Abscisic Acid Synthesis and Signaling during the Ripening of Raspberry (Rubus idaeus ‘Heritage’) Fruit
Abstract
1. Introduction
2. Results
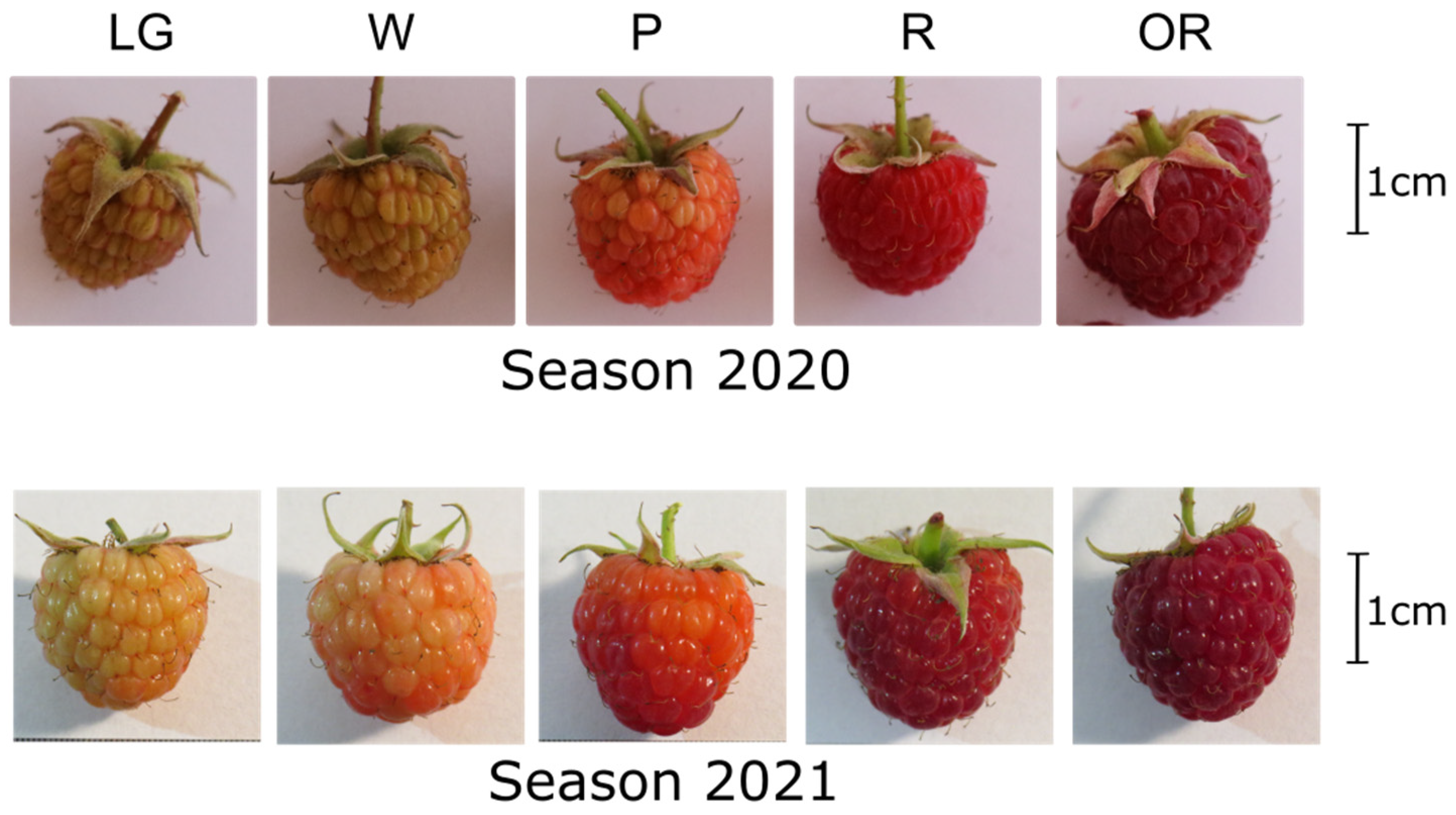
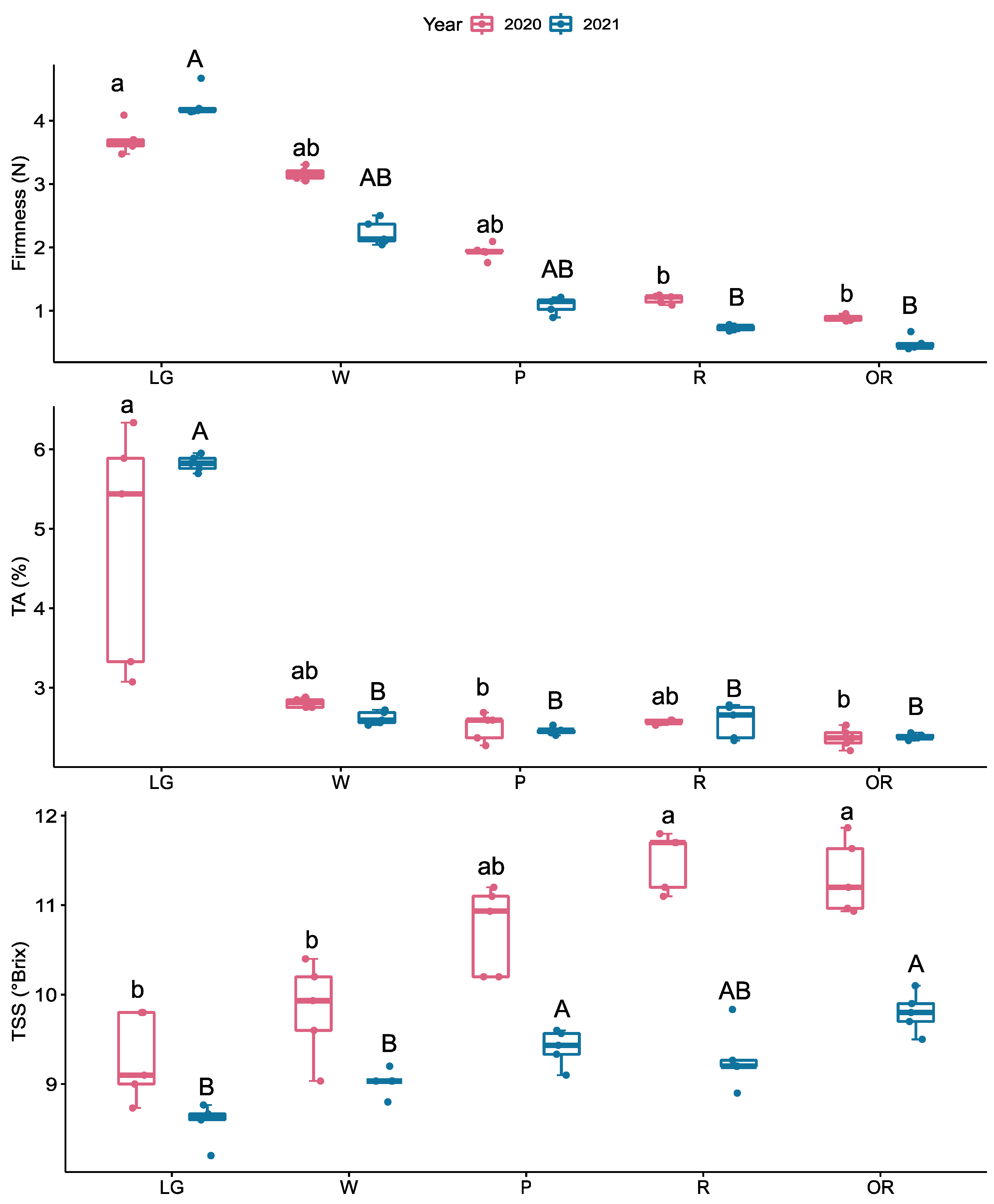
2.1. Fruit Quality Measurements
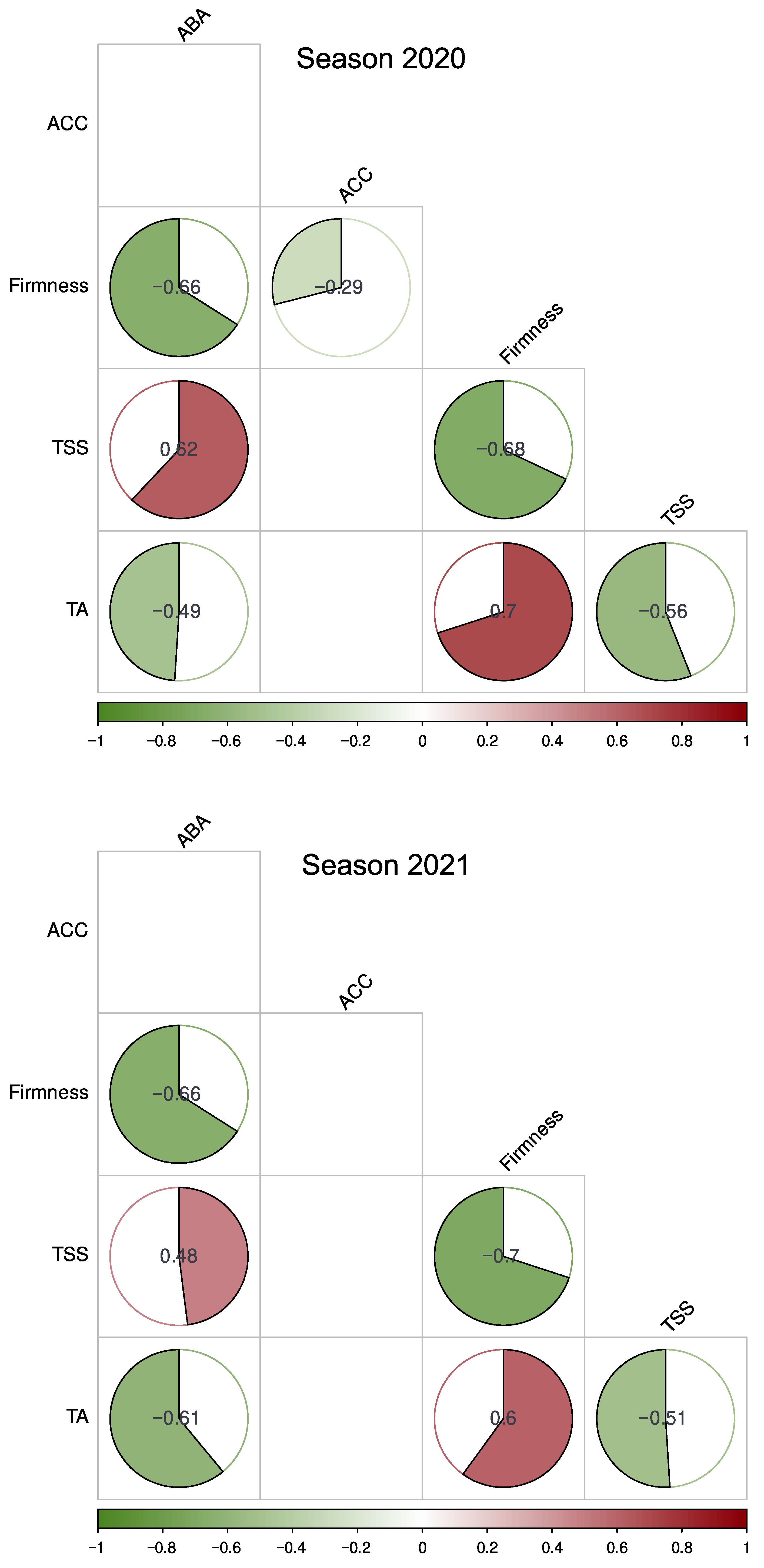
2.2. ABA and ACC Dynamics during Raspberry Fruit Development
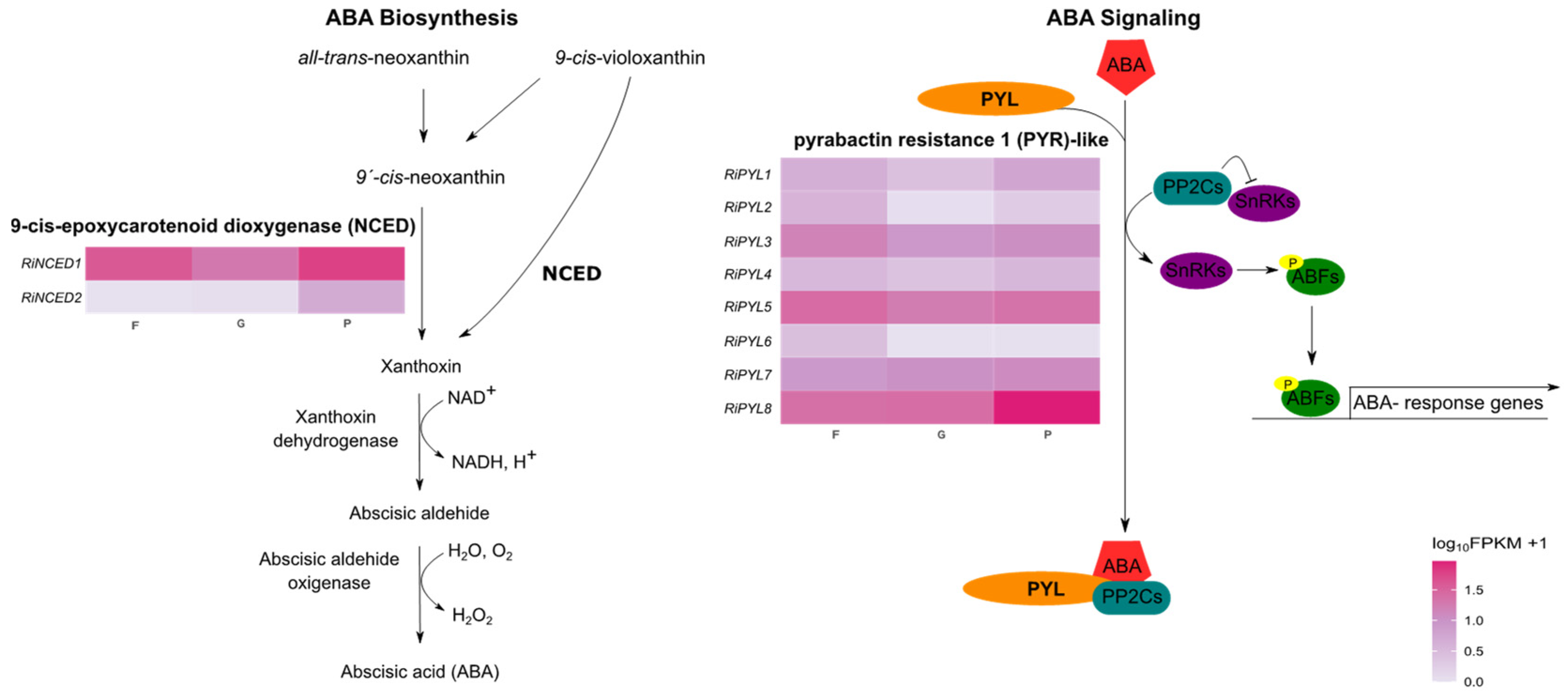
2.3. Identification of RiNCED and RiPYL Genes in the Raspberry Genome
2.4. Structure, Conserved Motifs and Promoter Analysis of RiNCED and RiPYL Genes
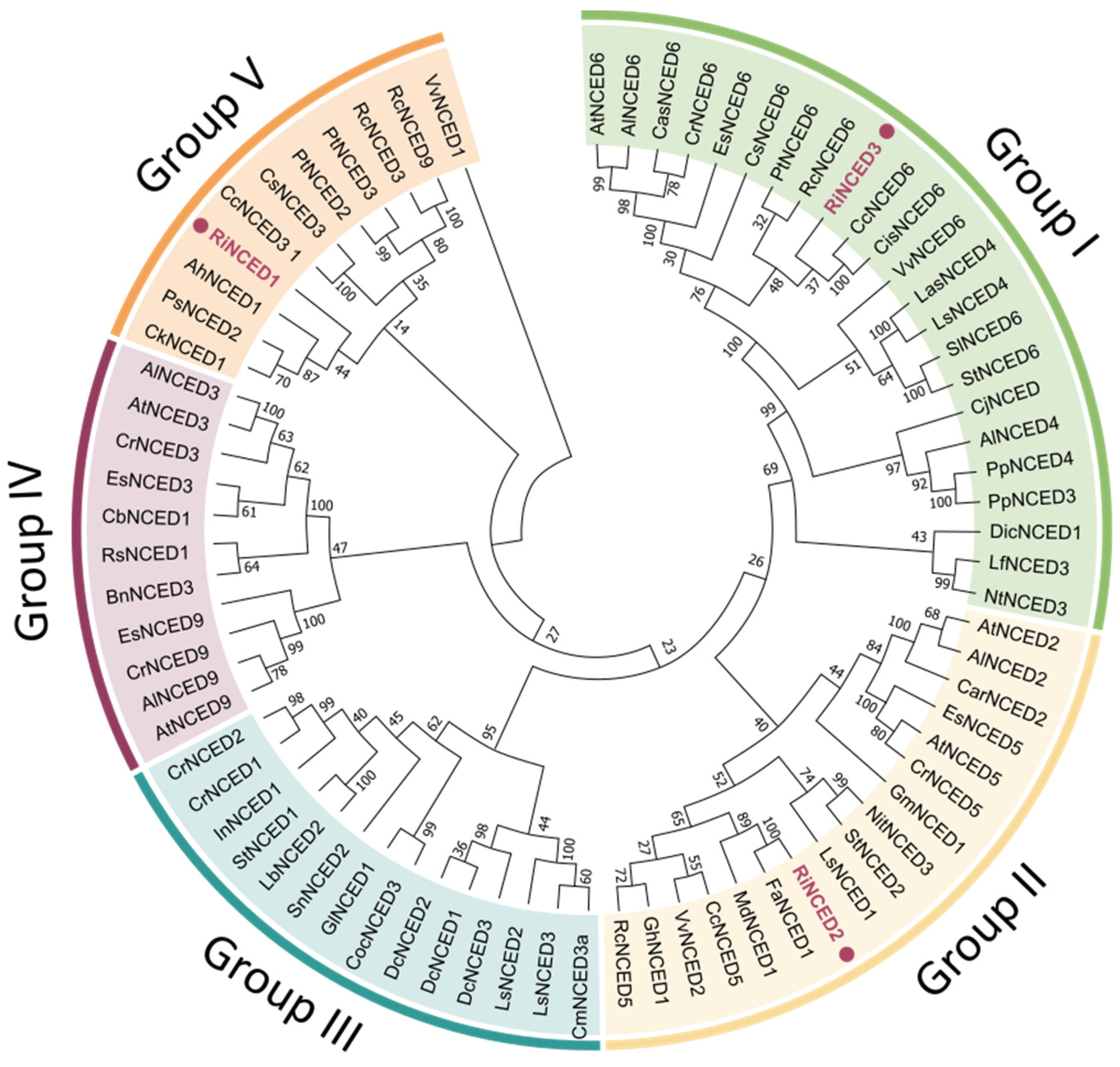
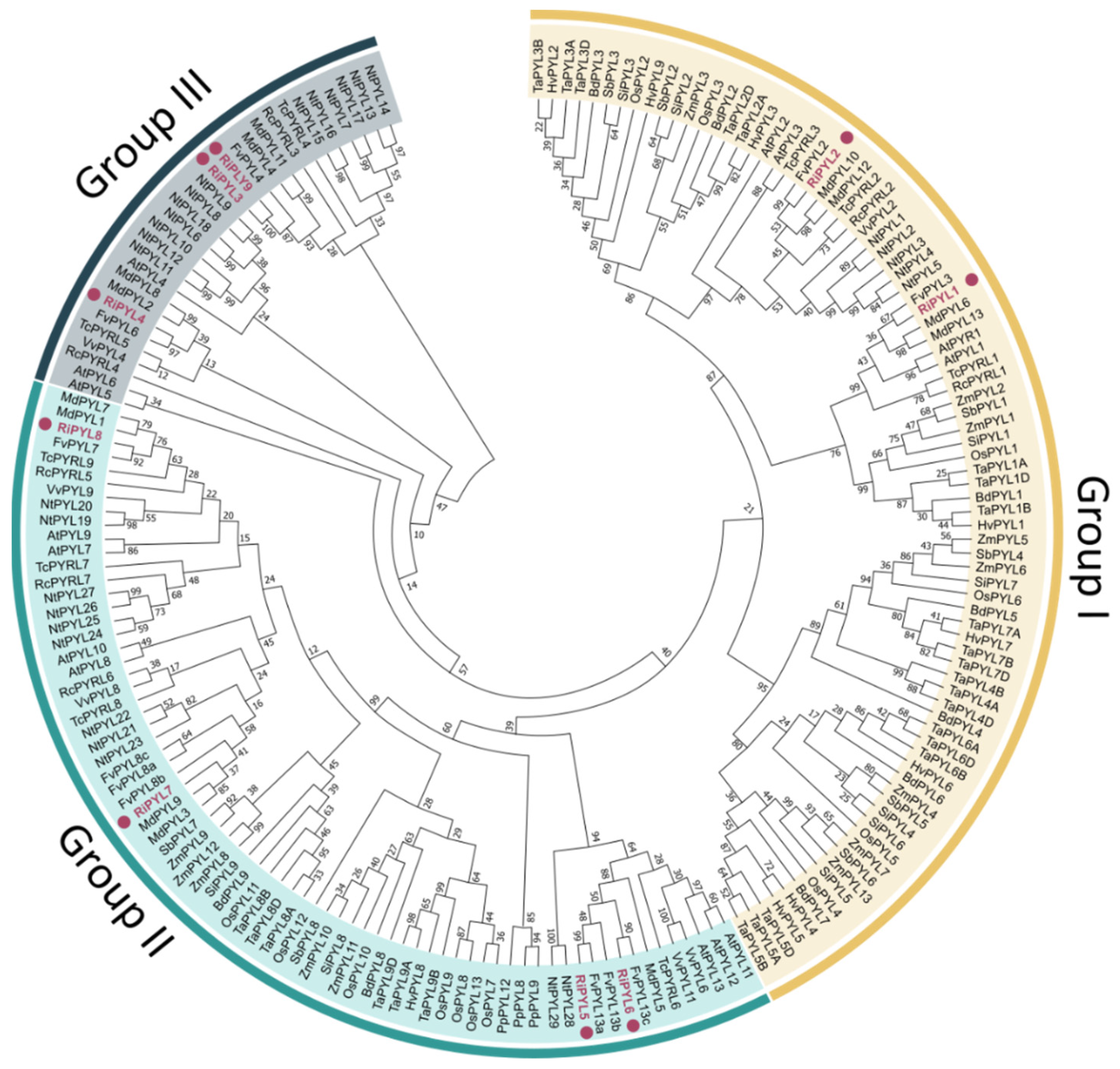
2.5. Phylogenetic Analysis and Classification of RiNCED and RiPYL Deduced Proteins
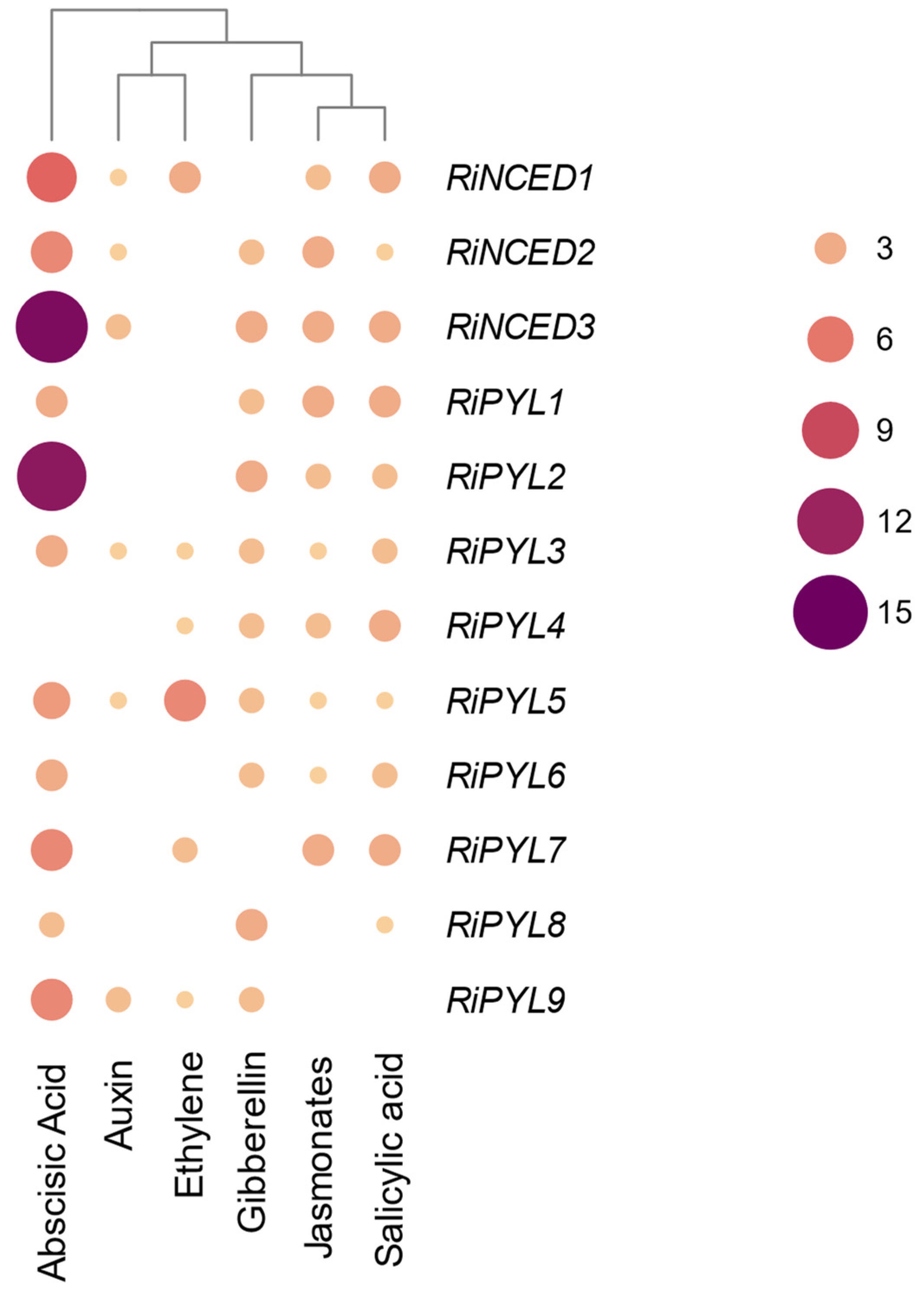
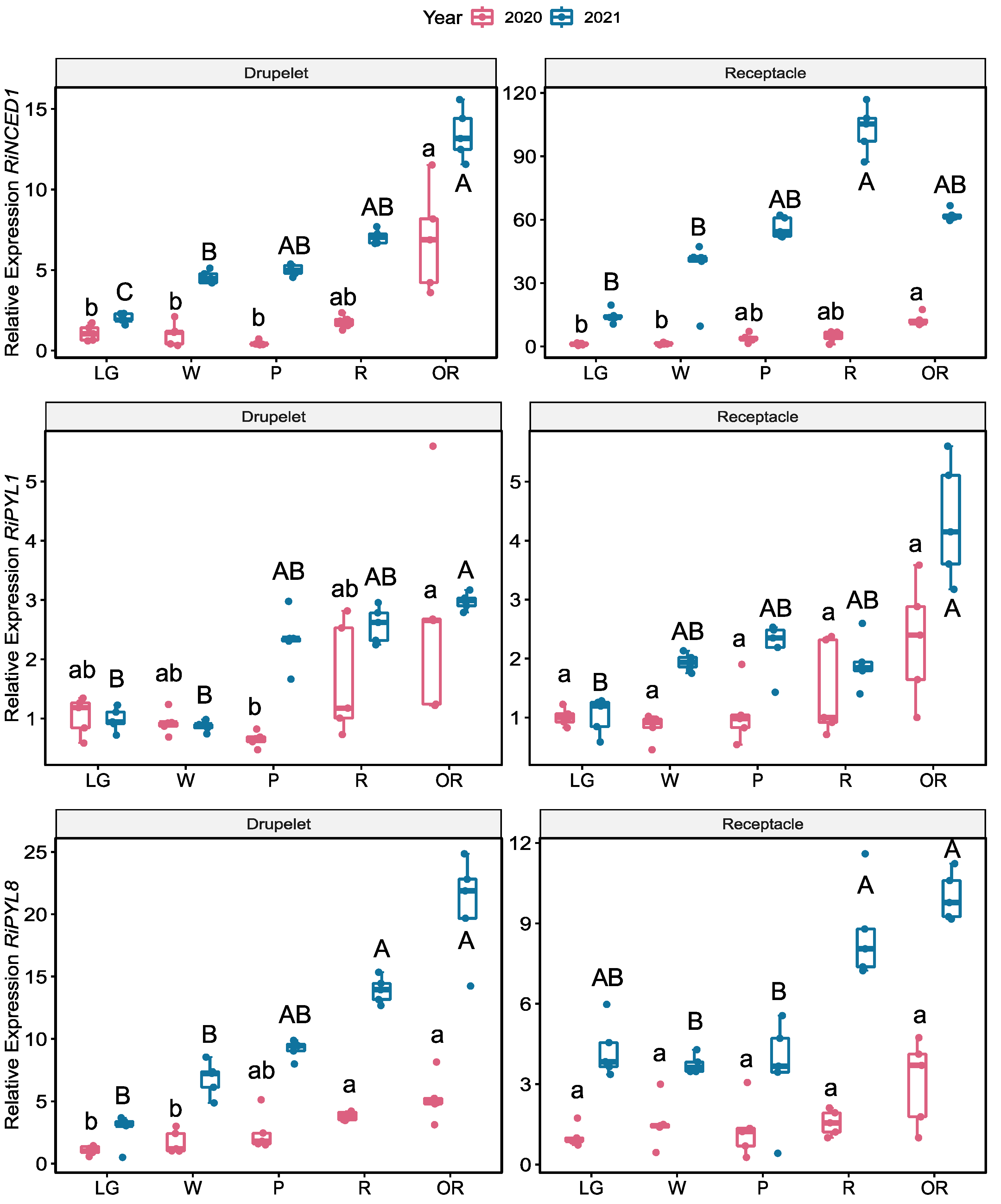
2.6. Expression of RiNCED and RiPYL Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fruit Quality and Physiological Measurements
4.3. Determination of ABA and ACC Contents
4.4. Identification of RiNCED and RiPYL Genes in the Raspberry Genome
4.5. Gene Structure and Promoter Analysis of RiNCED and RiPYL Genes
4.6. Characterization of RiNCED- and RiPYL-Deduced Protein Sequences
4.7. Identification of the Differentially Expressed RiNCED and RiPYL Genes in the Transcriptomic Data
4.8. RNA Isolation and Reverse Transcription Quatitative PCR (RT-qPCR) Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, J.; Simpson, C. Chapter 14: Developmental Transitions to Fruiting in Red Raspberry. In The Genomes of Rosaceous Berries and Their Wild Relatives; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 199–212. [Google Scholar]
- Popović, T.; Šarić, B.; Martačić, J.D.; Arsić, A.; Jovanov, P.; Stokić, E.; Mišan, A.; Mandić, A. Potential health benefits of blueberry and raspberry pomace as functional food ingredients: Dietetic intervention study on healthy women volunteers. Front. Nutr. 2022, 9, 969996. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Iannetta, P.P.; Davies, H.V. Ripening-related changes in raspberry cell wall composition and structure. Phytochemistry 2001, 56, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.; Ortungo, C.; Powell, A.; Greve, L.; Labavitch, J. Temporal sequence of cell wall disassembly events in developing fruits. 1. Analysis of raspberry (Rubus idaeus). J. Agric. Food Chem. 2007, 55, 4119–4124. [Google Scholar] [CrossRef]
- Fuentes, L.; Monsalve, L.; Morales-Quintana, L.; Valdenegro, M.; Martínez, J.P.; Defilippi, B.G.; González-Agüero, M. Differential expression of ethylene biosynthesis genes in drupelets and receptacle of raspberry (Rubus idaeus). J. Plant Physiol. 2015, 179, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.G.; Cullen, D.W.; Hackett CASmith, K.; Hallett, P.D.; McNicol, J.; Woodhead, M.; Graham, J. Mapping and expression of genes associated with raspberry fruit ripening and softening. Theor. Appl. Genet. 2017, 130, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Pritts, M.P. Raspberries and related fruits. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Kent, UK, 2003. [Google Scholar]
- Blanpied, G.D. A study of ethylene in apple, red raspberry and cherry. Plant Physiol. 1972, 29, 627–630. [Google Scholar] [CrossRef]
- Monsalve, L.; Bernales, M.; Ayala-Raso, A.; Álvarez, F.; Valdenegro, M.; Alvaro, J.-E.; Figueroa, C.R.; Defilippi, B.G.; Fuentes, L. Relationship between endogenous ethylene production and firmness during the ripening and cold storage of raspberry (Rubus idaeus ‘Heritage’) Fruit. Horticulturae 2022, 8, 262. [Google Scholar] [CrossRef]
- Iannetta, P.P.M.; Wyman, M.; Neelam, A.; Jones, C.; Davies, H.V.; Sexton, R. A causal role for ethylene and endo-b-1,4-glucanase in the abscission of red-raspberry (Rubus idaeus L.) drupelets. Physiol. Plant. 2000, 110, 535–543. [Google Scholar] [CrossRef]
- Bernales, M.; Monsalve, L.; Ayala-Raso, A.; Valdenegro, M.; Martínez, J.P.; Travisany, D.; Defilippi, B.; González-Agüero, M.; Cherian, S.; Fuentes, L. Expression of two indole-3-acetic acid (IAA)-amido synthetases (GH3) genes during fruit development and ripening of raspberry (Rubus idaeus Heritage). Sci. Hortic. 2019, 246, 168–175. [Google Scholar] [CrossRef]
- Yang, G.; Li, H.; Xin, Y.; Yu, H.; Chen, L.; Li, L.; Han, D. Effect of abscisic acid on expression of its key enzyme synthesis genes, RiNCED1 and RiCYP707A1, and quality of raspberry (Rubus idaeus) fruits. Int. J. Agric. Biol. 2020, 23, 269–278. [Google Scholar]
- Travisany, D.; Ayala-Raso, A.; Di Genova, A.; Monsalve, L.; Bernales, M.; Martínez, J.P.; González-Agüero, M.; Defilippi, B.; Cherian, S.; Maass, A.; et al. RNA-Seq analysis and transcriptome assembly of raspberry fruit (Rubus idaeus ¨Heritage¨) revealed several candidate genes involved in fruit development and ripening. Sci. Hort. 2019, 254, 26–34. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Ren, J.; Qi, J.; Zhang, G.; Leng, P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.F.; Chai, Y.M.; Li, C.L.; Lu, D.; Luo, J.J.; Qin, L.; Shen, Y.Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef]
- Lurie, S.; Ovadia, R.; Nissim-Levi, A.; Oren-Shamir, M.; Kaplunov, T.; Zutahy, Y.; Weksler, H.; Lichter, A. Abscisic acid improves colour development in ‘Crimson Seedless’ grapes in the vineyard and on detached berries. J. Hortic. Sci 2009, 84, 639–644. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003, 39, 171–174. [Google Scholar] [CrossRef]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M. Recent advances in hormonal regulation and cross-talk during non-climacteric fruit development and ripening. Horticulturae 2019, 5, 45. [Google Scholar] [CrossRef]
- Jia, H.; Li, C.; Chai, Y.; Xing, Y.; Shen, Y. Sucrose promotes strawberry fruit ripening by stimulation of abscisic acid biosynthesis. Pak. J. Bot. 2013, 45, 169–175. [Google Scholar]
- Li, D.; Mou, W.; Xia, R.; Li, L.; Zawora, C.; Ying, T.; Mao, L.; Liu, Z.; Luo, Z. Integrated analysis of high-throughput sequencing data shows abscisic acid-responsive genes and miRNAs in strawberry receptacle fruit ripening. Hortic. Res. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Degu, A.; Hochberg, U.; Sikron, N.; Venturini, L.; Buson, G.; Ghan, R.; Plaschkes, I.; Batushansky, A.; Chalifa-Caspi, V.; Mattivi, F.; et al. Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol. 2014, 14, 188. [Google Scholar] [CrossRef]
- Li, C.; Jia, H.; Chai, Y.; Shen, Y. Abscisic acid perception and signaling transduction in strawberry: A model for non-climacteric fruit ripening. Plant Signal. Behav. 2011, 12, 1950–1953. [Google Scholar] [CrossRef]
- Yang, J.; Wang, M.; Zhou, S.; Xu, B.; Chen, P.; Ma, F.; Mao, K. The ABA receptor gene MdPYL9 confers tolerance to drought stress in transgenic apple (Malus domestica). Environ. Exp. Bot. 2022, 194, 104695. [Google Scholar] [CrossRef]
- González-Guzmán, M.; Rodríguez, L.; Lorenzo-Orts, L.; Pons, C.; Sarrión-Perdigones, A.; Fernández, M.A.; Peirats-Llobet, M.; Forment, J.; Moreno-Alvero, M.; Cutler, S.R.; et al. Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J. Exp. Bot. 2014, 65, 4451–4464. [Google Scholar] [CrossRef]
- Hou, B.Z.; Chen, X.H.; Shen, Y.Y. Interactions between strawberry ABA receptor PYR/PYLs and protein phosphatase PP2Cs on basis of transcriptome and yeast two-hybrid analyses. J. Plant Growth Regul. 2021, 40, 594–602. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Q.; Li, J.; Chen, Y.; Luo, M.; Li, H.; Wang, J.; Wu, Y.; Duan, S.; Wang, L.; et al. Characterization of the ABA receptor VlPYL1 that regulates anthocyanin accumulation in grape berry skin. Front. Plant Sci. 2018, 9, 592. [Google Scholar] [CrossRef]
- El-Kayal, W.E.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Phospholipase D inhibition by hexanal is associated with calcium signal transduction events in raspberry. Hortic. Res. 2017, 4, 17042. [Google Scholar] [CrossRef]
- Wight, H.; Zhou, J.; Li, M.; Hannenhalli, S.; Mount, S.M.; Liu, Z. Draft genome assembly and annotation of red raspberry Rubus idaeus. BioRxiv 2019, 546135. [Google Scholar] [CrossRef]
- Available online: https://www.rosaceae.org/analysis/329 (accessed on 29 April 2023).
- Han, S.Y.; Kitahata, N.; Sekimata, K.; Saito, T.; Kobayashi, M.; Nakashima, K.; Asami, T. A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiol. 2004, 135, 1574–1582. [Google Scholar] [CrossRef]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The Role of Stress-Responsive Transcription Factors in Modulating Abiotic Stress Tolerance in Plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Contreras, C.; Hermosilla, A.; Contreras, E.; Naranjo, P.; Zoffoli, J.P.; Gambardella, M. Postharvest physiology and storage potential of new Chilean raspberry cultivars. Chil. J. Agric. Res. 2021, 81, 161–171. [Google Scholar] [CrossRef]
- Chervin, C.; El-Kereamy, A.; Roustan, J.-P.; Latché, A.; Lamon, J.; Bouzayen, M. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 2004, 167, 1301–1305. [Google Scholar] [CrossRef]
- Figueroa, N.E.; Gatica-Meléndez, C.; Figueroa, C.R. Ethylene application at the immature stage of Fragaria chiloensis fruit represses the anthocyanin biosynthesis with a concomitant accumulation of lignin. Food Chem. 2021, 358, 129913. [Google Scholar] [CrossRef] [PubMed]
- Iannetta, P.P.M.; van den Berg, J.; Wheatley, R.E.; McNicol, R.J.; Davies, H.V. The role of ethylene and cell wall modifying enzymes in raspberry (Rubus idaeus) fruit ripening. Physiol. Plant. 1999, 105, 338–347. [Google Scholar] [CrossRef]
- Zheng, D.; Hrazdina, G. Cloning and characterization of an expansin gene, RiEXP1, and a 1-aminocyclopropane-1-carboxylic acid synthase gene, RiACS1 in ripening fruit of raspberry (Rubus idaeus L.). Plant Sci. 2010, 179, 133–139. [Google Scholar] [CrossRef]
- Monsalve, L.; Ayala-Raso, A.; Bernales, M.; Valdenegro, M.; Defilippi, B.; González-Agüero, M.; Cherian, S.; Fuentes, L. Dataset on quality and physiological changes of raspberry fruit during their development and under auxin in-vitro assay. Data Brief 2018, 21, 1521–1525. [Google Scholar] [CrossRef]
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Asahira, T. Roles of cytokinin and abscisic acid in the maturing of strawberry fruits. J. Jpn. Soc. Hortic. Sci. 1981, 50, 31–36. [Google Scholar] [CrossRef]
- Gagné, S.; Cluzet, S.; Mérillon, J.M.; Gény, L. ABA initiates anthocyanin production in grape cell cultures. J. Plant Growth Regul. 2011, 30, 1–10. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2013, 65, 821–832. [Google Scholar] [CrossRef]
- Kühn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic acid is a major regulator of grape berry ripening onset: New insights into ABA signaling network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, W.; Ding, Y.; Wang, Y.; Niu, L.; Yao, J.L.; Pan, L.; Lu, Z.; Cui, G.; Li, G.; et al. PpERF3 positively regulates ABA biosynthesis by activating PpNCED2/3 transcription during fruit ripening in peach. Hortic. Res. 2019, 6, 19. [Google Scholar] [CrossRef]
- Garrido-Bigotes, A.; Figueroa, P.M.; Figueroa, C.R. Jasmonate Metabolism and Its Relationship with Abscisic Acid during Strawberry Fruit Development and Ripening. J. Plant Growth Regul. 2018, 37, 101–113. [Google Scholar] [CrossRef]
- Hou, H.; Lv, L.; Huo, H.; Dai, H.; Zhang, Y. Genome-wide identification of the ABA receptors genes and their response to abiotic stress in apple. Plants 2020, 9, 1028. [Google Scholar] [CrossRef]
- Zhang, M.; Leng, P.; Zhang, G.; Li, X. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J. Plant Physiol. 2009, 166, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.geneious.com (accessed on 4 April 2023).
- Available online: https://bioinf.uni-greifswald.de/augustus/submission.php (accessed on 4 April 2023).
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Available online: http://gsds.gao-lab.org/ (accessed on 4 April 2023).
- Available online: http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 21 October 2022).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://meme-suite.org/meme/tools/meme (accessed on 4 April 2023).
- Available online: https://www.rosaceae.org/blast/nucleotide/nucleotide (accessed on 29 April 2023).
- Goff, L.; Trapnell, C.; Kelley, D. cummeRbund: Analysis, exploration, manipulation, and visualization of Cufflinks high-throughput sequencing data. R package version 2.40.0. Bioconductor 2022. [Google Scholar] [CrossRef]
- Available online: https://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi (accessed on 29 April 2023).
- Delgado, L.D.; Zúñiga, P.E.; Figueroa, N.E.; Pastene, E.; Escobar-Sepúlveda, H.F.; Figueroa, P.M.; Garrido-Bigotes, A.; Figueroa, C.R. Application of a JA-Ile biosynthesis inhibitor to methyl jasmonate-treated strawberry fruit induces upregulation of specific MBW complex-related ge nes and accumulation of proanthocyanidins. Molecules 2018, 23, 1433. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 29 April 2023).
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Kassambara:, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests_ R Package Version 0.7.1. 2022. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 29 April 2023).

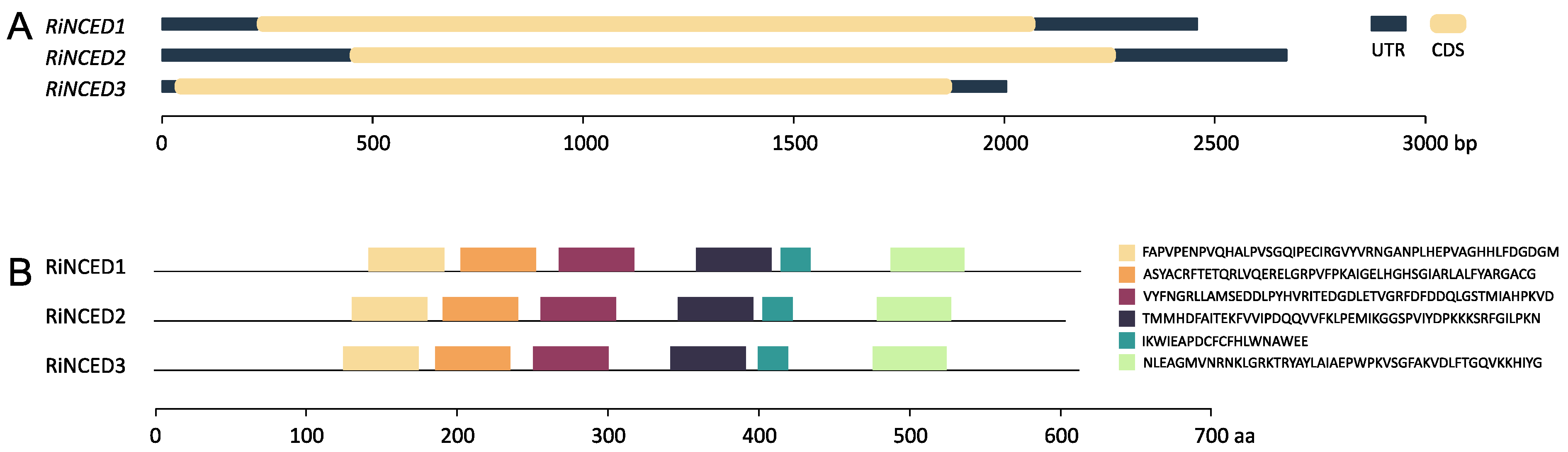
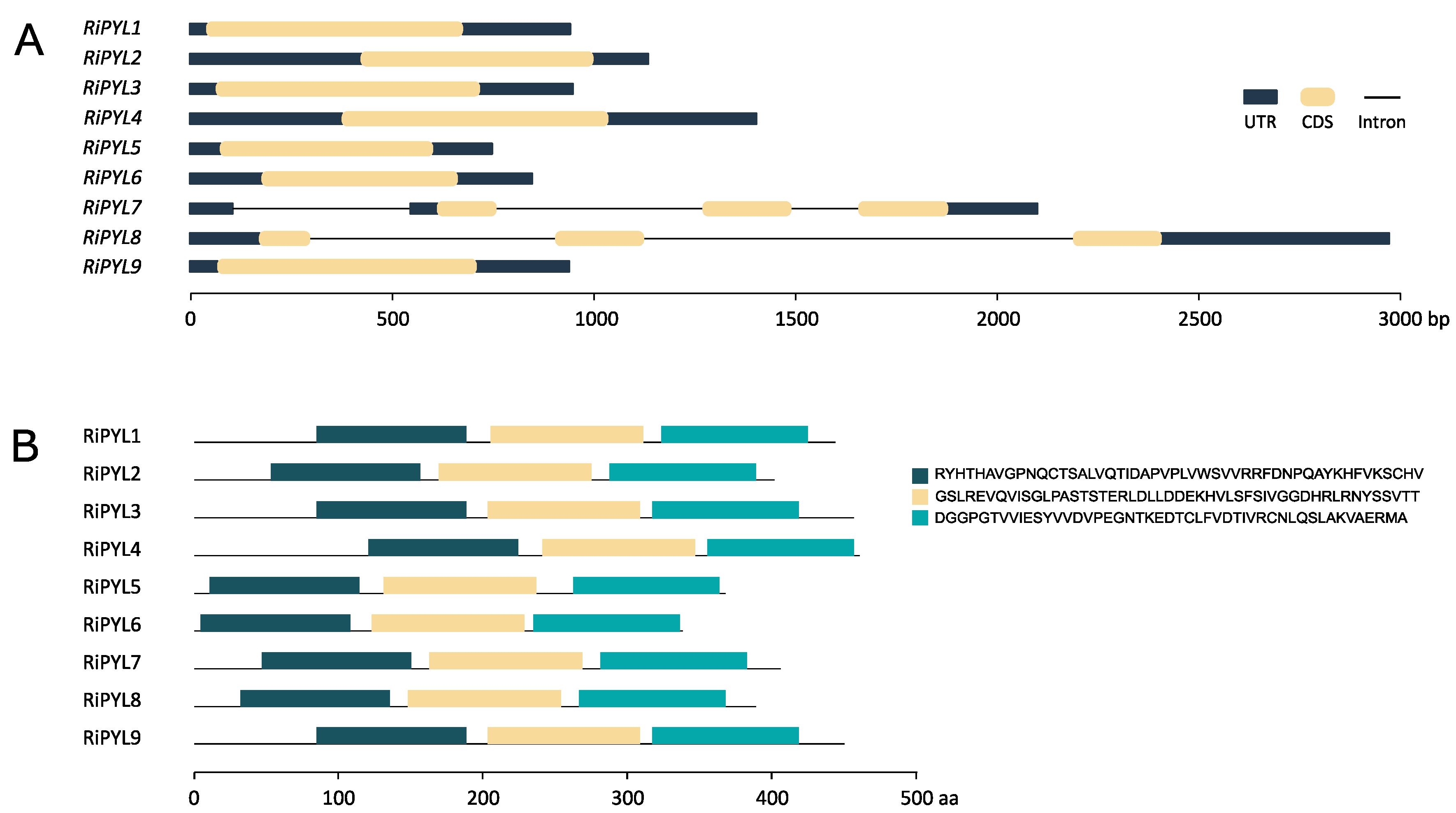

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, F.; Moya, M.; Rivera-Mora, C.; Zúñiga, P.E.; Jara-Cornejo, K.; Muñoz, P.; Ayala-Raso, A.; Munné-Bosch, S.; Figueroa, C.R.; Figueroa, N.E.; et al. Abscisic Acid Synthesis and Signaling during the Ripening of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Plants 2023, 12, 1882. https://doi.org/10.3390/plants12091882
Álvarez F, Moya M, Rivera-Mora C, Zúñiga PE, Jara-Cornejo K, Muñoz P, Ayala-Raso A, Munné-Bosch S, Figueroa CR, Figueroa NE, et al. Abscisic Acid Synthesis and Signaling during the Ripening of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Plants. 2023; 12(9):1882. https://doi.org/10.3390/plants12091882
Chicago/Turabian StyleÁlvarez, Fernanda, Mario Moya, Claudia Rivera-Mora, Paz E. Zúñiga, Karla Jara-Cornejo, Paula Muñoz, Aníbal Ayala-Raso, Sergi Munné-Bosch, Carlos R. Figueroa, Nicolás E. Figueroa, and et al. 2023. "Abscisic Acid Synthesis and Signaling during the Ripening of Raspberry (Rubus idaeus ‘Heritage’) Fruit" Plants 12, no. 9: 1882. https://doi.org/10.3390/plants12091882
APA StyleÁlvarez, F., Moya, M., Rivera-Mora, C., Zúñiga, P. E., Jara-Cornejo, K., Muñoz, P., Ayala-Raso, A., Munné-Bosch, S., Figueroa, C. R., Figueroa, N. E., Valdenegro, M., Alvaro, J. E., Schwab, W., Defilippi, B. G., & Fuentes, L. (2023). Abscisic Acid Synthesis and Signaling during the Ripening of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Plants, 12(9), 1882. https://doi.org/10.3390/plants12091882








