Tolerance with High Yield Potential Is Provided by Lower Na+ Ion Accumulation and Higher Photosynthetic Activity in Tolerant YNU31-2-4 Rice Genotype under Salinity and Multiple Heat and Salinity Stress
Abstract
1. Introduction
2. Results
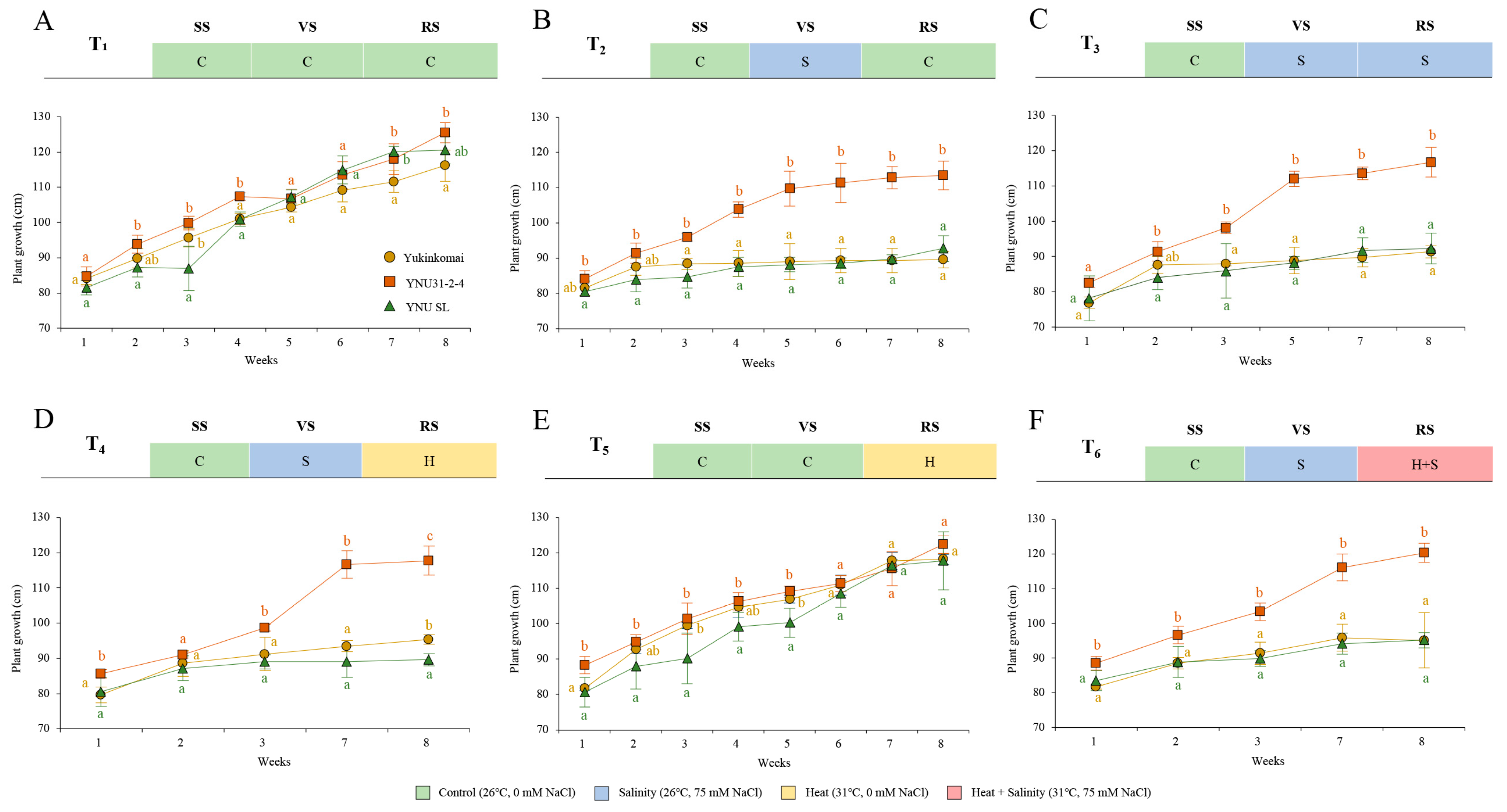
2.1. YNU31-2-4 Rice Genotype Shows Higher Growth Performance under Salinity and Heat and Salinity Stress
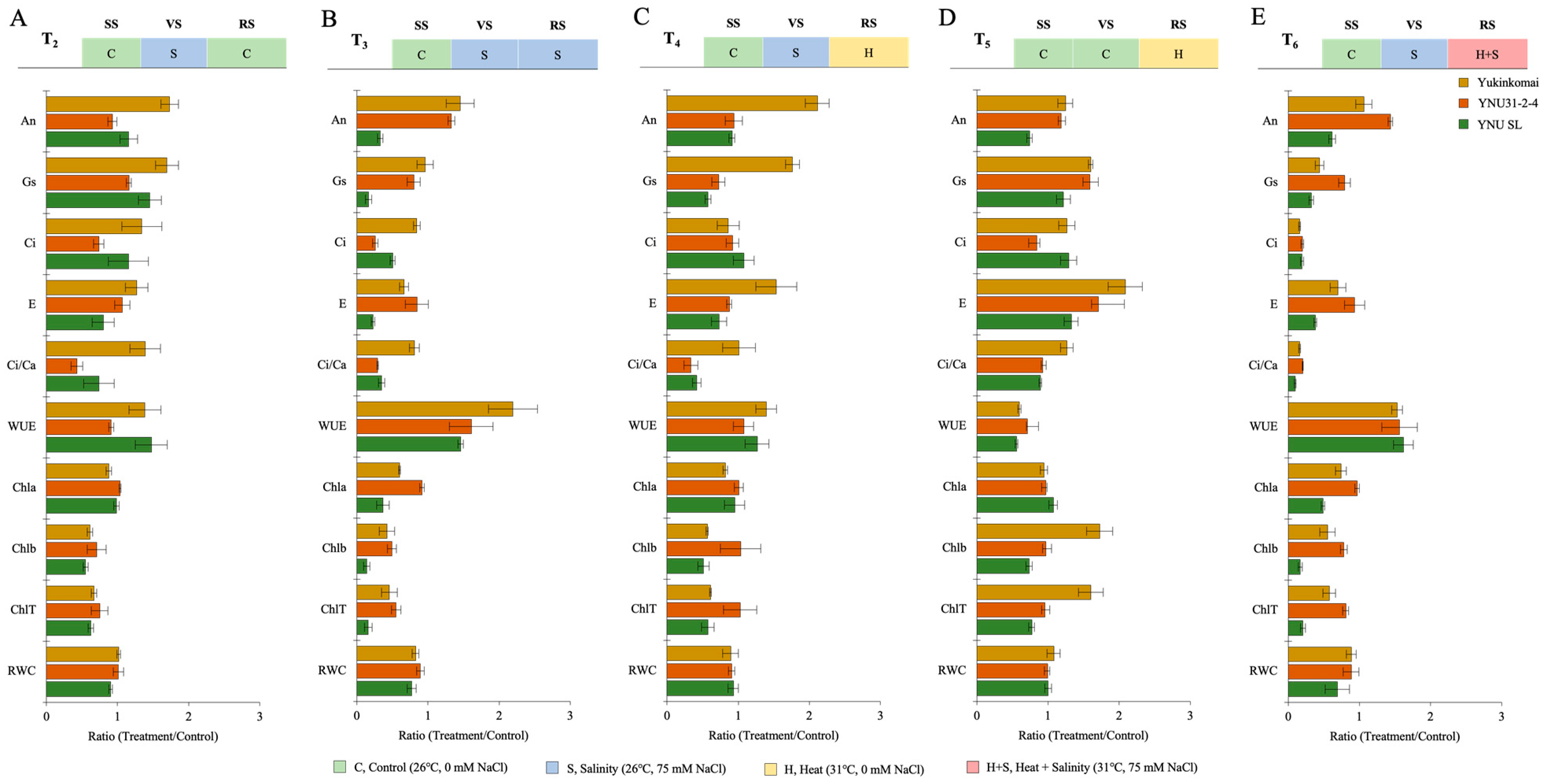
2.2. YNU31-2-4 Rice Genotype Defense Photosynthetic Activity by Higher Chlorophyll Content and Stomatal Conductance from Heat and Salinity Stress Damage
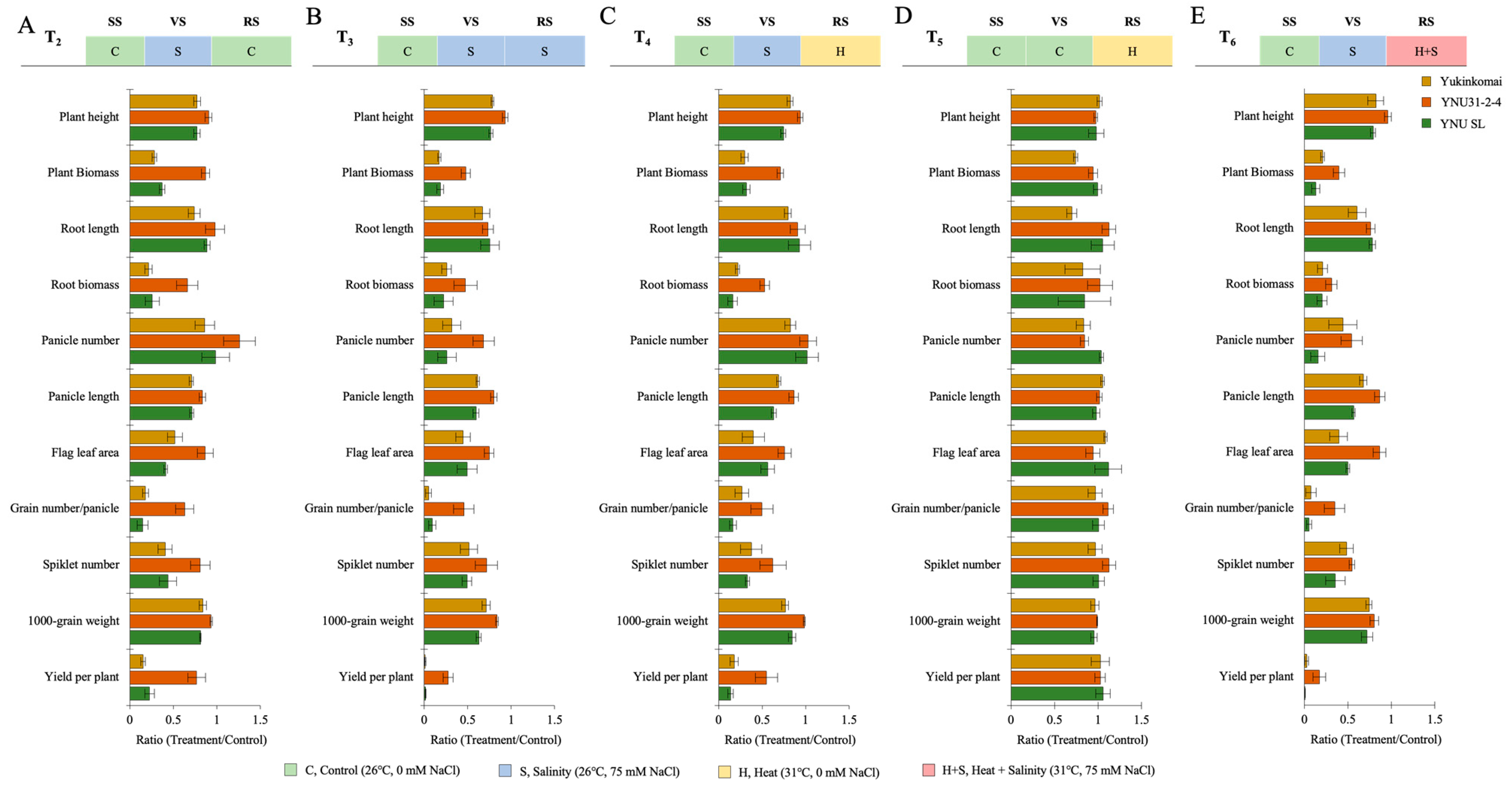
2.3. YNU31-2-4 Genotype Shows Higher Plant Growth and Yield Performance under Salt, Heat, and Heat + Salt Stress Treatments
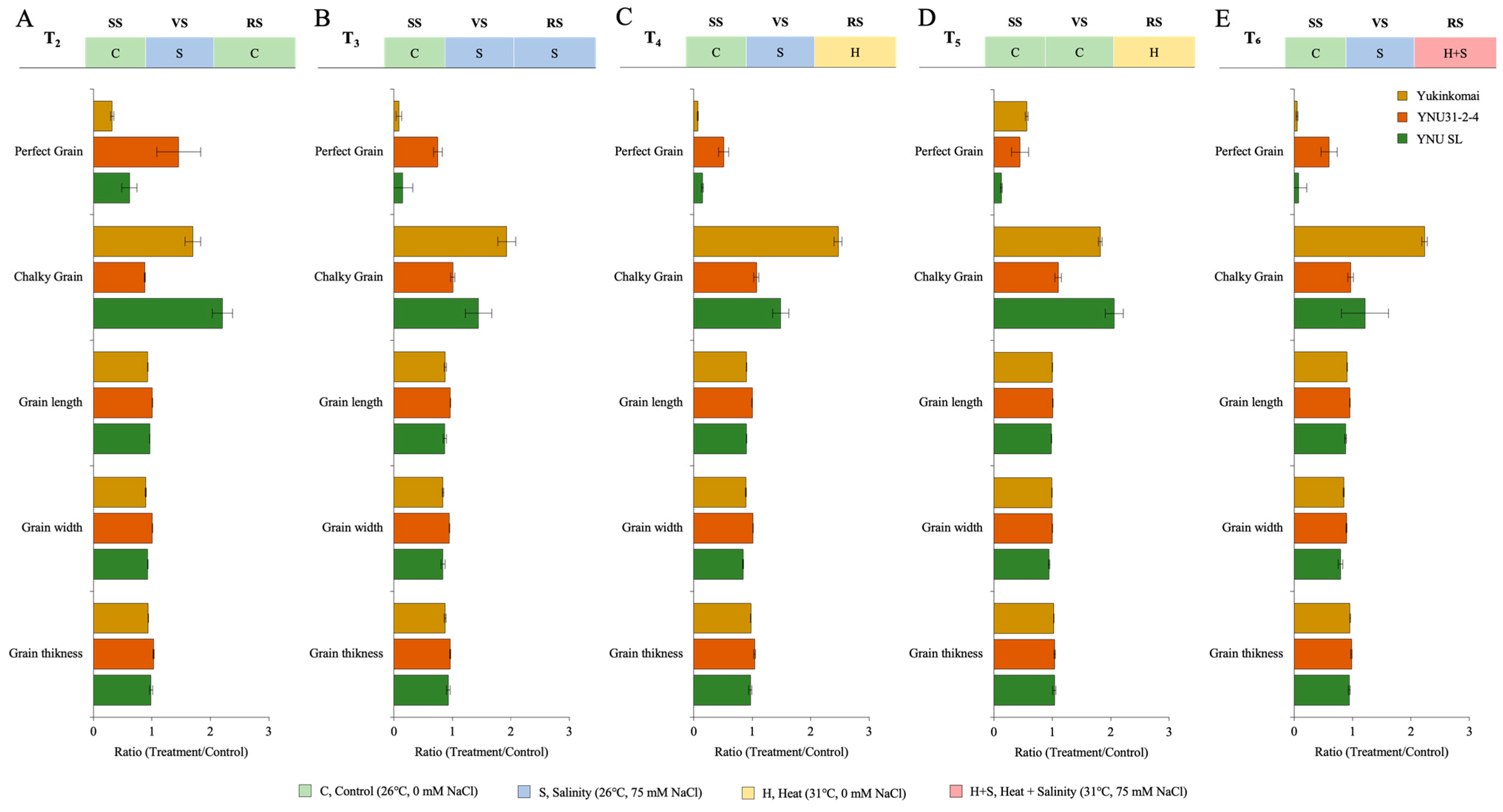
2.4. YNU31-2-4 Genotype Has High Perfect Grain Potential under Salinity and Heat and Salinity Stress Treatments
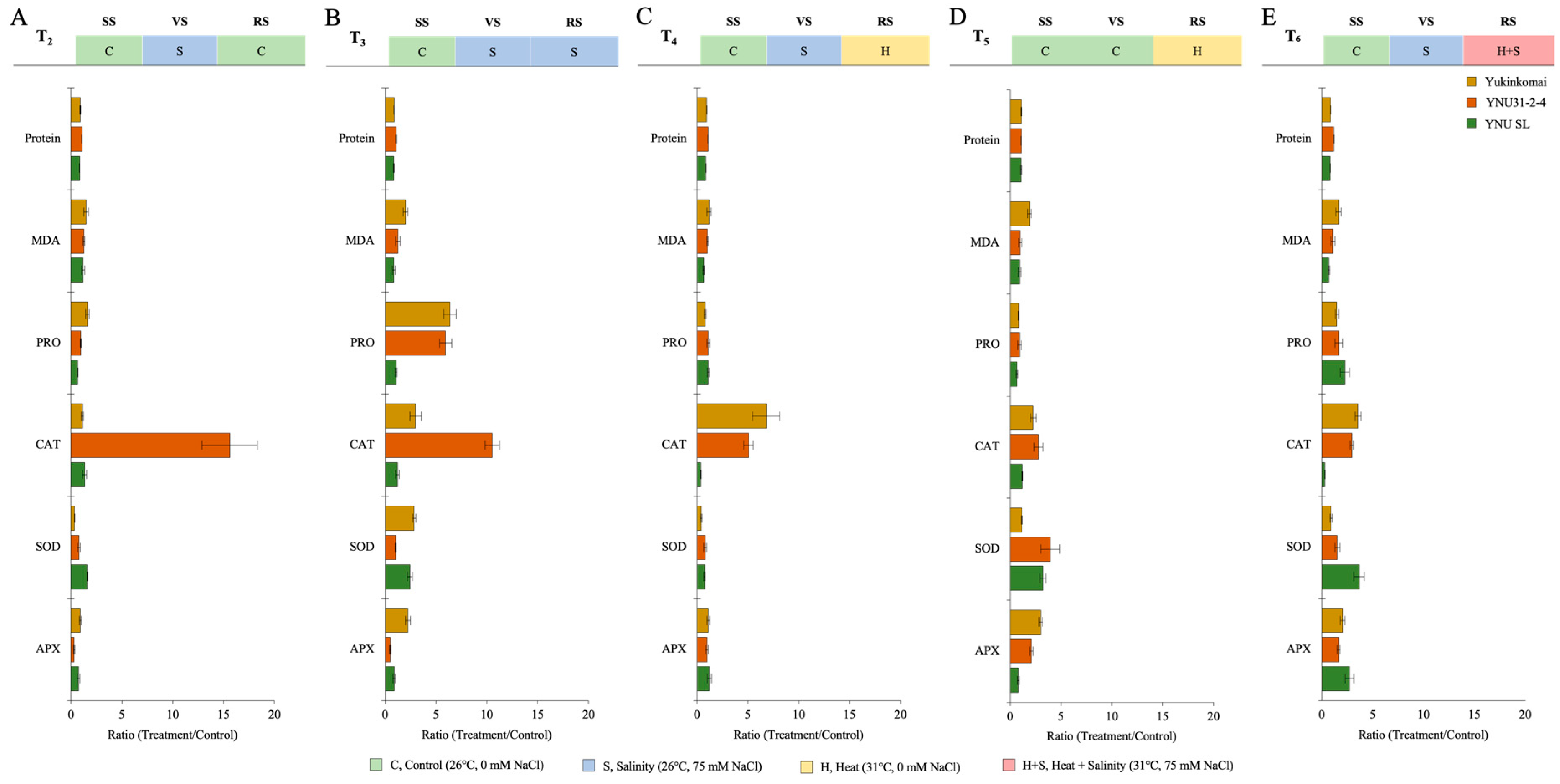
2.5. YNU31-2-4 Genotype Increased CAT Activity under Stress Conditions
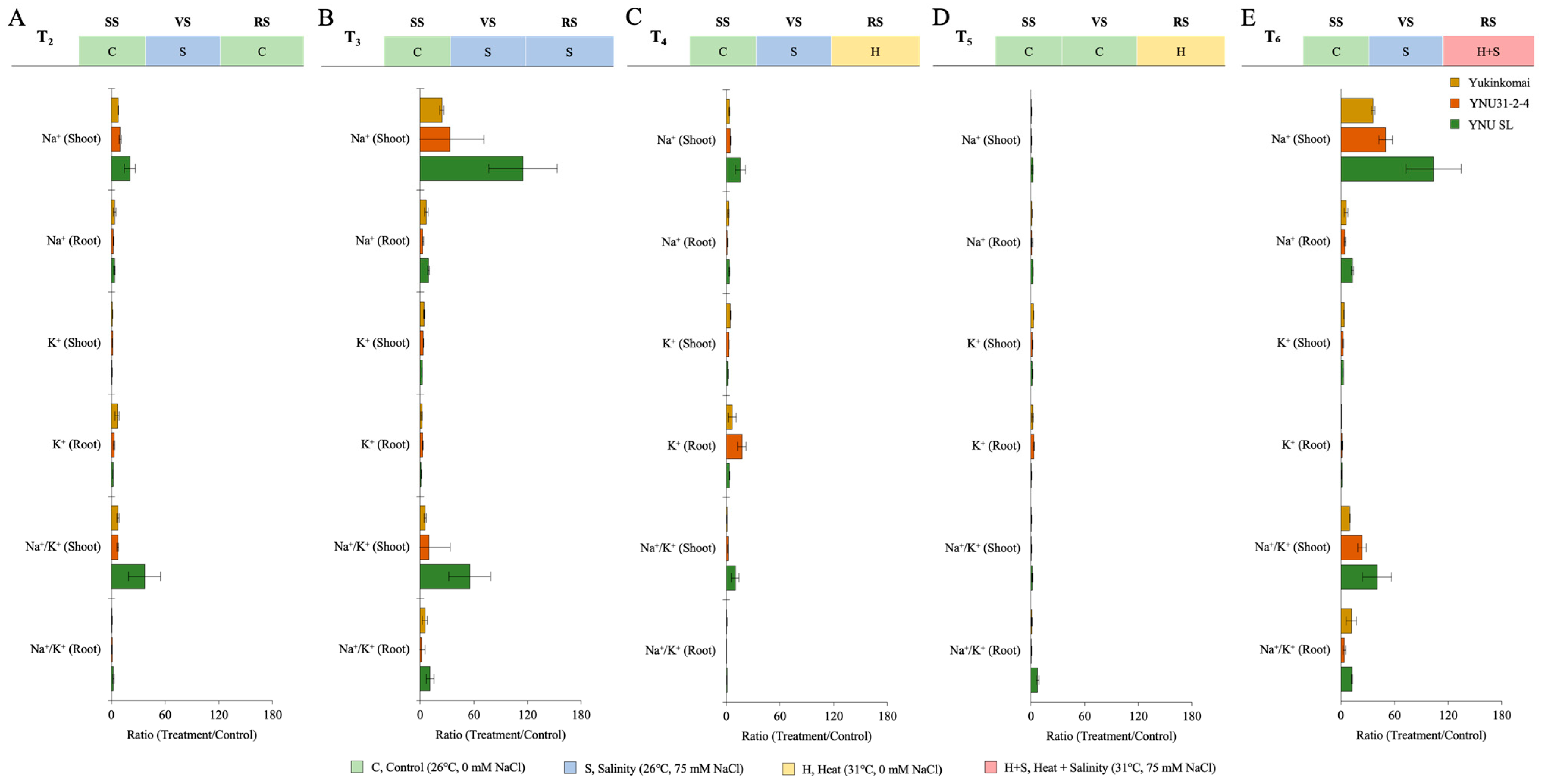
2.6. YNU31-2-4 Genotype Has Lower Na+ Ion Accumulation under Salinity Stress Conditions
2.7. Salinity Reduces Glucose Accumulation in Dry Seeds, but the YNU31-2-4 Genotype Has Higher Glucose Accumulation under Salinity and Heat and Salinity Stress
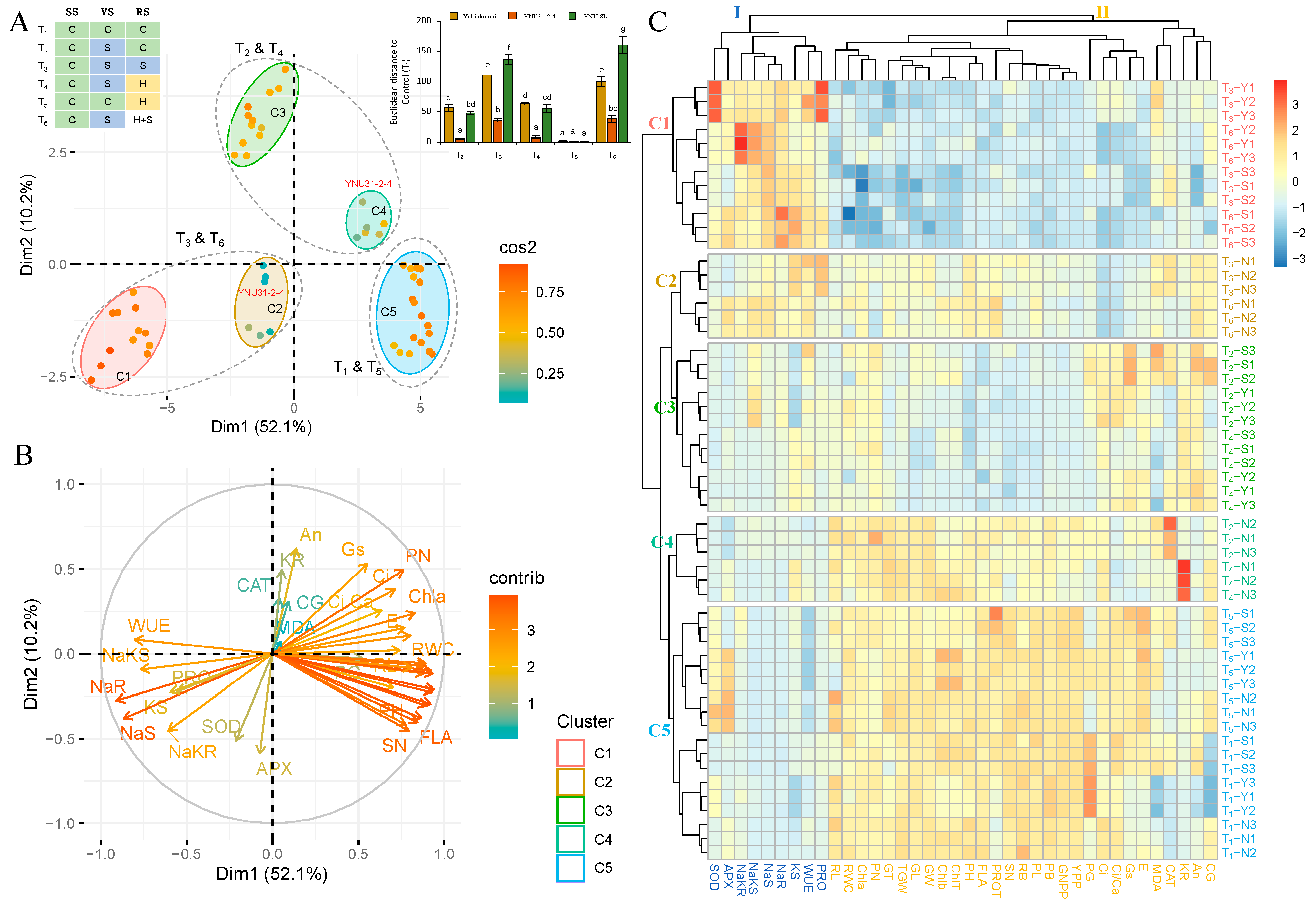
2.8. YNU31-2-4 Genotype Showed Higher Salinity Stress and Heat and Salinity Stress Tolerance according to the Overall Result Evaluation by PCA and HCA Clustering
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Sampling, Phenotyping, and Harvesting Determination
4.3. Chlorophyll Content and Leaf Gas Exchange Measurements
4.4. Malondialdehyde (MDA), Proline, Protein, and Antioxidant Enzyme Activities
4.5. Na+ and K+ Measurement
4.6. Measurement of Glucose Content
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| An | gs | Ci | E | Ci/Ca | ||
|---|---|---|---|---|---|---|
| T1 | Yukinkomai | 27.24 ± 2.143 b | 0.262 ± 0.008 ce | 195.094 ± 15.722 ce | 0.005 ± 0.001 cd | 0.502 ± 0.038 fg |
| YNU31-2-4 | 36.565 ± 0.735 df | 0.294 ± 0.014 de | 270.645 ± 16.142 i | 0.006 ± 0.001 d | 0.646 ± 0.024 hi | |
| YNU SL | 51.712 ± 4.159 hi | 0.447 ± 0.016 g | 186.313 ± 17.761 cd | 0.008 ± 0.000 fg | 0.762 ± 0.021 j | |
| T2 | Yukinkomai | 47.030 ± 0.903 gh | 0.444 ± 0.040 g | 258.815 ± 32.877 hi | 0.006 ± 0.001 def | 0.692 ± 0.051 ij |
| YNU31-2-4 | 35.554 ± 3.414 bde | 0.341 ± 0.027 ef | 198.411 ± 9.735 ce | 0.006 ± 0.001 def | 0.275 ± 0.043 cd | |
| YNU SL | 59.379 ± 3.999 j | 0.651 ± 0.033 i | 213.957 ± 10.706 deg | 0.006 ± 0.001 def | 0.563 ± 0.029 gh | |
| T3 | Yukinkomai | 39.280 ± 2.290 ef | 0.251 ± 0.024 cd | 164.123 ± 9.049 c | 0.003 ± 0.000 ac | 0.404 ± 0.039 ef |
| YNU31-2-4 | 48.602 ± 2.046 gh | 0.235 ± 0.017 cd | 69.973 ± 7.970 ab | 0.005 ± 0.001 bcd | 0.190 ± 0.014 bc | |
| YNU SL | 17.033 ± 1.216 a | 0.073 ± 0.020 a | 93.351 ± 3.303 b | 0.002 ± 0.000 a | 0.266 ± 0.027 cd | |
| T4 | Yukinkomai | 57.288 ± 1.646 ij | 0.462 ± 0.026 g | 166.029 ± 16.803 c | 0.008 ± 0.001 eg | 0.502 ± 0.074 fg |
| YNU31-2-4 | 34.367 ± 5.005 cde | 0.212 ± 0.027 bc | 247.628 ± 14.765 gi | 0.005 ± 0.001 cd | 0.214 ± 0.055 bd | |
| YNU SL | 47.027 ± 2.426 gh | 0.258 ± 0.026 cd | 199.569 ± 17.701 cef | 0.006 ± 0.001 de | 0.317 ± 0.039 de | |
| T5 | Yukinkomai | 33.770 ± 1.422 bde | 0.420 ± 0.011 fg | 245.587 ± 1.999 gi | 0.011 ± 0.000 h | 0.633 ± 0.007 hi |
| YNU31-2-4 | 43.255 ± 1.740 fg | 0.468 ± 0.018 fh | 228.309 ± 3.413 egh | 0.010 ± 0.001 gh | 0.598 ± 0.010 gi | |
| YNU SL | 38.179 ± 1.264 df | 0.546 ± 0.062 h | 239.284 ± 2.934 fgi | 0.010 ± 0.000 h | 0.681 ± 0.020 ij | |
| T6 | Yukinkomai | 28.802 ± 0.807 bc | 0.115 ± 0.018 a | 30.912 ± 2.471 a | 0.004 ± 0.000 ac | 0.080 ± 0.005 a |
| YNU31-2-4 | 52.535 ± 1.267 hj | 0.233 ± 0.029 cd | 53.508 ± 2.453 ab | 0.005 ± 0.000 cd | 0.131 ± 0.010 ab | |
| YNU SL | 31.766 ± 0.875 bd | 0.144 ± 0.009 ab | 35.912 ± 1.767 a | 0.003 ± 0.000 ab | 0.074 ± 0.009 a |
| WUE | Chla | Chlb | ChlT | RWC | ||
|---|---|---|---|---|---|---|
| T1 | Yukinkomai | 5356.450 ± 222.552 acd | 173.194 ± 0.716 e | 153.654 ± 3.269 fgh | 151.35 ± 2.741 fghi | 82.158 ± 4.546 bcd |
| YNU31-2-4 | 6420.039 ± 658.935 bce | 168.620 ± 3.417 e | 214.242 ± 22.012 ik | 199.261 ± 17.123 ijl | 86.986 ± 2.844 d | |
| YNU SL | 6525.314 ± 363.796 bce | 164.792 ± 4.350 de | 236.165 ± 21.879 jk | 216.246 ± 16.866 kl | 81.667 ± 2.357 cd | |
| T2 | Yukinkomai | 7405.811 ± 1249.448 deg | 152.335 ± 6.312 de | 93.577 ± 5.494 bcde | 101.040 ± 4.813 cde | 83.397 ± 5.656 cd |
| YNU31-2-4 | 5862.909 ± 808.315 ade | 174.102 ± 1.434 e | 148.976 ± 12.820 eg | 147.740 ± 10.525 egh | 87.774 ± 3.754 d | |
| YNU SL | 9635.331 ± 1925.732 fgi | 157.486 ± 6.956 de | 131.329 ± 33.090 dg | 136.017 ± 32.179 dg | 75.797 ± 9.239 bcd | |
| T3 | Yukinkomai | 11,742.127 ± 1884.126 i | 104.073 ± 1.979 b | 65.102 ± 15.081 ac | 69.014 ± 15.757 ac | 68.004 ± 7.417 ac |
| YNU31-2-4 | 10,252.976 ± 1707.586 gi | 154.695 ± 7.976 de | 105.15 ± 3.273 cdef | 109.415 ± 3.497 cdef | 77.548 ± 2.260 bcd | |
| YNU SL | 9511.533 ± 314.831 fgi | 59.195 ± 16.011 a | 33.682 ± 12.376 a | 35.349 ± 13.308 a | 65.027 ± 7.742 ab | |
| T4 | Yukinkomai | 7455.155 ± 686.156 deg | 141.545 ± 4.980 cd | 86.637 ± 0.722 ad | 92.099 ± 0.676 bcd | 73.158 ± 6.835 ad |
| YNU31-2-4 | 6860.766 ± 627.490 cef | 169.760 ± 7.829 e | 217.640 ± 37.459 ik | 201.802 ± 28.990 jl | 79.069 ± 4.280 bcd | |
| YNU SL | 8246.898 ± 1111.925 egh | 151.440 ± 14.435 ce | 119.849 ± 10.642 cdg | 123.383 ± 12.547 dg | 78.278 ± 2.683 bcd | |
| T5 | Yukinkomai | 3198.187 ± 24.146 a | 163.181 ± 8.136 de | 265.235 ± 28.815 k | 242.345 ± 25.235 l | 88.549 ± 2.595 d |
| YNU31-2-4 | 4514.127 ± 518.664 ac | 163.873 ± 2.306 de | 206.358 ± 13.852 hij | 189.627 ± 14.032 hjk | 86.706 ± 2.406 d | |
| YNU SL | 3646.112 ± 265.588 ab | 172.111 ± 0.592 e | 173.085 ± 9.185 gi | 166.777 ± 7.280 gj | 84.553 ± 2.596 cd | |
| T6 | Yukinkomai | 8192.701 ± 337.352 degh | 128.363 ± 12.348 | 84.637 ± 17.380 ad | 87.566 ± 14.384 bcd | 72.563 ± 1.532 ad |
| YNU31-2-4 | 9935.842 ± 518.927 gi | 163.280 ± 2.355 | 168.113 ± 27.65 gi | 161.552 ± 22.384 gj | 76.905 ± 8.693 bcd | |
| YNU SL | 10,534.637 ± 282.673 hi | 78.732 ± 7.221 | 39.708 ± 4.669 ab | 44.599 ± 4.878 ab | 57.663 ± 11.171 a |
| Plant Height | Plant Biomass | Root Length | Root Biomass | Panicle Number | Panicle Length | ||
|---|---|---|---|---|---|---|---|
| T1 | Yukinkomai | 116.120 ± 4.348 b | 125.366 ± 4.282 k | 34.440 ± 1.766 h | 17.792 ± 2.179 eg | 22.400 ± 1.517 f | 17.791 ± 0.335 h |
| YNU31-2-4 | 125.480 ± 2.777 c | 112.752 ± 3.730 j | 34.800 ± 1.483 hi | 19.830 ± 1.598 fg | 21.800 ± 0.837 f | 18.313 ± 0.591 hi | |
| YNU SL | 120.540 ± 3.957 bc | 104.315 ± 2.263 ij | 28.200 ± 2.168 df | 16.127 ± 2.948 def | 21.000 ± 1.581 f | 18.929 ± 0.634 i | |
| T2 | Yukinkomai | 89.620 ± 2.309 a | 35.323 ± 2.772 ce | 25.440 ± 1.901 ade | 3.774 ± 0.522 a | 19.200 ± 1.643 ef | 12.565 ± 0.237 de |
| YNU31-2-4 | 113.480 ± 3.989 b | 98.184 ± 3.310 hi | 33.970 ± 2.815 gh | 13.127 ± 2.666 cd | 27.400 ± 3.209 g | 15.281 ± 0.238 fg | |
| YNU SL | 92.740 ± 3.686 a | 38.495 ± 3.370 de | 25.000 ± 2.000 ade | 4.004 ± 0.641 a | 20.600 ± 2.793 f | 13.500 ± 0.141 e | |
| T3 | Yukinkomai | 91.340 ± 1.685 a | 21.620 ± 2.534 ab | 22.900 ± 1.949 abc | 4.520 ± 0.820 a | 7.000 ± 1.732 ab | 10.953 ± 0.345 ab |
| YNU31-2-4 | 116.680 ± 4.218 bc | 53.845 ± 4.672 f | 25.400 ± 1.380 ade | 9.350 ± 2.448 bc | 14.800 ± 2.280 de | 14.639 ± 0.232 f | |
| YNU SL | 92.320 ± 4.322 a | 19.351 ± 4.236 ab | 21.200 ± 2.308 a | 3.359 ± 1.300 a | 5.600 ± 2.702 ab | 11.282 ± 0.824 ac | |
| T4 | Yukinkomai | 95.400 ± 1.319 a | 37.731 ± 6.384 de | 27.420 ± 1.675 cdf | 3.856 ± 0.137 a | 18.400 ± 1.140 ef | 12.307 ± 0.358 cd |
| YNU31-2-4 | 117.760 ± 4.091 bc | 79.920 ± 4.778 g | 31.600 ± 2.074 fh | 10.491 ± 1.124 c | 22.400 ± 1.342 f | 15.827 ± 0.685 g | |
| YNU SL | 89.640 ± 1.713 a | 33.379 ± 4.449 cd | 26.000 ± 1.837 bde | 2.584 ± 0.975 a | 21.200 ± 1.789 f | 11.965 ± 0.272 bcd | |
| T5 | Yukinkomai | 118.140 ± 1.435 bc | 93.131 ± 3.345 h | 24.000 ± 1.581 ad | 14.375 ± 2.385 de | 18.600 ± 1.673 ef | 18.721 ± 0.137 hi |
| YNU31-2-4 | 122.300 ± 2.432 bc | 106.441 ± 5.899 ij | 39.200 ± 2.588 i | 20.162 ± 1.942 g | 18.400 ± 0.894 ef | 18.595 ± 0.165 hi | |
| YNU SL | 117.720 ± 8.164 bc | 103.708 ± 3.477 ij | 29.600 ± 2.881 efg | 12.959 ± 1.989 cd | 21.800 ± 1.304 f | 18.517 ± 0.287 hi | |
| T6 | Yukinkomai | 95.120 ± 8.007 a | 25.953 ± 2.461 bc | 20.800 ± 2.864 a | 3.683 ± 1.056 a | 10.000 ± 3.937 bc | 12.018 ± 0.602 bcd |
| YNU31-2-4 | 120.340 ± 2.791 bc | 44.673 ± 6.520 ef | 26.440 ± 1.170 bde | 6.147 ± 1.218 ab | 11.800 ± 2.588 cd | 15.825 ± 0.812 g | |
| YNU SL | 95.180 ± 2.194 a | 13.672 ± 4.876 a | 22.000 ± 1.581 ab | 3.223 ± 0.703 a | 3.200 ± 1.643 a | 10.716 ± 0.612 a |
| Flag Leaf Area | Grain Number per Panicle | Spikelet Number | 1000-Grain Weight | Yield per Plant | ||
|---|---|---|---|---|---|---|
| T1 | Yukinkomai | 48.637 ± 2.186 bd | 75.260 ± 5.974 gh | 76.894 ± 6.279 e | 26.677 ± 0.911 jk | 40.502 ± 3.234 gh |
| YNU31-2-4 | 52.717 ± 2.521 d | 66.495 ± 3.762 g | 71.588 ± 4.566 e | 28.055 ± 0.298 k | 36.941 ± 1.740 g | |
| YNU SL | 46.600 ± 2.623 bd | 80.511 ± 6.279 h | 82.908 ± 5.714 e | 25.673 ± 0.645 ij | 40.822 ± 1.943 gh | |
| T2 | Yukinkomai | 25.153 ± 3.798 a | 13.523 ± 2.564 ac | 30.755 ± 3.580 ab | 22.340 ± 0.352 efg | 6.069 ± 0.678 cd |
| YNU31-2-4 | 45.527 ± 2.781 bd | 41.892 ± 5.801 f | 57.665 ± 7.070 d | 26.186 ± 0.242 ik | 28.347 ± 4.024 f | |
| YNU SL | 19.183 ± 1.207 a | 11.775 ± 4.717 ab | 36.068 ± 7.582 ab | 20.837 ± 0.577 cdf | 9.211 ± 1.930 d | |
| T3 | Yukinkomai | 21.560 ± 3.175 a | 3.677 ± 2.505 a | 39.364 ± 6.884 abc | 18.918 ± 0.671 bc | 0.430 ± 0.288 ab |
| YNU31-2-4 | 39.233 ± 1.405 b | 30.127 ± 7.227 de | 50.889 ± 6.692 cd | 23.421 ± 0.242 gh | 10.024 ± 1.773 d | |
| YNU SL | 22.720 ± 4.095 a | 7.447 ± 3.387 a | 40.917 ± 7.252 abc | 16.083 ± 0.520 a | 0.606 ± 0.265 ab | |
| T4 | Yukinkomai | 19.520 ± 6.323 a | 19.751 ± 4.089 bcd | 28.338 ± 7.168 a | 20.399 ± 0.401 bde | 7.189 ± 1.643 d |
| YNU31-2-4 | 39.973 ± 3.470 bc | 32.939 ± 7.145 ef | 44.211 ± 8.162 bd | 27.615 ± 0.511 jk | 20.247 ± 4.246 e | |
| YNU SL | 26.173 ± 2.317 a | 13.260 ± 3.501 ac | 27.477 ± 1.645 a | 21.715 ± 0.537 dfg | 5.619 ± 1.221 bcd | |
| T5 | Yukinkomai | 52.857 ± 3.170 d | 72.552 ± 4.786 gh | 74.229 ± 4.866 e | 25.744 ± 0.671 ij | 41.552 ± 4.508 gh |
| YNU31-2-4 | 49.530 ± 1.924 cd | 74.066 ± 2.634 gh | 80.502 ± 3.817 e | 27.760 ± 0.147 k | 37.811 ± 1.754 gh | |
| YNU SL | 51.910 ± 4.556 d | 80.670 ± 2.711 h | 83.154 ± 2.068 e | 24.467 ± 0.298 hi | 43.067 ± 1.837 h | |
| T6 | Yukinkomai | 19.093 ± 4.575 a | 5.503 ± 4.605 a | 37.059 ± 6.249 ab | 19.809 ± 0.632 bd | 1.135 ± 0.787 ac |
| YNU31-2-4 | 45.493 ± 3.169 bd | 23.268 ± 8.025 ce | 39.022 ± 3.049 abc | 22.557 ± 1.242 fh | 6.386 ± 2.514 cd | |
| YNU SL | 23.233 ± 0.907 a | 4.300 ± 2.273 a | 29.030 ± 7.712 a | 18.475 ± 1.305 b | 0.230 ± 0.200 a |
| Perfect Grain | Chalky Grain | Grain Length | Grain Width | Grain Thickness | ||
|---|---|---|---|---|---|---|
| T1 | Yukinkomai | 62.350 ± 0.071 f | 34.533 ± 0.321 a | 5.127 ± 0.016 h | 2.737 ± 0.006 g | 2.007 ± 0.006 ghi |
| YNU31-2-4 | 16.567 ± 3.353 bd | 83.100 ± 3.504 fhj | 5.080 ± 0.010 h | 2.853 ± 0.006 hi | 2.057 ± 0.015 ij | |
| YNU SL | 53.433 ± 3.355 f | 44.167 ± 3.528 ab | 5.073 ± 0.006 h | 2.770 ± 0.010 gh | 1.967 ± 0.058 eh | |
| T2 | Yukinkomai | 19.900 ± 1.637 cd | 58.667 ± 5.033 bcd | 4.750 ± 0.020 de | 2.440 ± 0.017 d | 1.880 ± 0.000 bcd |
| YNU31-2-4 | 24.133 ± 1.159 de | 72.667 ± 3.512 deh | 5.100 ± 0.000 h | 2.867 ± 0.006 hi | 2.117 ± 0.006 jk | |
| YNU SL | 32.867 ± 5.129 e | 97.333 ± 15.144 j | 4.873 ± 0.012 eg | 2.567 ± 0.021 e | 1.933 ± 0.006 def | |
| T3 | Yukinkomai | 5.433 ± 2.892 a | 66.600 ± 5.717 cefg | 4.510 ± 0.095 ac | 2.300 ± 0.026 ab | 1.760 ± 0.035 a |
| YNU31-2-4 | 12.467 ± 1.686 abc | 83.667 ± 1.650 ghj | 4.897 ± 0.021 fg | 2.710 ± 0.000 fg | 1.983 ± 0.006 fh | |
| YNU SL | 8.000 ± 10.046 ab | 63.933 ± 8.701 ce | 4.420 ± 0.140 a | 2.327 ± 0.090 bc | 1.830 ± 0.017 ab | |
| T4 | Yukinkomai | 4.167 ± 0.321 a | 85.233 ± 1.595 hj | 4.603 ± 0.006 bc | 2.433 ± 0.012 cd | 1.957 ± 0.006 efg |
| YNU31-2-4 | 8.500 ± 1.609 ab | 89.000 ± 1.179 hj | 5.067 ± 0.006 h | 2.883 ± 0.006 i | 2.140 ± 0.010 k | |
| YNU SL | 7.867 ± 0.351 ab | 65.567 ± 1.79 cef | 4.547 ± 0.015 ac | 2.333 ± 0.021 bd | 1.900 ± 0.010 be | |
| T5 | Yukinkomai | 35.033 ± 1.401 e | 63.000 ± 0.721 ce | 5.133 ± 0.006 h | 2.713 ± 0.015 fg | 2.057 ± 0.006 ij |
| YNU31-2-4 | 7.433 ± 2.228 ab | 91.267 ± 2.228 ij | 5.103 ± 0.012 h | 2.850 ± 0.000 hi | 2.143 ± 0.006 k | |
| YNU SL | 6.767 ± 0.850 ab | 90.967 ± 0.950 ij | 5.000 ± 0.000 gh | 2.623 ± 0.015 ef | 2.037 ± 0.006 hi | |
| T6 | Yukinkomai | 3.000 ± 1.000 a | 77.133 ± 0.902 ehi | 4.637 ± 0.021 cd | 2.330 ± 0.030 bc | 1.920 ± 0.017 cef |
| YNU31-2-4 | 9.933 ± 0.503 abc | 80.567 ± 1.007 ehj | 4.850 ± 0.017 ef | 2.557 ± 0.012 e | 2.020 ± 0.010 ghi | |
| YNU SL | 4.167 ± 7.217 a | 53.633 ± 13.359 bc | 4.470 ± 0.092 ab | 2.200 ± 0.104 a | 1.857 ± 0.060 bc |
| Protein | MDA | PRO | CAT | SOD | APX | ||
|---|---|---|---|---|---|---|---|
| T1 | Yukinkomai | 3050.106 ± 68.337 bd | 6.237 ± 0.453 a | 0.533 ± 0.024 ad | 2.401 ± 0.337 ab | 23.676 ± 1.074 ef | 157.972 ± 6.912 bc |
| YNU31-2-4 | 2938.287 ± 78.909 abc | 9.281 ± 0.901 bd | 0.409 ± 0.055 a | 2.045 ± 0.013 a | 10.386 ± 0.913 abc | 254.73 ± 14.490 d | |
| YNU SL | 3361.854 ± 39.895 ef | 11.452 ± 1.137 df | 0.739 ± 0.034 bd | 15.632 ± 1.557 e | 7.023 ± 0.267 a | 169.462 ± 20.805 bc | |
| T2 | Yukinkomai | 2782.591 ± 65.920 ab | 9.274 ± 0.980 bd | 0.859 ± 0.066 d | 2.671 ± 0.430 ab | 8.539 ± 0.103 a | 145.742 ± 7.114 bc |
| YNU31-2-4 | 3178.910 ± 142.256 cdf | 11.741 ± 0.979 df | 0.385 ± 0.039 a | 31.873 ± 5.326 h | 8.106 ± 0.773 a | 79.500 ± 11.281 a | |
| YNU SL | 2868.295 ± 74.170 ab | 13.467 ± 1.310 f | 0.495 ± 0.018 abc | 20.659 ± 1.769 fg | 11.198 ± 0.645 abc | 125.077 ± 10.497 ab | |
| T3 | Yukinkomai | 2703.326 ± 68.249 a | 12.519 ± 0.943 ef | 3.401 ± 0.301 g | 7.077 ± 0.516 bd | 67.569 ± 1.149 h | 354.956 ± 48.535 ef |
| YNU31-2-4 | 3144.940 ± 49.216 cde | 11.607 ± 0.862 df | 2.411 ± 0.094 f | 21.514 ± 1.351 g | 10.682 ± 0.676 abc | 125.488 ± 11.578 ab | |
| YNU SL | 2833.404 ± 124.041 ab | 9.667 ± 0.757 bd | 0.787 ± 0.047 cd | 18.677 ± 0.735 eg | 17.053 ± 1.275 cde | 147.717 ± 5.814 bc | |
| T4 | Yukinkomai | 2853.503 ± 74.106 ab | 7.393 ± 1.054 ab | 0.430 ± 0.060 ab | 16.007 ± 0.795 ef | 9.761 ± 1.454 ab | 176.568 ± 25.973 bc |
| YNU31-2-4 | 3224.983 ± 84.522 df | 9.571 ± 0.334 bd | 0.446 ± 0.023 ab | 10.385 ± 0.903 d | 8.358 ± 1.377 a | 251.031 ± 21.805 d | |
| YNU SL | 2844.215 ± 55.318 ab | 7.504 ± 0.862 ab | 0.809 ± 0.034 cd | 5.658 ± 0.450 abc | 5.236 ± 0.495 a | 204.629 ± 8.371 cd | |
| T5 | Yukinkomai | 3363.057 ± 78.570 ef | 11.763 ± 0.312 df | 0.446 ± 0.033 ab | 5.408 ± 0.216 abc | 27.371 ± 0.74 f | 475.974 ± 18.797 gh |
| YNU31-2-4 | 3238.358 ± 50.660 df | 9.178 ± 0.640 bd | 0.378 ± 0.032 a | 5.697 ± 0.895 ad | 40.458 ± 7.777 g | 527.775 ± 16.803 h | |
| YNU SL | 3650.955 ± 191.165 g | 10.659 ± 0.634 de | 0.497 ± 0.073 abc | 18.724 ± 1.190 eg | 22.567 ± 1.508 df | 134.957 ± 4.541 ab | |
| T6 | Yukinkomai | 2683.227 ± 58.142 a | 10.200 ± 0.954 cde | 0.795 ± 0.090 cd | 8.535 ± 1.023 cd | 21.685 ± 1.503 df | 322.044 ± 24.748 e |
| YNU31-2-4 | 3431.423 ± 94.090 fg | 10.000 ± 0.794 bde | 0.667 ± 0.060 ad | 6.048 ± 0.376 ad | 15.865 ± 1.491 bd | 418.579 ± 38.202 fg | |
| YNU SL | 2762.774 ± 37.099 a | 7.882 ± 1.148 abc | 1.664 ± 0.264 e | 4.710 ± 0.679 abc | 25.791 ± 3.874 f | 457.374 ± 20.386 g |
| Na⁺ (Shoot) | Na⁺ (Root) | K⁺ (Shoot) | K⁺ (Root) | Na⁺/K⁺ (Shoot) | Na⁺/K⁺ (Root) | ||
|---|---|---|---|---|---|---|---|
| T1 | Yukinkomai | 7.153 ± 0.307 ab | 6.443 ± 1.821 ab | 36.999 ± 0.320 a | 9.979 ± 3.646 ab | 0.193 ± 0.007 ab | 0.756 ± 0.486 ab |
| YNU31-2-4 | 4.015 ± 0.461 a | 5.815 ± 0.209 a | 73.580 ± 10.387 bc | 9.540 ± 2.286 ab | 0.055 ± 0.006 a | 0.638 ± 0.180 ab | |
| YNU SL | 2.395 ± 0.623 a | 4.278 ± 0.475 a | 103.244 ± 4.470 d | 19.745 ± 3.525 bce | 0.024 ± 0.007 a | 0.224 ± 0.065 a | |
| T2 | Yukinkomai | 52.891 ± 3.861 e | 21.774 ± 3.349 c | 39.277 ± 7.142 a | 58.186 ± 6.054 fg | 1.370 ± 0.204 h | 0.374 ± 0.042 a |
| YNU31-2-4 | 38.461 ± 1.138 de | 12.693 ± 1.506 ac | 101.262 ± 11.89 d | 25.517 ± 1.060 ce | 0.383 ± 0.035 bc | 0.496 ± 0.039 a | |
| YNU SL | 46.925 ± 0.740 e | 14.504 ± 2.699 ac | 59.984 ± 8.697 ab | 33.589 ± 0.869 e | 0.793 ± 0.115 d | 0.431 ± 0.076 a | |
| T3 | Yukinkomai | 173.428 ± 10.181 g | 42.970 ± 2.422 d | 163.663 ± 20.843 gh | 14.358 ± 1.173 acd | 1.074 ± 0.185 fg | 3.008 ± 0.330 d |
| YNU31-2-4 | 131.200 ± 13.936 f | 17.729 ± 0.633 bc | 237.981 ± 2.467 i | 26.135 ± 3.356 ce | 0.551 ± 0.062 cd | 0.687 ± 0.104 ab | |
| YNU SL | 259.658 ± 12.609 j | 38.881 ± 1.899 d | 215.377 ± 9.604 i | 17.085 ± 2.165 acd | 1.209 ± 0.112 gh | 2.301 ± 0.323 cd | |
| T4 | Yukinkomai | 24.048 ± 3.714 bcd | 14.335 ± 0.665 ac | 165.506 ± 2.965 gh | 54.866 ± 12.976 f | 0.145 ± 0.024 ab | 0.270 ± 0.055 a |
| YNU31-2-4 | 18.563 ± 2.238 ac | 6.056 ± 1.404 c | 185.275 ± 8.845 h | 158.934 ± 10.385 h | 0.100 ± 0.015 a | 0.038 ± 0.008 a | |
| YNU SL | 34.999 ± 3.478 ce | 14.026 ± 1.653 ac | 162.721 ± 1.295 gh | 69.164 ± 3.512 g | 0.215 ± 0.023 ab | 0.203 ± 0.015 a | |
| T5 | Yukinkomai | 2.655 ± 0.138 a | 6.403 ± 0.243 ab | 100.987 ± 5.386 cd | 14.312 ± 2.986 acd | 0.026 ± 0.000 a | 0.459 ± 0.086 a |
| YNU31-2-4 | 2.129 ± 0.216 a | 6.159 ± 3.117 ab | 108.075 ± 4.447 de | 28.459 ± 0.918 de | 0.020 ± 0.002 a | 0.215 ± 0.102 a | |
| YNU SL | 3.720 ± 0.494 a | 7.716 ± 1.824 ab | 124.550 ± 3.831 de | 4.760 ± 0.505 a | 0.030 ± 0.003 a | 1.626 ± 0.367 bc | |
| T6 | Yukinkomai | 256.632 ± 3.638 j | 35.668 ± 3.350 g | 132.577 ± 7.727 ef | 5.065 ± 0.248 a | 1.939 ± 0.084 i | 7.062 ± 0.886 e |
| YNU31-2-4 | 198.885 ± 8.74 h | 26.292 ± 3.969 e | 155.934 ± 8.892 fg | 12.507 ± 1.528 ac | 1.280 ± 0.127 gh | 2.140 ± 0.503 cd | |
| YNU SL | 235.424 ± 10.79 i | 55.548 ± 9.217 f | 268.199 ± 15.224 j | 20.706 ± 1.664 bce | 0.880 ± 0.075 ef | 2.718 ± 0.674 d |
References
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards Food Security: Current State and Future Prospects of Agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Future of Food and Agriculture: Trends and Challenges. Available online: http://www.fao.org/3/a-I6583e.pdf (accessed on 8 January 2023).
- UN. United Nations Set Out 17 Sustainable Development Goals (SDGs). Available online: https://www.un.org/sustainabledevelopment/hunger/ (accessed on 2 February 2023).
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Uri, N. Cropland Soil Salinization and Associated Hydrology: Trends, Processes and Examples. Water 2018, 10, 1030. [Google Scholar] [CrossRef]
- Singh, A. Soil Salinization Management for Sustainable Development: A Review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, P.K. Soil Salinity and Food Security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Bannari, A.; Al-Ali, Z.M. Assessing Climate Change Impact on Soil Salinity Dynamics between 1987-2017 in Arid Landscape Using Landsat TM, ETM+ and OLI Data. Remote Sens. 2020, 12, 2794. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/SYR_AR5_FINAL_full.pdf (accessed on 12 January 2023).
- Friedlingstein, P.; Houghton, R.A.; Marland, G.; Hackler, J.; Boden, T.A.; Conway, T.J.; Canadell, J.G.; Raupach, M.R.; Ciais, P.; le Quéré, C. Update on CO2 Emissions. Nat. Geosci. 2010, 3, 811–812. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration to Mitigate Climate Change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising Temperatures Reduce Global Wheat Production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A Meta-Analysis of Crop Yield under Climate Change and Adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Gupta, B.K.; Sahoo, K.K.; Anwar, K.; Nongpiur, R.C.; Deshmukh, R.; Pareek, A.; Singla-Pareek, S.L. Silicon Nutrition Stimulates Salt-Overly Sensitive (SOS) Pathway to Enhance Salinity Stress Tolerance and Yield in Rice. Plant Physiol. Biochem. 2021, 166, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an Inflorescence Meristem-Specific Cytokinin Oxidase—OsCKX2 in Rice Reduces Yield Penalty under Salinity Stress Condition. Plant Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 871, 1705. [Google Scholar] [CrossRef]
- Aghamolki, M.T.K.; Yusop, M.K.; Oad, F.C.; Zakikhani, H.; Jaafar, H.Z.; Kharidah, S.; Musa, M.H. Heat Stress Effects on Yield Parameters of Selected Rice Cultivars at Reproductive Growth Stages. J. Food Agric. Environ. 2014, 12, 741–746. [Google Scholar]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of Drought and/or Heat Stress on Physiological, Developmental, Growth, and Yield Processes of Crop Plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; American Society of Agronomy: Madison, WI, USA, 2015. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Sewelam, N.; Oshima, Y.; Mitsuda, N.; Ohme-Takagi, M. A Step towards Understanding Plant Responses to Multiple Environmental Stresses: A Genome-Wide Study. Plant Cell Environ. 2014, 37, 20024–22035. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The Combined Effect of Salinity and Heat Reveals a Specific Physiological, Biochemical and Molecular Response in Tomato Plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium Transport and Salt Tolerance in Plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Benlloch, R.; Lois, L.M. Sumoylation in Plants: Mechanistic Insights and Its Role in Drought Stress. J. Exp. Bot. 2018, 69, 4539–4554. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of Progressive Drought Stress on Growth, Leaf Gas Exchange, and Antioxidant Production in Two Maize Cultivars. Environ. Sci. Pollut. Res. 2016, 23, 17132–17141. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Cao, W.; Wu, L.; Geng, M. Seed Priming Alters the Production and Detoxification of Reactive Oxygen Intermediates in Rice Seedlings Grown under Sub-Optimal Temperature and Nutrient Supply. Front. Plant Sci. 2016, 7, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ye, C.; Lü, H.; Chen, X.; Chai, G.; Chen, J.; Wang, C. Identification and Characterization of a Novel Heat Shock Transcription Factor Gene, GmHsfA1, in Soybeans (Glycine max). J. Plant Res. 2006, 119, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Chopra, P.; Chhillar, H.; Ahanger, M.A.; Hussain, S.J.; Maheshwari, C. Regulatory Hubs and Strategies for Improving Heavy Metal Tolerance in Plants: Chemical Messengers, Omics and Genetic Engineering. Plant Physiol. Biochem. 2021, 164, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Asgher, M.; Fatma, M.; Per, T.S.; Khan, N.A. Drought Stress Vis a Vis Plant Functions in the Era of Climate Change. Clim. Chang. Environ. Sustain. 2015, 3, 13–25. [Google Scholar] [CrossRef]
- Nahar, L.; Aycan, M.; Hanamata, S.; Baslam, M.; Mitsui, T. Impact of Single and Combined Salinity and High-Temperature Stresses on Agro-Physiological, Biochemical, and Transcriptional Responses in Rice and Stress-Release. Plants 2022, 11, 501. [Google Scholar] [CrossRef]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Tasaka, K. Raising and Transplanting Technology for Long Mat with Hydroponically Grown Rice Seedlings. Jpn. Agric. Res. Q. 1999, 33, 31–37. [Google Scholar]
- Grattan, S.R.; Zeng, L.; Shannon, M.C.; Roberts, S.R. Rice Is More Sensitive to Salinity than Previously Thought. Calif. Agric. 2002, 56, 189–198. [Google Scholar] [CrossRef]
- Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Umali, D.L. Irrigation-Induced Salinity: A Growing Problem for Development and the Environment; World Bank Technical Paper; World Bank Publications: Herndon, VA, USA, 1993; Volume 215. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar] [CrossRef]
- Chagué-Goff, C.; Niedzielski, P.; Wong, H.K.Y.; Szczuciński, W.; Sugawara, D.; Goff, J. Environmental Impact Assessment of the 2011 Tohoku-Oki Tsunami on the Sendai Plain. Sediment. Geol. 2012, 282, 175–187. [Google Scholar] [CrossRef]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaegashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E.; et al. MutMap Accelerates Breeding of a Salt-Tolerant Rice Cultivar. Nat. Biotechnol. 2015, 33, 445–449. [Google Scholar] [CrossRef]
- Das, S.; Krishnan, P.; Nayak, M.; Ramakrishnan, B. High Temperature Stress Effects on Pollens of Rice (Oryza sativa L.) Genotypes. Environ. Exp. Bot. 2014, 101, 36–46. [Google Scholar] [CrossRef]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.V.K.; Kusolwa, P.; Rathinasabapathi, B. Heat Stress Tolerance in Rice (Oryza sativa L.): Identification of Quantitative Trait Loci and Candidate Genes for Seedling Growth under Heat Stress. Front. Plant Sci. 2018, 871, 1578. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers of IPCC Special Report on Global Warming of 1.5 °C Approved by Governments. Available online: https://www.ipcc.ch/2018/10/08/summary-for-policymakers-of-ipcc-special-report-on-global-warming-of-1-5c-approved-by-governments/ (accessed on 12 February 2023).
- Xu, Y.; Chu, C.; Yao, S. The Impact of High-Temperature Stress on Rice: Challenges and Solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Blum, A. Osmotic Adjustment Is a Prime Drought Stress Adaptive Engine in Support of Plant Production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of Proline, Sugars, and Anthocyanins during Photosynthetic Acclimation of Arabidopsis thaliana to Drought Stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef]
- Sultan, S.E. Plant Developmental Responses to the Environment: Eco-Devo Insights. Curr. Opin. Plant Biol. 2010, 13, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, J.; Sun, L.; Wang, H.; Lv, Y.; Li, Y.; Hameed, F. Effects of Straw Returning on Rice Growth and Yield under Water-Saving Irrigation. Chil. J. Agric. Res. 2019, 79, 66–74. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription Factors and Regulation of Photosynthetic and Related Metabolism under Environmental Stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, A.; Poustini, K.; Tavakkol-Afshari, R.; Tavakoli, A. Growth and Stomatal Responses of Bread Wheat Genotypes in Tolerance to Salt Stress. World Acad. Sci. Eng. Technol. 2010, 4, 787–792. [Google Scholar]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of Photosynthesis of C3 Plants in Response to Progressive Drought: Stomatal Conductance as a Reference Parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Medici, L.O.; Azevedo, R.A.; Canellas, L.P.; Machado, A.T.; Pimentel, C. Stomatal Conductance of Maize under Water and Nitrogen Deficits. Pesqui. Agropecuária Bras. 2007, 42, 599–601. [Google Scholar] [CrossRef]
- Dodd, I.C. Hormonal Interactions and Stomatal Responses. J. Plant Growth Regul. 2003, 22, 32–46. [Google Scholar] [CrossRef]
- Omoto, E.; Taniguchi, M.; Miyake, H. Effects of Salinity Stress on the Structure of Bundle Sheath and Mesophyll Chloroplasts in NAD-Malic Enzyme and PCK Type C4 Plants. Plant Prod. Sci. 2010, 13, 169–176. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Adaptive Responses of Birch-Leaved Pear (Pyrus betulaefolia) Seedlings to Salinity Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 133–138. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; Silva, J.V.; Endres, L.; Ferreira, V.M.; Câmara, C.d.A.; Cabral, F.F.; Oliveira, J.F.; de Carvalho, L.W.T.; dos Santos, J.M.; Filho, B.G.d.S. Leaf Gas Exchange, Chloroplastic Pigments and Dry Matter Accumulation in Castor Bean (Ricinus communis L.) Seedlings Subjected to Salt Stress Conditions. Ind. Crop. Prod. 2008, 27, 385–392. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Liu, H.; Wu, Y.T.; Li, W.B.; Zhang, H.X. Stable Expression of Arabidopsis Vacuolar Na+/H+ Antiporter Gene AtNHX1, and Salt Tolerance in Transgenic Soybean for over Six Generations. Chin. Sci. Bull. 2010, 55, 1127–1134. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zheng, W.; Tian, Y.; Wu, Y.; Zhou, D.W. Effects of Various Mixed Salt-Alkaline Stresses on Growth, Photosynthesis, and Photosynthetic Pigment Concentrations of Medicago ruthenica Seedlings. Photosynthetica 2011, 49, 275–284. [Google Scholar] [CrossRef]
- Aycan, M.; Erkilic, E.G.; Ozgen, Y.; Poyraz, I.; Yildiz, M. The Response of Sugar Beet (Beta vulgaris L.) Genotypes at Different Ploidy Levels to Salt (NaCl) Stress. Int. J. Plant Biol. 2023, 14, 199–217. [Google Scholar] [CrossRef]
- Akram, M.S.; Ashraf, M. Exogenous Application of Potassium Dihydrogen Phosphate Can Alleviate the Adverse Effects of Salt Stress on Sunflower. J. Plant Nutr. 2011, 34, 1041–1057. [Google Scholar] [CrossRef]
- Khan, M.A.; Shirazi, M.U.; Khan, M.A.; Mujtaba, S.M.; Islam, E.; Mumtaz, S.; Shereen, A.; Ansari, R.U.; Yasin Ashraf, M. Role of Proline, K/NA Ratio and Chlorophyll Content in Salt Tolerance of Wheat (Triticum aestivum L.). Pak. J. Bot. 2009, 41, 633–638. [Google Scholar]
- Aycan, M.; Baslam, M.; Asiloglu, R.; Mitsui, T.; Yildiz, M. Development of New High-Salt Tolerant Bread Wheat (Triticum aestivum L.) Genotypes and Insight into the Tolerance Mechanisms. Plant Physiol. Biochem. 2021, 166, 314–327. [Google Scholar] [CrossRef]
- Lam, H.-M.; Xu, X.; Liu, X.; Chen, W.; Yang, G.; Wong, F.-L.; Li, M.-W.; He, W.; Qin, N.; Wang, B.; et al. Resequencing of 31 Wild and Cultivated Soybean Genomes Identifies Patterns of Genetic Diversity and Selection. Nat. Genet. 2010, 42, 1053–1059. [Google Scholar] [CrossRef]
- Ambardekar, A.A.; Siebenmorgen, T.J.; Counce, P.A.; Lanning, S.B.; Mauromoustakos, A. Impact of Field-Scale Nighttime Air Temperatures during Kernel Development on Rice Milling Quality. Field Crop. Res. 2011, 122, 179–185. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose Accumulation in Rice Plants Confers High Tolerance Levels to Different Abiotic Stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef]
- James, R.A.; Rivelli, A.R.; Munns, R.; von Caemmerer, S. Factors Affecting CO2 Assimilation, Leaf Injury and Growth in Salt-Stressed Durum Wheat. Funct. Plant Biol. 2002, 29, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Grzesiak, S.; Hura, K.; Grzesiak, M.; Rzepka, A. Differences in the Physiological State between Triticale and Maize Plants during Drought Stress and Followed Rehydration Expressed by the Leaf Gas Exchange and Spectrofluorimetric Methods. Acta Physiol. Plant 2006, 28, 433–443. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic Mechanisms of Abiotic Stress Tolerance That Translate to Crop Yield Stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Porch, T.G.; Jahn, M. Effects of High-Temperature Stress on Microsporogenesis in Heat-Sensitive and Heat-Tolerant Genotypes of Phaseolus Vulgaris. Plant Cell Environ. 2001, 24, 723–731. [Google Scholar] [CrossRef]
- Akbar, A.; Manohar, S.S.; Variath, M.T.; Kurapati, S.; Pasupuleti, J. Efficient Partitioning of Assimilates in Stress-Tolerant Groundnut Genotypes under High-Temperature Stress. Agronomy 2017, 7, 30. [Google Scholar] [CrossRef]
- Karkute, S.G.; Ansari, W.A.; Singh, A.K.; Singh, P.M.; Rai, N.; Bahadur, A.; Singh, J. Characterization of High-Temperature Stress-Tolerant Tomato (Solanum lycopersicum L.) Genotypes by Biochemical Analysis and Expression Profiling of Heat-Responsive Genes. 3 Biotech 2021, 11, 45. [Google Scholar] [CrossRef]
- Yogeesh, L.N.; Naryanareddy, A.B.; Nanjareddy, Y.A.; Channabyre Gowda, M.V. High Temperature Tolerant Genotypes of Finger Millet (Eleusine coracana L.). Nat. Environ. Pollut. Technol. 2016, 15, 1293–1296. [Google Scholar]
- Qureshi, A.S.; Daba, A.W. Evaluating Growth and Yield Parameters of Five Quinoa (Chenopodium quinoa W.) Genotypes under Different Salt Stress Conditions. J. Agric. Sci. 2020, 12, 128–140. [Google Scholar] [CrossRef]
- Razzaq, A.; Saleem, F.; Wani, S.H.; Abdelmohsen, S.A.M.; Alyousef, H.A.; Abdelbacki, A.M.M.; Alkallas, F.H.; Tamam, N.; Elansary, H.O. De-Novo Domestication for Improving Salt Tolerance in Crops. Front. Plant Sci. 2021, 12, 681367. [Google Scholar] [CrossRef]
- Quan, X.; Liang, X.; Li, H.; Xie, C.; He, W.; Qin, Y. Identification and Characterization of Wheat Germplasm for Salt Tolerance. Plants 2021, 10, 268. [Google Scholar] [CrossRef]
- Moustafa, E.S.A.; Ali, M.M.A.; Kamara, M.M.; Awad, M.F.; Hassanin, A.A.; Mansour, E. Field Screening of Wheat Advanced Lines for Salinity Tolerance. Agronomy 2021, 11, 281. [Google Scholar] [CrossRef]
- Aycan, M.; Nahar, L.; Baslam, M.; Mitsui, T. B-Type Response Regulator hst1 Controls Salinity Tolerance in Rice by Regulating Transcription Factors and Antioxidant Mechanisms. Plant Physiol. Biochem. 2023, 196, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; Takamatsu, T.; Baslam, M.; Kaneko, K.; Itoh, K.; Harada, N.; Sugiyama, T.; Ohnishi, T.; Kinoshita, T.; Takagi, H.; et al. Salt Tolerance Improvement in Rice through Efficient SNP Marker-Assisted Selection Coupled with Speed-Breeding. Int. J. Mol. Sci. 2019, 20, 2585. [Google Scholar] [CrossRef]
- Sami, F.; Siddiqui, H.; Alam, P.; Hayat, S. Glucose-Induced Response on Photosynthetic Efficiency, ROS Homeostasis, and Antioxidative Defense System in Maintaining Carbohydrate and Ion Metabolism in Indian Mustard (Brassica juncea L.) under Salt-Mediated Oxidative Stress. Protoplasma 2021, 258, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Aycan, M.; Baslam, M.; Ozdemir, B.; Asiloglu, R.; Mitsui, T.; Yildiz, M. Direct Contribution of the Maternal Genotype on the Transgenerational Salinity Tolerance in Wheat (Triticum aestivum L.). Environ. Exp. Bot. 2021, 192, 104648. [Google Scholar] [CrossRef]
- Aycan, M.; Baslam, M.; Mitsui, T.; Yildiz, M. The TaGSK1, TaSRG, TaPTF1, and TaP5CS Gene Transcripts Confirm Salinity Tolerance by Increasing Proline Production in Wheat (Triticum aestivum L.). Plants 2022, 11, 3401. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, K.; Matsui, T.; Kaneda, S.; Kobayashi, K.; Kasaneyama, H.; Abe, S.; Azuma, S.; Hoshi, T.; Sasaki, Y.; Hirao, K. A New Rice Cultivar “Yukinkomai”. J. Niigata Agric. Res. Inst. 2008, 9, 89–98. [Google Scholar]
- Horii, A.; McCue, P.; Shetty, K. Seed Vigour Studies in Corn, Soybean and Tomato in Response to Fish Protein Hydrolysates and Consequences on Phenolic-Linked Responses. Bioresour. Technol. 2007, 98, 2170–2177. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence against Lipid Peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Tejera García, N.A.; Olivera, M.; Iribarne, C.; Lluch, C. Partial Purification and Characterization of a Non-Specific Acid Phosphatase in Leaves and Root Nodules of Phaseolus vulgaris. Plant Physiol. Biochem. 2004, 42, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Amako, K.; Chen, G.X.; Asada, K. Separate Assays Specific for Ascorbate Peroxidase and Guaiacol Peroxidase and for the Chloroplastic and Cytosolic Isozymes of Ascorbate Peroxidase in Plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar] [CrossRef]
- Pequerul, A.; Pérez, C.; Madero, P.; Val, J.; Monge, E. A Rapid Wet Digestion Method for Plant Analysis. Optim. Plant Nutr. 1993, 2, 3–6. [Google Scholar] [CrossRef]
- Kaneko, K.; Sasaki, M.; Kuribayashi, N.; Suzuki, H.; Sasuga, Y.; Shiraya, T.; Inomata, T.; Itoh, K.; Baslam, M.; Mitsui, T. Proteomic and Glycomic Characterization of Rice Chalky Grains Produced under Moderate and High-Temperature Conditions in Field System. Rice 2016, 9, 26. [Google Scholar] [CrossRef]
- Guglielminetti, M.; de Giuli Morghen, C.; Radaelli, A.; Bistoni, F.; Carruba, G.; Spera, G.; Caretta, G. Mycological and Ultrastructural Studies to Evaluate Biodeterioration of Mural Paintings. Detection of Fungi and Mites in Frescos of the Monastery of St Damian in Assisi. Int. Biodeterior. Biodegrad. 1994, 33, 269–283. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 January 2023).
- Kolde, R. Pretty Heatmaps. R Package Version 1.0.10. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 20 January 2023).
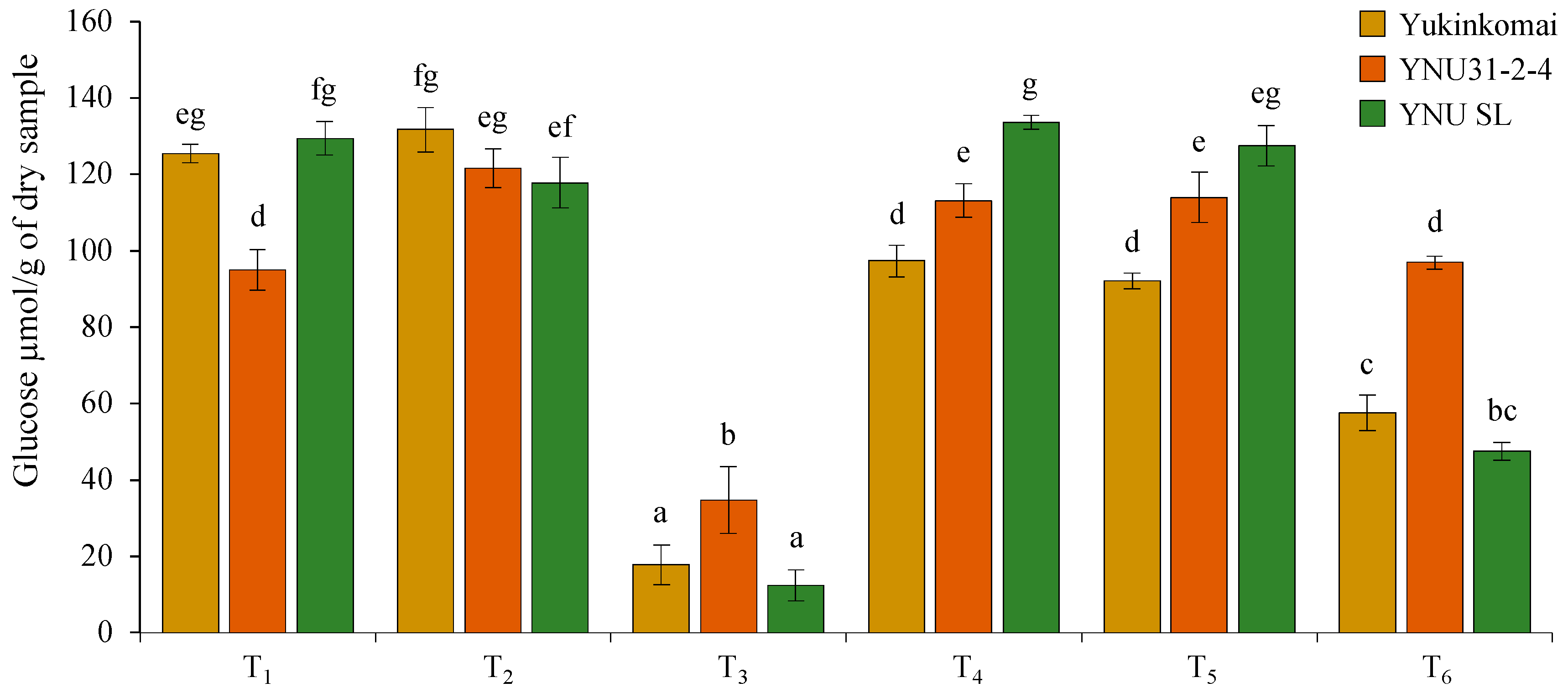
| Treatment (T) | Seedling Stage (SS) | Vegetative Stage (VS) | Reproductive Stage (RS) |
|---|---|---|---|
| T1 | Control (C; 26 ℃, 0 mM NaCl) | Control (C; 26 °C, 0 mM NaCl) | Control (C; 26 ℃, 0 mM NaCl) |
| T2 | Control (C; 26 ℃, 0 mM NaCl) | Salinity (S; 26 ℃, 75 mM NaCl) | Control (C; 26 ℃, 0 mM NaCl) |
| T3 | Control (C; 26 ℃, 0 mM NaCl) | Salinity (S; 26 ℃, 75 mM NaCl) | Salinity (S; 26 ℃, 75 mM NaCl) |
| T4 | Control (C; 26 ℃, 0 mM NaCl) | Salinity (S; 26 ℃, 75 mM NaCl) | Heat (H; 31 ℃, 0 mM NaCl) |
| T5 | Control (C; 26 ℃, 0 mM NaCl) | Control (C; 26 ℃, 0 mM NaCl) | Heat (H; 31 ℃, 0 mM NaCl) |
| T6 | Control (C; 26 ℃, 0 mM NaCl) | Salinity (S; 26 ℃, 75 mM NaCl) | Heat and Salinity (H + S; 31 ℃, 75 mM NaCl) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, L.; Aycan, M.; Lopes Hornai, E.M.; Baslam, M.; Mitsui, T. Tolerance with High Yield Potential Is Provided by Lower Na+ Ion Accumulation and Higher Photosynthetic Activity in Tolerant YNU31-2-4 Rice Genotype under Salinity and Multiple Heat and Salinity Stress. Plants 2023, 12, 1910. https://doi.org/10.3390/plants12091910
Nahar L, Aycan M, Lopes Hornai EM, Baslam M, Mitsui T. Tolerance with High Yield Potential Is Provided by Lower Na+ Ion Accumulation and Higher Photosynthetic Activity in Tolerant YNU31-2-4 Rice Genotype under Salinity and Multiple Heat and Salinity Stress. Plants. 2023; 12(9):1910. https://doi.org/10.3390/plants12091910
Chicago/Turabian StyleNahar, Lutfun, Murat Aycan, Ermelinda Maria Lopes Hornai, Marouane Baslam, and Toshiaki Mitsui. 2023. "Tolerance with High Yield Potential Is Provided by Lower Na+ Ion Accumulation and Higher Photosynthetic Activity in Tolerant YNU31-2-4 Rice Genotype under Salinity and Multiple Heat and Salinity Stress" Plants 12, no. 9: 1910. https://doi.org/10.3390/plants12091910
APA StyleNahar, L., Aycan, M., Lopes Hornai, E. M., Baslam, M., & Mitsui, T. (2023). Tolerance with High Yield Potential Is Provided by Lower Na+ Ion Accumulation and Higher Photosynthetic Activity in Tolerant YNU31-2-4 Rice Genotype under Salinity and Multiple Heat and Salinity Stress. Plants, 12(9), 1910. https://doi.org/10.3390/plants12091910










