisia vulgaris Leaves as a Source of Antioxidant Phenolic Compounds. J. Funct. Foods 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Reale, S.; Pace, L.; D’Archivio, A.A.; De Angelis, F.; Marcozzi, G. Volatiles Fingerprint of Artemisia umbelliformis subsp. eriantha by Headspace-Solid Phase Microextraction GC–MS. Nat. Prod. Res. 2014, 28, 61–66. [Google Scholar] [CrossRef]
- Walch, S.G.; Kuballa, T.; Stühlinger, W.; Lachenmeier, D.W. Determination of the Biologically Active Flavour Substances Thujone and Camphor in Foods and Medicines Containing Sage (Salvia officinalis L.). Chem. Cent. J. 2011, 5, 44. [Google Scholar] [CrossRef]
- Pace, L.; Grandi, S.; Marotti, M.; Piccaglia, R.; Pacioni, G.; Spanò, L. Terpenoid Profiles of in Vitro Regenerated Artemisia petrosa subsp. eriantha (Apennines’ Genepì) *. Ann. Appl. Biol. 2010, 157, 309–316. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Accardo, F.; Leni, G.; Tedeschi, T.; Prandi, B.; Sforza, S. Structural and Chemical Changes Induced by Temperature and PH Hinder the Digestibility of Whey Proteins. Food Chem. 2022, 387, 132884. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In Vitro Bioaccessibility Assessment as a Prediction Tool of Nutritional Efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and Fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Lucas-Gonzalez, R.; Fernández-López, J.; Ricci, A.; Pérez-Álvarez, J.A.; Sterzo, C.L.; Viuda-Martos, M. Bioaccessibility of Polyphenolic Compounds of Six Quinoa Seeds during in Vitro Gastrointestinal Digestion. J. Funct. Foods 2017, 38, 77–88. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Lima, K.; Silva, O.; Figueira, M.E.; Pires, C.; Cruz, D.; Gomes, S.; Maurício, E.M.; Duarte, M.P. Influence of the in Vitro Gastrointestinal Digestion on the Antioxidant Activity of Artemisia gorgonum Webb and Hyptis pectinata (L.) Poit. Infusions from Cape Verde. Food Res. Int. 2019, 115, 150–159. [Google Scholar] [CrossRef]
- Udomwasinakun, N.; Saha, S.; Mulet-Cabero, A.-I.; Wilde, P.J.; Pirak, T. Assessment of Polyphenols Bioaccessibility, Stability, and Antioxidant Activity of White Mugwort (Artemisia lactiflora Wall.) during Static In Vitro Gastrointestinal Digestion. Foods 2023, 12, 949. [Google Scholar] [CrossRef]
- Pace, L.; Pacioni, G.; Spano, L. In Vitro Propagation of Artemisia petrosa ssp. eriantha: Potential for the Preservation of an Endangered Species. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2004, 138, 291–294. [Google Scholar] [CrossRef]
- Liu, W.; Dongxue, Y.; Li, N.; Xiaogai, H.; Dongmei, W.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ma, X.; Wei, Q.; Peng, S.; Zhang, S. Effects of Growing Location on the Contents of Secondary Metabolites in the Leaves of Four Selected Superior Clones of Eucommia ulmoides. Ind. Crops Prod. 2011, 34, 1607–1614. [Google Scholar] [CrossRef]
- Zidorn, C.; Stuppner, H. Evaluation of Chemosystematic Characters in the Genus Leontodon (Asteraceae). Taxon 2001, 50, 115. [Google Scholar] [CrossRef]
- Bilger, W.; Rolland, M.; Nybakken, L. UV Screening in Higher Plants Induced by Low Temperature in the Absence of UV-B Radiation. Photochem. Photobiol. Sci. 2007, 6, 190–195. [Google Scholar] [CrossRef]
- Bautista, I.; Boscaiu, M.; Lidon, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Mayoral, O.; Vicente, O. Environmentally Induced Changes in Antioxidant Phenolic Compounds Levels in Wild Plants. Acta Physiol. Plant. 2016, 38, 9. [Google Scholar] [CrossRef]
- Rasera, G.B.; de Camargo, A.C.; de Castro, R.J.S. Bioaccessibility of Phenolic Compounds Using the Standardized INFOGEST Protocol: A Narrative Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 260–286. [Google Scholar] [CrossRef]
- Özkan, G.; Aras, A.; Çapanoğlu Güven, E. Investigating the Antioxidant Properties of Some Herbal Infusions During In Vitro Digestion. J. Apitherapy Nat. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Quintriqueo-Cid, A.; Rueda-Robles, A.; Robert, P.; Borrás-Linares, I.; Lozano-Sánchez, J. Evaluation of Different Advanced Approaches to Simulation of Dynamic In Vitro Digestion of Polyphenols from Different Food Matrices—A Systematic Review. Antioxidants 2022, 12, 101. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, H.; McClements, D.J. Application of Static in Vitro Digestion Models for Assessing the Bioaccessibility of Hydrophobic Bioactives: A Review. Trends Food Sci. Technol. 2022, 122, 314–327. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The Reciprocal Interaction between Polyphenols and Other Dietary Compounds: Impact on Bioavailability, Antioxidant Capacity and Other Physico-Chemical and Nutritional Parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Record, I.R.; Lane, J.M. Simulated Intestinal Digestion of Green and Black Teas. Food Chem. 2001, 73, 481–486. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Andrade, P.B.; Valentão, P.; Romano, A. Effect of in Vitro Gastrointestinal Digestion on the Total Phenolic Contents and Antioxidant Activity of Wild Mediterranean Edible Plant Extracts. Eur. Food Res. Technol. 2019, 245, 753–762. [Google Scholar] [CrossRef]
- Pavan, V.; Sancho, R.S.A.; Pastore, G.M. The Effect of In Vitro Digestion on the Antioxidant Activity of Fruit Extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT-Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. Impacts of Processing Conditions on Digestive Recovery of Polyphenolic Compounds and Stability of the Antioxidant Activity of Green Tea Infusion during in Vitro Gastrointestinal Digestion. LWT-Food Sci. Technol. 2018, 89, 648–656. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic Composition and Antioxidant Activity of Aqueous Infusions from Capparis spinosa L. and Crithmum maritimum L. before and after Submission to a Two-Step In Vitro Digestion Model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aranda-Ledesma, N.E.; Rojas, R.; Tafolla-Arellano, J.C.; Martínez-Ávila, G.C.G. Antioxidant Activity of Polyphenolic Compounds Obtained from Euphorbia antisyphilitica By-Products. Heliyon 2021, 7, e06734. [Google Scholar] [CrossRef]
- Baeza, G.; Sarriá, B.; Bravo, L.; Mateos, R. Polyphenol Content, In Vitro Bioaccessibility and Antioxidant Capacity of Widely Consumed Beverages. J. Sci. Food Agric. 2018, 98, 1397–1406. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Bioaccessibility and Antioxidant Potential of Millet Grain Phenolics as Affected by Simulated in Vitro Digestion and Microbial Fermentation. J. Funct. Foods 2012, 4, 226–237. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Barber, X.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility, Changes in the Antioxidant Potential and Colonic Fermentation of Date Pits and Apple Bagasse Flours Obtained from Co-Products during Simulated In Vitro Gastrointestinal Digestion. Food Res. Int. 2015, 78, 169–176. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzalez, R.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility of Phenolic Compounds and Antioxidant Capacity of Chia (Salvia hispanica L.) Seeds. Plant Foods Hum. Nutr. 2018, 73, 47–53. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Zhao, Y.-Y.; Luo, C.-X.; Li, J.; Gao, Y.-Q. Total Phenolic Contents of 33 Fruits and Their Antioxidant Capacities before and after In Vitro Digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of Polyphenolic Profile Stability and Changes in the Antioxidant Potential of Maqui Berry (Aristotelia chilensis (Molina) Stuntz) during in Vitro Gastrointestinal Digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST In Vitro Digestion Model to Foods: A Review. Annu. Rev. Food Sci. Technol. 2023, 14, 135–156. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The Harmonized INFOGEST in Vitro Digestion Method: From Knowledge to Action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In Vitro Gastrointestinal Digestion of Pomegranate Peel (Punica granatum) Flour Obtained from Co-Products: Changes in the Antioxidant Potential and Bioactive Compounds Stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction: Antioxidative Activity of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
| Compound | Wild Genepì | Micropropagated Genepì | ||||||
|---|---|---|---|---|---|---|---|---|
| Undigested | Control | Digested | LSD | Undigested | Control | Digested | LSD | |
| Caffeic acid | - | - | 24.8 | 67.0 a | 69.1 a | 75.1 a | 9.2 | |
| Catechin | 172.5 a | 71.9 b | 11.0 c | 42.2 | - | - | - | |
| Chicoric acid | - | - | 7.9 | - | 8.2 b | 10.5 a | 2.6 | |
| Chlorogenic acid | 200.4 c | 355.2 b | 523.6 a | 15.8 | 48.8 b | 48.7 b | 63.0 a | 9.9 |
| Cinnamic acid | 45.6 a | 13.9 b | 10.0 b | 1.2 | 79.5 a | 23.7 b | 14.6 c | 2.5 |
| Epicatechin | - | - | - | 89.1 a | 15.5 b | 4.0 c | 2.4 | |
| Ferulic acid | 163.8 a | 156.3 a | 132.9 b | 6.2 | 109.4 a | 89.7 b | 52.4 c | 2.6 |
| Kaempferol | - | - | - | - | 20.9 b | 29.6 a | 5.9 | |
| o-Coumaric acid | 33.5 b | 39.1 a | 41.7 a | 10.4 | 48.0 b | 51.5 a | 51.9 a | 2.9 |
| p-Coumaric acid | 770.4 a | 65.3 b | 42.3 c | 19.5 | 107.2 a | 45.5 b | - | 11.8 |
| Protocatechuic acid | - | - | - | - | 3.8 b | 12.9 a | 4.7 | |
| Quercetin | - | - | - | - | 74.3 b | 127.7 a | 35.1 | |
| Rosmarinic acid | 84.5 a | 38.4 b | 44.5 b | 8 | 79.2 a | 51.4 b | 45.2 b | 13.8 |
| Rutin | - | - | 105 | - | - | - | ||
| Sinapic acid | 5.5 c | 26.9 b | 63.8 a | 5.7 | 18.6 a | 8.3 b | 1.7 c | 2.5 |
| Syringic acid | 36.0 a | - | 6.3 b | 7 | - | - | - | |
| Vanillic acid | 18.2 a | - | 13.6 b | 2.7 | 120.4 a | 29.4 b | 9.4 c | 7.3 |
| TOT | 1530.5 a | 767.0 c | 1027.5 b | 77.9 | 767.1 a | 540.2 b | 498.0 b | 46.5 |
| TPC | ABTS | DPPH | FRAP | |||||||||
| (mg GAE g−1 dw) | (IC50) | (IC50) | (mg TE g−1) | |||||||||
| U | D | p-Value | U | D | p-Value | U | D | p-Value | U | D | p-Value | |
| Wild Genepì | 11.48 | 14.13 | *** | 1.56 | 4.11 | * | 2.70 | 4.64 | *** | 23.62 | 5.68 | ** |
| Micropropagated Genepì | 5.29 | 7.86 | *** | 1.83 | 4.13 | * | 3.37 | 4.88 | *** | 16.26 | 5.49 | ** |
| p-value | ** | ** | *** | ns | ns | ns | *** | ns | ||||
| Pearson’s correlation coefficient | ||||||||||||
| Assay | ABTS | DPPH | FRAP | |||||||||
| TPC | 0.33 | 0.12 | −0.13 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchi, R.; Pellegrini, M.; Pittia, P.; Pace, L. Wild and Micropropagated Artemisia eriantha Infusions: In Vitro Digestion Effects on Phenolic Pattern and Antioxidant Activity. Plants 2024, 13, 85. https://doi.org/10.3390/plants13010085
Rocchi R, Pellegrini M, Pittia P, Pace L. Wild and Micropropagated Artemisia eriantha Infusions: In Vitro Digestion Effects on Phenolic Pattern and Antioxidant Activity. Plants. 2024; 13(1):85. https://doi.org/10.3390/plants13010085
Chicago/Turabian StyleRocchi, Rachele, Marika Pellegrini, Paola Pittia, and Loretta Pace. 2024. "Wild and Micropropagated Artemisia eriantha Infusions: In Vitro Digestion Effects on Phenolic Pattern and Antioxidant Activity" Plants 13, no. 1: 85. https://doi.org/10.3390/plants13010085
APA StyleRocchi, R., Pellegrini, M., Pittia, P., & Pace, L. (2024). Wild and Micropropagated Artemisia eriantha Infusions: In Vitro Digestion Effects on Phenolic Pattern and Antioxidant Activity. Plants, 13(1), 85. https://doi.org/10.3390/plants13010085