Monitoring of Volatile Organic Compounds in Strawberry Genotypes over the Harvest Period
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Volatile Organic Compounds
2.2. Effect of Harvest Time on Strawberry Volatiles
3. Materials and Methods
3.1. Plant Material
3.2. Fruit Measurements and Experimental Design
3.3. Reagents and Chemicals
3.4. Preparation of Samples for Analysis of Strawberry Volatiles
3.5. Gas Chromatography–Mass Spectrometry (GC-MS)
3.6. Identification and Relative Quantification of Strawberry Volatiles
3.7. Statistical Analysis of Strawberry Volatiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Xiong, J.; Shu, Z.; Dong, C.; Gu, T.; Sun, P.; He, S.; Jiang, M.; Xia, Z.; Xue, J.; et al. The Telomere-to-Telomere Genome of Fragaria vesca Reveals the Genomic Evolution of Fragaria and the Origin of Cultivated Octoploid Strawberry. Hortic. Res. 2023, 10, uhad027. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAO Statistical Database (FAOSTAT). 2020. Available online: http://data.un.org/Data.aspx?d=FAO&f=itemCode%3A544 (accessed on 3 March 2023).
- Ferrão, L.F.V.; Sater, H.; Lyrene, P.; Amadeu, R.R.; Sims, C.A.; Tieman, D.M.; Munoz, P.R. Terpene Volatiles Mediates the Chemical Basis of Blueberry Aroma and Consumer Acceptability. Food Res. Int. 2022, 158, 111468. [Google Scholar] [CrossRef]
- Rubinstein, J. Fragaria Xananassa: Past, Present and Future Production of the Modern Strawberry; University of Minnesota: Minneapolis, MN, USA, 2015. [Google Scholar]
- Mezzetti, B.; Giampieri, F.; Zhang, Y.T.; Zhong, C.F. Status of Strawberry Breeding Programs and Cultivation Systems in Europe and the Rest of the World. J. Berry Res. 2018, 8, 205–221. [Google Scholar] [CrossRef]
- Ulrich, D.; Kecke, S.; Olbricht, K. What Do We Know about the Chemistry of Strawberry Aroma? J. Agric. Food Chem. 2018, 66, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, I.; Holden, M.A. Strawberry Flavour: Analysis and Biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Rohloff, J. Impact of Agricultural and Environmental Factors on Strawberry (Fragaria x Ananassa Duch.) Aroma-A Review. Eur. J. Plant Sci. Biotechnol. 2011, 5, 17–34. [Google Scholar]
- Tressl, R.; Drawert, F.; Heimann, W. Notizen: Gaschromatographisch-Massenspektrometrische Bestandsaufnahme von Erdbeer-Aromastoffen. Z. Nat. B 1969, 24, 1201–1202. [Google Scholar] [CrossRef]
- Cannon, R.J.; Agyemang, D.; Curto, N.L.; Yusuf, A.; Chen, M.Z.; Janczuk, A.J. In-Depth Analysis of Ciflorette Strawberries (Fragaria × Ananassa ‘Ciflorette’) by Multidimensional Gas Chromatography and Gas Chromatography-Olfactometry. Flavour Fragr. J. 2015, 30, 302–319. [Google Scholar] [CrossRef]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of Selected Aroma-Active Compounds in Strawberries by Headspace Solid-Phase Microextraction Gas Chromatography and Correlation with Sensory Descriptive Analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Schieberle, P.; Hofmann, T. Evaluation of the Character Impact Odorants in Fresh Strawberry Juice by Quantitative Measurements and Sensory Studies on Model Mixtures. J. Agric. Food Chem. 1997, 45, 227–232. [Google Scholar] [CrossRef]
- Larsen, M.; Poll, L.; Erik Olsen, C. Evaluation of the Aroma Composition of Some Strawberry (Fragaria Ananassa Duch) Cultivars by Use of Odour Threshold Values. Z. Lebensm-Unters. Forsch. 1992, 195, 536–539. [Google Scholar] [CrossRef]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of Volatiles from Two Subtropical Strawberry Cultivars Using GC-Olfactometry, GC-MS Odor Activity Values, and Sensory Analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef] [PubMed]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 20 October 2022).
- Fan, Z.; Plotto, A.; Bai, J.; Whitaker, V.M. Volatiles Influencing Sensory Attributes and Bayesian Modeling of the Soluble Solids–Sweetness Relationship in Strawberry. Front. Plant Sci. 2021, 12, 640704. [Google Scholar] [CrossRef]
- Urün, I.; Attar, S.H.; Sönmez, D.A.; Gündeşli, M.A.; Ercişli, S.; Kafkas, N.E.; Bandić, L.M.; Duralija, B. Comparison of Polyphenol, Sugar, Organic Acid, Volatile Compounds, and Antioxidant Capacity of Commercially Grown Strawberry Cultivars in Turkey. Plants 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Bruaut, M.; Chalot, G.; Cottet, V. Impact of Maturity Stage at Harvest on the Main Physicochemical Characteristics, the Levels of Vitamin C, Polyphenols and Volatiles and the Sensory Quality of Gariguette Strawberry. Eur. Food Res. Technol. 2021, 247, 37–49. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Liu, X.; Xiao, Y.; Zhang, Z.; Shi, Y.; Kong, W.; Yang, X.; Jiang, G.; Zhang, B.; et al. Cultivation Conditions Change Aroma Volatiles of Strawberry Fruit. Horticulturae 2021, 7, 81. [Google Scholar] [CrossRef]
- Rey-Serra, P.; Mnejja, M.; Monfort, A. Inheritance of Esters and Other Volatile Compounds Responsible for the Fruity Aroma in Strawberry. Front. Plant Sci. 2022, 13, 959155. [Google Scholar] [CrossRef]
- Douillard, C.; Guichard, E. The Aroma of Strawberry (Fragaria Ananassa): Characterisation of Some Cultivars and Influence of Freezing. J. Sci. Food Agric. 1990, 50, 517–531. [Google Scholar] [CrossRef]
- Larsen, M.; Poll, L. Changes in the Composition of Aromatic Compounds and Other Quality Parameters of Strawberries during Freezing and Thawing. Z. Lebensm-Unters. Forsch. 1995, 201, 205–207. [Google Scholar] [CrossRef]
- Akhatou, I.; Fernández-Recamales, Á. Nutritional and Nutraceutical Quality of Strawberries in Relation to Harvest Time and Crop Conditions. J. Agric. Food Chem. 2014, 62, 5749–5760. [Google Scholar] [CrossRef]
- Watson, R.; Wright, C.J.; McBurney, T.; Taylor, A.J.; Linforth, R.S.T. Influence of Harvest Date and Light Integral on the Development of Strawberry Flavour Compounds. J. Exp. Bot. 2002, 53, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Pelayo-Zaldívar, C.; Ebeler, S.E.; Kader, A.A. Cultivar and Harvest Date Effects on Flavor and Other Quality Attributes of California Strawberries. J. Food Qual. 2005, 28, 78–97. [Google Scholar] [CrossRef]
- Jouquand, C.; Chandler, C.; Plotto, A.; Goodner, K. A Sensory and Chemical Analysis of Fresh Strawberries Over Harvest Dates and Seasons Reveals Factors that Affect Eating Quality. J. Am. Soc. Hortic. Sci. 2008, 133, 859–867. [Google Scholar] [CrossRef]
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry Flavor: Diverse Chemical Compositions, a Seasonal Influence, and Effects on Sensory Perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Methyl Jasmonate in Conjunction with Ethanol Treatment Increases Antioxidant Capacity, Volatile Compounds and Postharvest Life of Strawberry Fruit. Eur. Food Res. Technol. 2005, 221, 731–738. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of Storage Temperatures on Antioxidant Capacity and Aroma Compounds in Strawberry Fruit. LWT 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Leonardou, V.K.; Doudoumis, E.; Tsormpatsidis, E.; Vysini, E.; Papanikolopoulos, T.; Papasotiropoulos, V.; Lamari, F.N. Quality Traits, Volatile Organic Compounds, and Expression of Key Flavor Genes in Strawberry Genotypes over Harvest Period. Int. J. Mol. Sci. 2021, 22, 13499. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Sayago, A.; Akhatou, I.; Fernández-Recamales, Á. Volatile Profiling of Strawberry Fruits Cultivated in a Soilless System to Investigate Cultivar-Dependent Chemical Descriptors. Foods 2020, 9, 768. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Nicolai, B.M.; Hertog, M.L.A.T.M. Optimization of HS SPME Fast GC-MS for High-Throughput Analysis of Strawberry Aroma. Food Anal. Methods 2013, 6, 512–520. [Google Scholar] [CrossRef]
- Ozcan, G.; Barringer, S. Effect of Enzymes on Strawberry Volatiles during Storage, at Different Ripeness Level, in Different Cultivars, and during Eating. J. Food Sci. 2011, 76, C324–C333. [Google Scholar] [CrossRef]
- Azzi-Achkouty, S.; Estephan, N.; Ouaini, N.; Rutledge, D.N. Headspace Solid-Phase Microextraction for Wine Volatile Analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 2009–2020. [Google Scholar] [CrossRef]
- Prat, L.; Espinoza, M.I.; Agosin, E.; Silva, H. Identification of Volatile Compounds Associated with the Aroma of White Strawberries (Fragaria chiloensis). J. Sci. Food Agric. 2014, 94, 752–759. [Google Scholar] [CrossRef]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The Aroma Volatile Repertoire in Strawberry Fruit: A Review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Olbricht, K.; Ulrich, D.; Weiss, K.; Grafe, C. Variation in the Amounts of Selected Volatiles in a Model Population of Fragaria × Ananassa Duch. As Influenced by Harvest Year. J. Agric. Food Chem. 2011, 59, 944–952. [Google Scholar] [CrossRef]
- Stopar, M.; Bolcina, U.; Vanzo, A.; Vrhovsek, U. Lower Crop Load for Cv. Jonagold Apples (Malus × Domestica Borkh.) Increases Polyphenol Content and Fruit Quality. J. Agric. Food Chem. 2002, 50, 1643–1646. [Google Scholar] [CrossRef]
- De Salvador, F.R.; Fisichella, M.; Fontanari, M. Correlations between Fruit Size and Fruit Quality in Apple Trees with High and Standard Crop Load Levels. J. Fruit Ornam. Plant Res. 2006, 14, 113–122. [Google Scholar]
- Correia, P.J.; Pestana, M.; Martinez, F.; Ribeiro, E.; Gama, F.; Saavedra, T.; Palencia, P. Relationships between Strawberry Fruit Quality Attributes and Crop Load. Sci. Hortic. 2011, 130, 398–403. [Google Scholar] [CrossRef]
- Ariza, M.T.; Martínez-Ferri, E.; Domínguez, P.; Medina, J.J.; Miranda, L.; Soria, C. Effects of Harvest Time on Functional Compounds and Fruit Antioxidant Capacity in Ten Strawberry Cultivars. J. Berry Res. 2015, 5, 71–80. [Google Scholar] [CrossRef]
- Tosetti, R.; Elmi, F.; Pradas, I.; Cools, K.; Terry, L.A. Continuous Exposure to Ethylene Differentially Affects Senescence in Receptacle and Achene Tissues in Strawberry Fruit. Front. Plant Sci. 2020, 11, 174. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
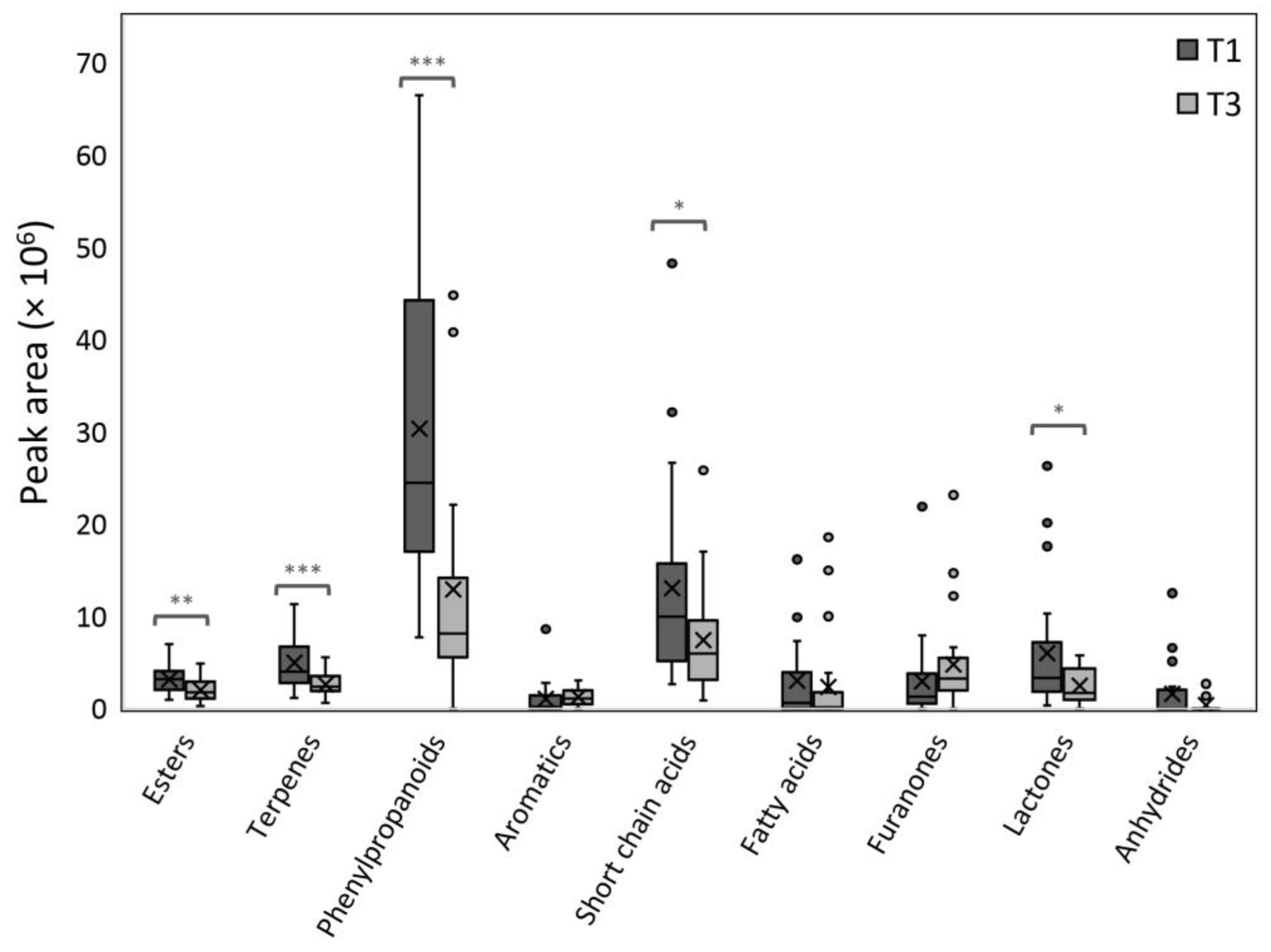
| Compound Name | Compound Category | RIexp | Range of Peak Area Percentages (%) a (T1) | Range of Peak Area Percentages (%) a (T3) | Genotype (F-Values) b |
|---|---|---|---|---|---|
| 2-Methylpropanoic acid | Acids | 777 | 0.29–1.36 | 0.10–1.83 | 1.742 |
| Ethyl butanoate | Esters | 804 | 0.38–2.48 | 0.32–0.99 | 0.732 |
| Butanoic acid | Acids | 808 | 0.71–6.08 | 0.17–4.27 | 2.989 ** |
| Ethyl 2-methylbutanoate | Esters | 849 | 0.12–0.86 | 0.06–0.20 d | 0.641 |
| Maleic anhydride c | Anhydrides | 862 | 0.86–6.12 | ≤1.22 e,f | 0.777 |
| 2-Methylbutanoic acid | Acids | 871 | 0.27–4.59 | 0.48–9.63 | 1.681 |
| Butyrolactone | Lactones | 916 | 0.17–7.83 | 0.18–6.72 | 0.771 |
| Methyl hexanoate | Esters | 926 | 0.24–2.54 | 0.34–4.93 | 1.053 |
| Citraconic anhydride c | Anhydrides | 948 | 0.85–2.93 | ≤2.23 e,f | 0.886 |
| Ethyl hexanoate | Esters | 1001 | 0.46–3.42 | 0.66–6.07 | 1.771 |
| Hexanoic acid | Acids | 1009 | 0.73–31.88 | 1.31–36.56 | 1.509 |
| Succinic anhydride c | Anhydrides | 1027 | 0.34–5.38 | ≤1.90 e,f | 0.834 |
| Limonene | Terpenes | 1028 | 0.17–1.01 | 0.09–0.97 | 1.468 |
| Benzyl Alcohol | Aromatic compounds | 1038 | 0.31–1.89 | 0.40–2.96 | 1.248 |
| Mesifurane | Furanones | 1063 | 0.09–9.92 | 0.37–7.36 | 2.450 * |
| Furaneol | Furanones | 1070 | 0.62–12.15 | 2.19–20.66 | 3.285 ** |
| trans-Linalool oxide | Terpenes | 1089 | 0.19–1.04 | 0.23–1.92 | 1.970 * |
| Linalool | Terpenes | 1101 | 0.39–3.91 | 0.26–3.85 | 2.167 * |
| Coumaran | Phenylpropanoids | 1227 | 1.30–5.07 | 1.82–7.22 | 1.316 |
| trans-Cinnamic acid b | Phenylpropanoids | 1481 | 6.96–64.42 | 9.99–55.42 | 0.516 |
| γ-Decalactone | Lactones | 1472 | 1.18–12.56 | 2.35–13.80 | 2.656 * |
| Levoglucosan | Others | 1553 | 0.27–9.48 | 0.51–11.83 | 0.757 |
| trans-(Ε)-Nerolidol | Terpenes | 1567 | 0.53–4.60 | 0.18–2.60 | 0.913 |
| Bisabolol oxide II | Terpenes | 1659 | 1.08–6.01 | 1.45–5.15 | 0.617 |
| γ-Dodecalactone | Lactones | 1682 | 0.22–0.97 | 0.13–1.08 e | 0.943 |
| Hexadecanoic acid | Acids/Fatty acids | 1968 | 0.79–9.85 | 0.34–12.52 | 0.637 |
| Oleic and Elaidic acid | Acids/Fatty acids | 2141 | 0.61–5.08 | 1.40–6.84 | 0.756 |
| Stearic acid | Acids/Fatty acids | 1682 | 0.22–0.97 | 0.38–3.19 | 0.754 |
| Volatile Constituent | F-Value (Significance) | |
|---|---|---|
| Harvest Date (Two-Point T1/T3) | Harvest Date (Four-Point T1/T2/T3/T4) | |
| Esters | 7.179 ** | 1.726 |
| Terpenes | 15.358 *** | 2.151 |
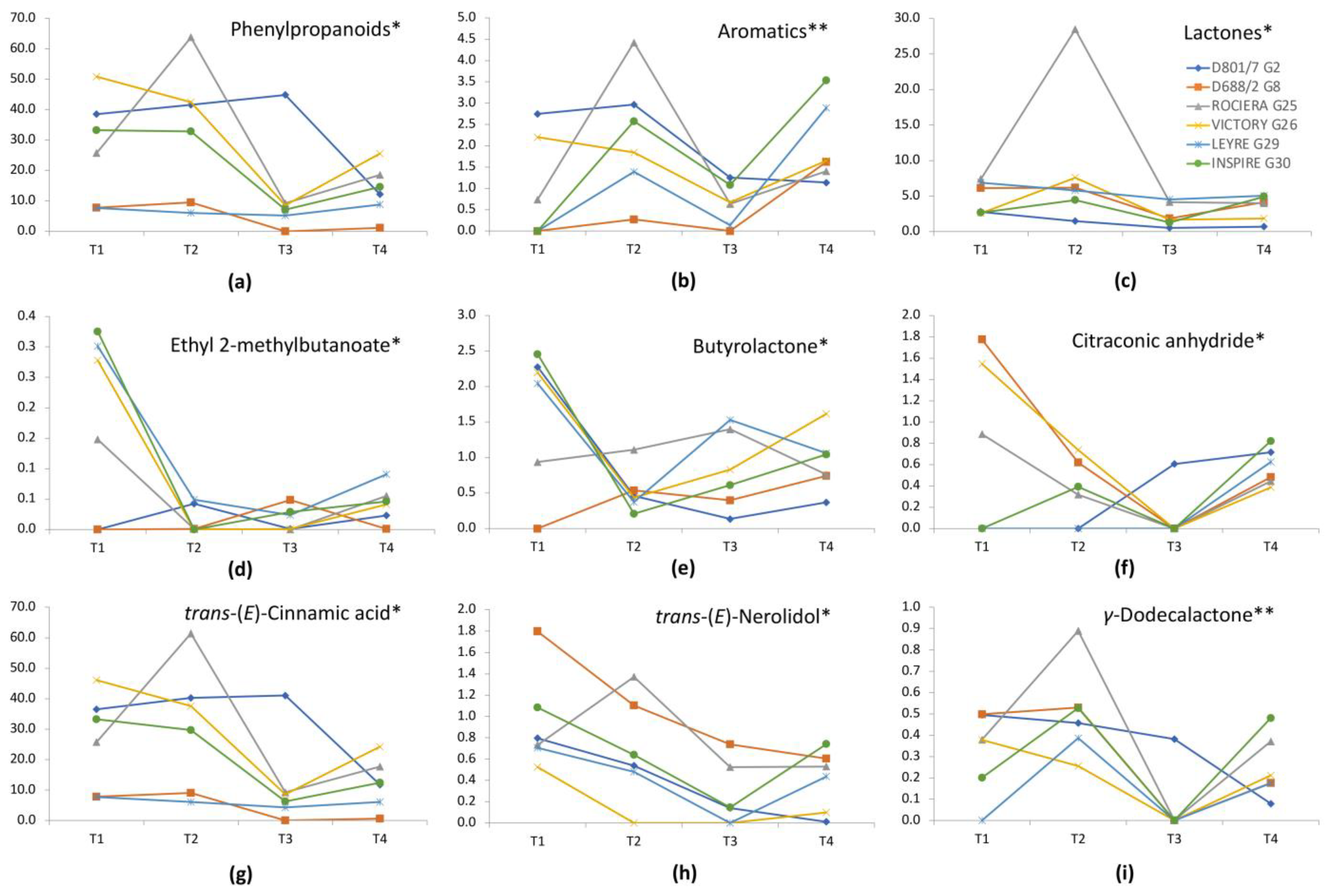
| Phenylpropanoids | 16.972 *** | 3.701 * |
| Aromatic compounds | 0.245 | 6.019 ** |
| Short Chain acids | 5.756 * | 2.433 |
| Lactones | 7.015 * | 4.229 * |
| Ethyl butanoate | 7.836 * | 0.467 |
| Butanoic acid | 5.659 * | 0.375 |
| Ethyl 2-methylbutanoate | 16.877 *** | 3.983 * |
| Butyrolactone | 10.797 ** | 3.857 * |
| Citraconic anhydride | 3.034 | 6.511 * |
| Ethyl hexanoate | 4.800 * | 2.537 |
| Hexanoic acid | 5.561 * | 2.573 |
| Limonene | 4.219 * | 1.456 |
| Furaneol | 4.813 ** | 0.019 |
| Linalool | 9.530 ** | 2.114 |
| trans-Cinnamic acid | 19.073 *** | 4.008 * |
| γ-Decalactone | 7.498 * | 1.736 |
| trans-(Ε)-Nerolidol | 15.152 *** | 4.307 * |
| Bisabolol oxide II | 11.866 ** | 1.416 |
| γ-Dodecalactone | 9.957 ** | 7.291 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passa, K.; Simal, C.; Tsormpatsidis, E.; Papasotiropoulos, V.; Lamari, F.N. Monitoring of Volatile Organic Compounds in Strawberry Genotypes over the Harvest Period. Plants 2023, 12, 1881. https://doi.org/10.3390/plants12091881
Passa K, Simal C, Tsormpatsidis E, Papasotiropoulos V, Lamari FN. Monitoring of Volatile Organic Compounds in Strawberry Genotypes over the Harvest Period. Plants. 2023; 12(9):1881. https://doi.org/10.3390/plants12091881
Chicago/Turabian StylePassa, Kondylia, Carmen Simal, Evangelos Tsormpatsidis, Vasileios Papasotiropoulos, and Fotini N. Lamari. 2023. "Monitoring of Volatile Organic Compounds in Strawberry Genotypes over the Harvest Period" Plants 12, no. 9: 1881. https://doi.org/10.3390/plants12091881
APA StylePassa, K., Simal, C., Tsormpatsidis, E., Papasotiropoulos, V., & Lamari, F. N. (2023). Monitoring of Volatile Organic Compounds in Strawberry Genotypes over the Harvest Period. Plants, 12(9), 1881. https://doi.org/10.3390/plants12091881








