Salt Tolerance of Limonium gmelinii subsp. hungaricum as a Potential Ornamental Plant for Secondary Salinized Soils
Abstract
1. Introduction
2. Results
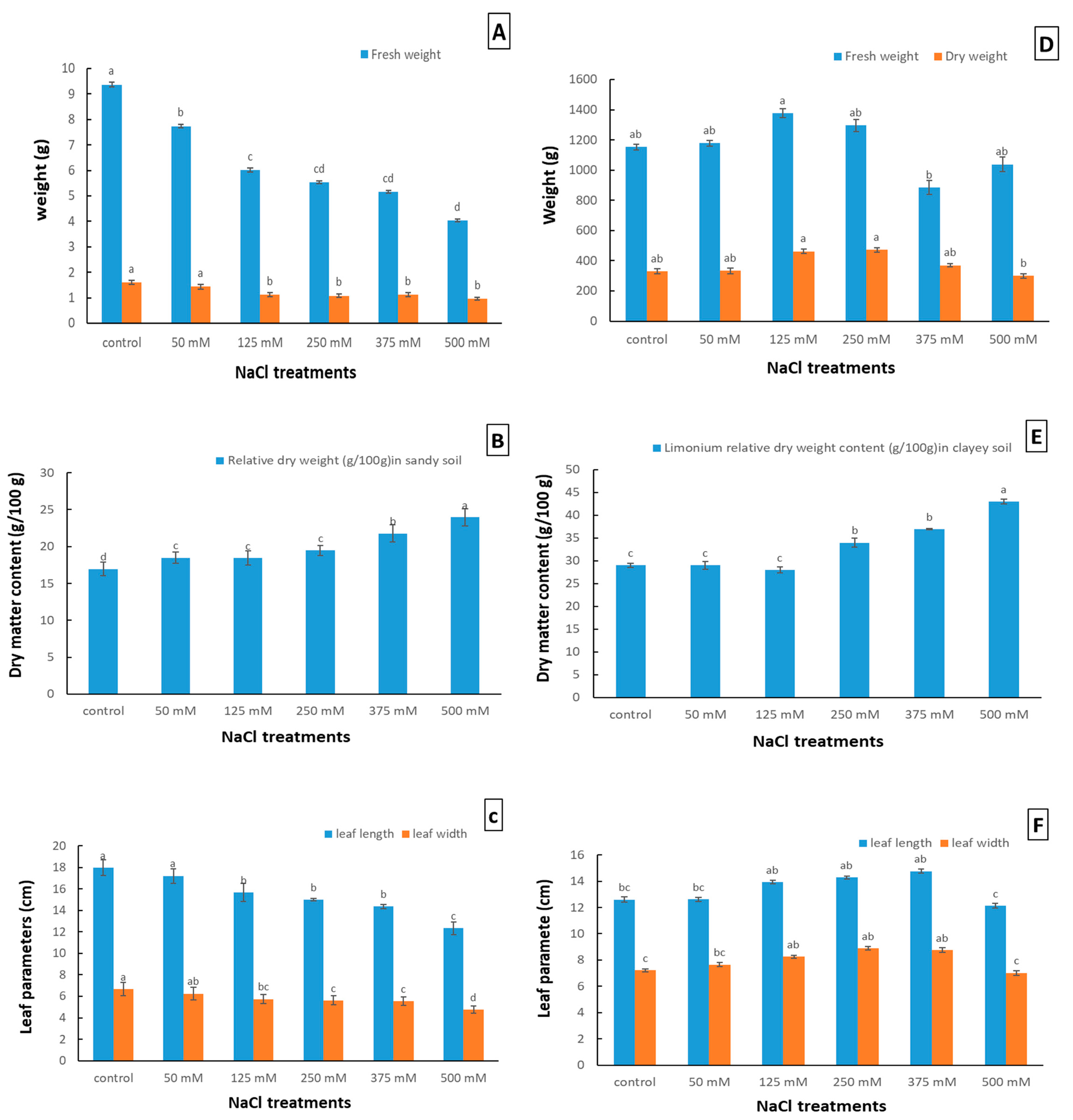
2.1. Effect of NaCl Stress on the Growth and Development of Limonium gmelinii subsp. hungaricum in Sandy and Clayey Soil
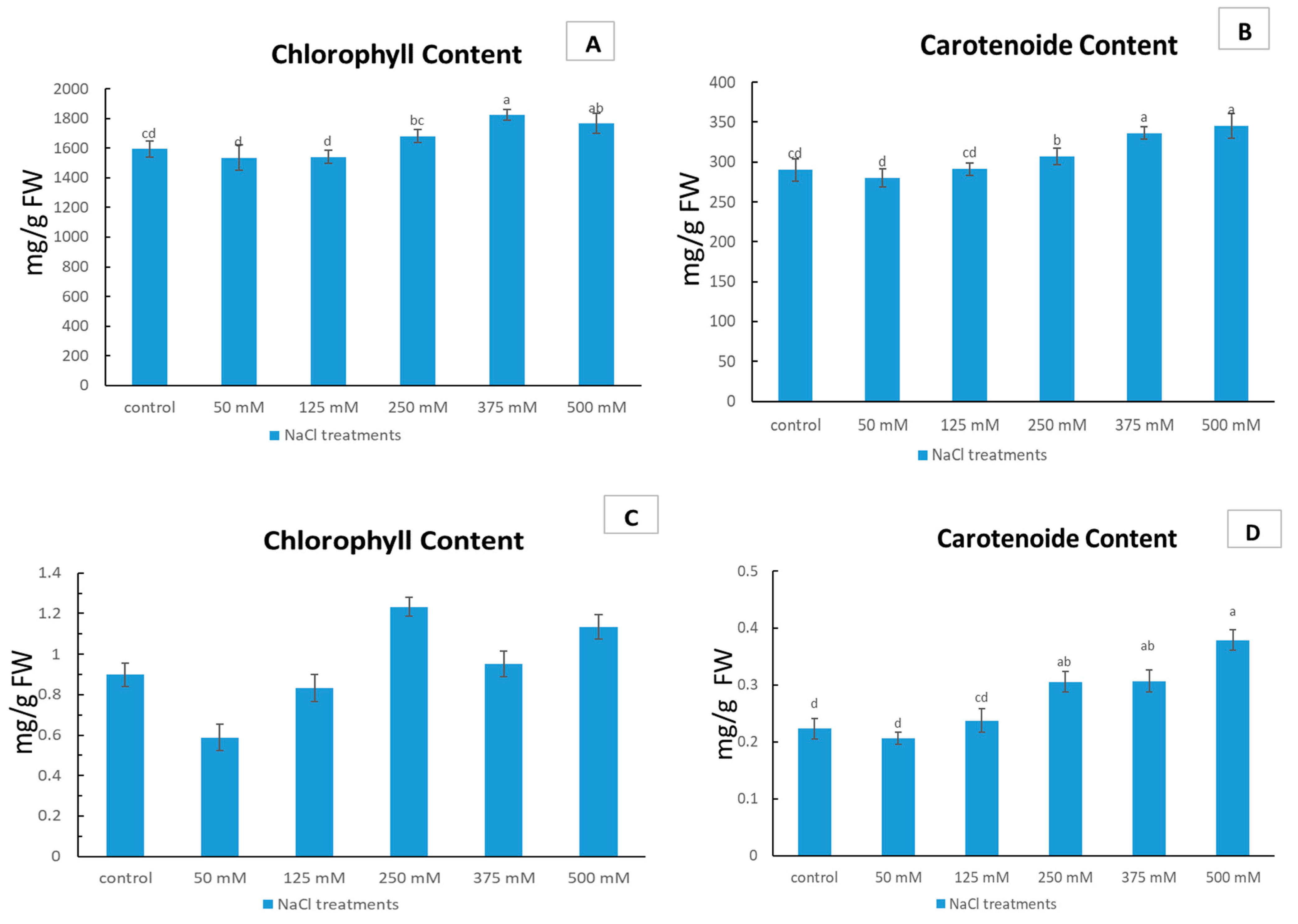
2.2. The Effects of NaCl Concentration on Chlorophyll and Carotenoid Content in Limonium gmelinii subsp. hungaricum Grown in Sandy and Clayey Soils
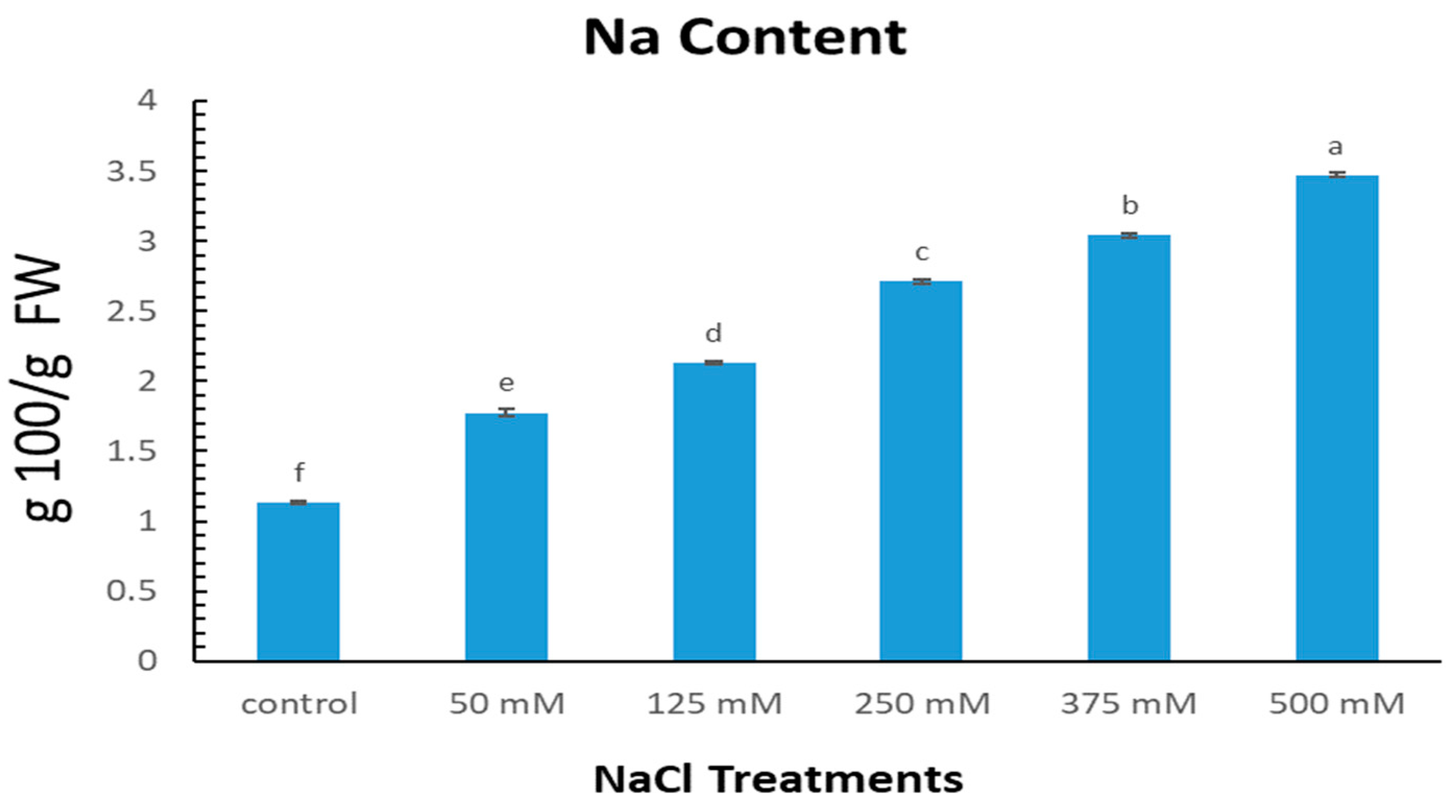
2.3. Effect of NaCl Levels on Sodium Content in Limonium gmelinii subsp. hungaricum Leaves
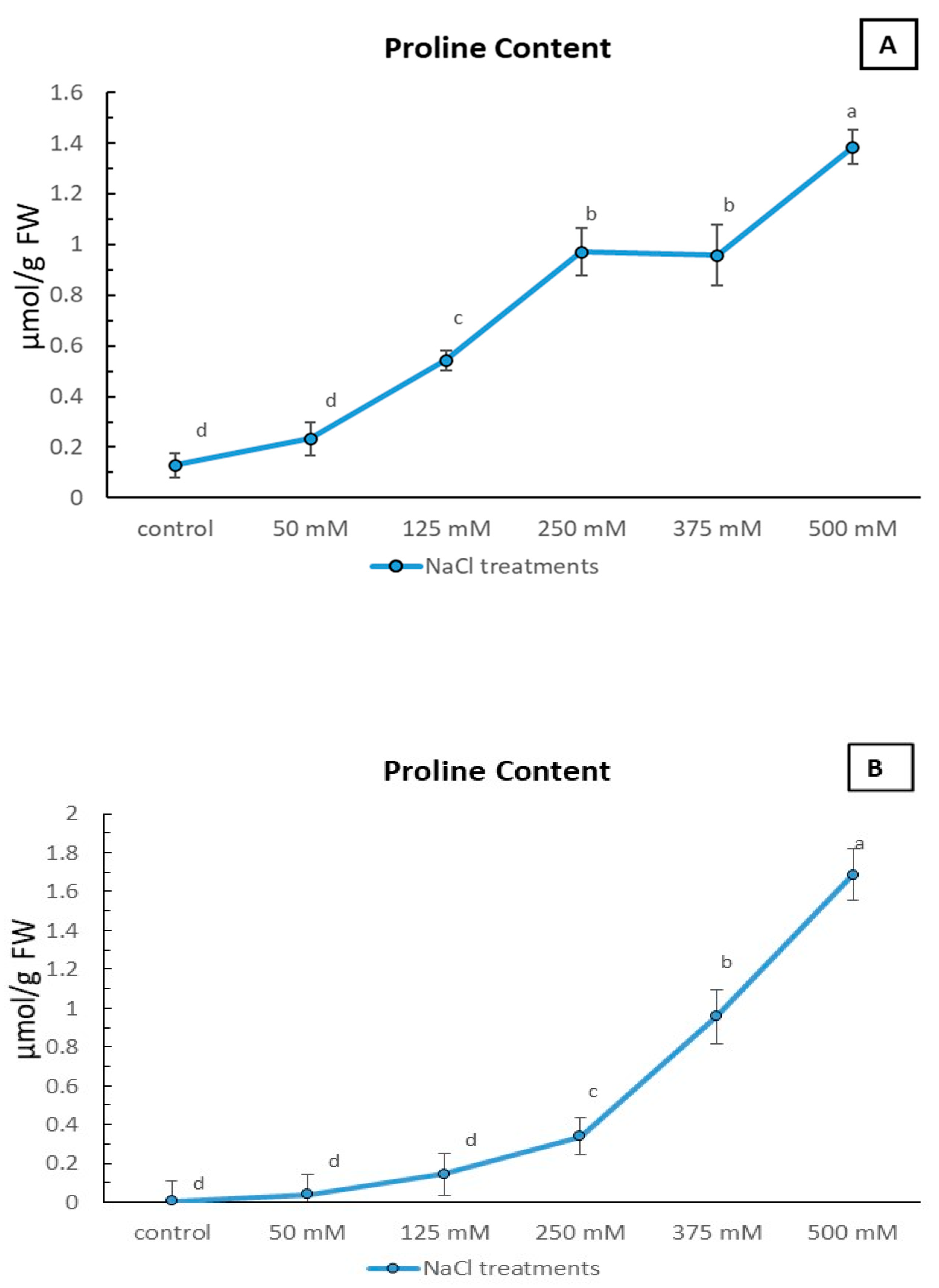
2.4. Proline Content Response of Limonium gmelinii subsp. hungaricum Leaves to Increasing NaCl Concentrations in Sandy and Clayey Soils
3. Discussion
4. Materials and Methods
4.1. Material, Cultivation Conditions, and Trial Layout
4.2. Morphological Characteristics
4.3. Chlorophyll, Carotenoid and Proline Determination
4.4. Determination of Sodium Content in Leaves Grown in Sand
4.5. Statical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential Use of Halophytes to Remediate Saline Soils. Biomed. Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant Cell-Surface GIPC Sphingolipids Sense Salt to Trigger Ca2+ Influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive Physiology of Halophytes: Current Standing. Front. Plant Sci. 2019, 9, 1954. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.-P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.-K.; Gong, Z. Reactive Oxygen Species Signaling and Stomatal Movement in Plant Responses to Drought Stress and Pathogen Attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blumwald, E. Developing Salt-Tolerant Crop Plants: Challenges and Opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using Euhalophytes to Understand Salt Tolerance and to Develop Saline Agriculture: Suaeda Salsa as a Promising Model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Wang, B. Progress in Studying Salt Secretion from the Salt Glands in Recretohalophytes: How Do Plants Secrete Salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef]
- Koutroumpa, K.; Theodoridis, S.; Warren, B.H.; Jiménez, A.; Celep, F.; Doğan, M.; Romeiras, M.M.; Santos-Guerra, A.; Fernández-Palacios, J.M.; Caujapé-Castells, J.; et al. An Expanded Molecular Phylogeny of Plumbaginaceae, with Emphasis on Limonium (Sea Lavenders): Taxonomic Implications and Biogeographic Considerations. Ecol. Evol. 2018, 8, 12397–12424. [Google Scholar] [CrossRef] [PubMed]
- González-Orenga, S.; Llinares, J.V.; Al Hassan, M.; Fita, A.; Collado, F.; Lisón, P.; Vicente, O.; Boscaiu, M. Physiological and Morphological Characterisation of Limonium Species in Their Natural Habitats: Insights into Their Abiotic Stress Responses. Plant Soil 2020, 449, 267–284. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Plantaginaceae to Compositae and Rubiaceae). In Flora Europaea; Cambridge University Press: Cambridge, UK, 1976; Volume 4. [Google Scholar]
- González-Orenga, S.; Grigore, M.-N.; Boscaiu, M.; Vicente, O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Salt Stress Effects on Growth, Pigments, Proteins and Lipid Peroxidation in Salicornia Persica and S. Europaea. Biol. Plant. 2009, 53, 243–248. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Hussain, T.; Huchzermeyer, B.; Khan, M.A. Photosynthetic and Growth Responses of a Perennial Halophytic Grass Panicum Turgidum to Increasing NaCl Concentrations. Environ. Exp. Bot. 2013, 91, 22–29. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive Oxygen Species Regulation and Antioxidant Defence in Halophytes. Funct. Plant Biol. 2013, 40, 832. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Yuan, F.; Lyu, M.-J.A.; Leng, B.-Y.; Zhu, X.-G.; Wang, B.-S. The Transcriptome of NaCl-Treated Limonium Bicolor Leaves Reveals the Genes Controlling Salt Secretion of Salt Gland. Plant Mol. Biol. 2016, 91, 241–256. [Google Scholar] [CrossRef]
- Hill, A.E. Ion and Water Transport in Limonium. III. Time Constants of the Transport System. Biochim. Biophys. Acta Biomembr. 1970, 196, 66–72. [Google Scholar] [CrossRef]
- Mi, P.; Yuan, F.; Guo, J.; Han, G.; Wang, B. Salt Glands Play a Pivotal Role in the Salt Resistance of Four Recretohalophyte Limonium Mill. Species. Plant Biol. 2021, 23, 1063–1073. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Bogdanova, E.S.; Ivanova, L.A.; Ivanov, L.A.; Tabalenkova, G.N.; Zakhozhiy, I.G.; Nesterov, V.N. Structural and Functional Organization of the Photosynthetic Apparatus in Halophytes with Different Strategies of Salt Tolerance. Photosynthetica 2016, 54, 405–413. [Google Scholar] [CrossRef]
- Daraban, I.N.; Mihali, C.V.; Turcus, V.; Ardelean, A.; Arsene, G.G. ESEM and EDAX Observations on Leaf and Stem Epidermal Structures (Stomata and Salt Glands) in Limonium Gmelinii (Willd.) Kuntze. Ann. Rom. Soc. Cell Biol. 2013, 18, 123. [Google Scholar]
- Roohi Aslam A Critical Review on Halophytes: Salt Tolerant Plants. J. Med. Plants Res. 2011, 5, 7108–7118. [CrossRef]
- Leng, B.Y.; Yuan, F.; Dong, X.X.; Wang, J.; Wang, B.S. Distribution Pattern and Salt Excretion Rate of Salt Glands in Two Recretohalophyte Species of Limonium (Plumbaginaceae). S. Afr. J. Bot. 2018, 115, 74–80. [Google Scholar] [CrossRef]
- Ghanem, A.E.M.F.M.; Mohamed, E.; Kasem, A.M.M.A.; El-Ghamery, A.A. Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, Distribution and Roles of Osmoprotective Compounds Accumulated in Halophytes under Abiotic Stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Kanber, R.; Ünlü, M.; Kapur, B.; Özekici, B.; Donma, S. Adaptation of Contemporary Irrigation Systems to Face the Challenges of Future Climate Changes in the Mediterranean Region: A Case Study of the Lower Seyhan Irrigation System. In Climate Change Impacts on Basin Agro-Ecosystems. The Anthropocene: Politik—Economics—Society—Science; Watanabe, T., Kapur, S., Aydın, M., Kanber, R., Akça, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 125–161. [Google Scholar]
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soil, 3rd ed.; Prentice Hall: Lebanon, IN, USA, 2009. [Google Scholar]
- Ondrasek, G.; Romic, D.; Rengel, Z. Interactions of Humates and Chlorides with Cadmium Drive Soil Cadmium Chemistry and Uptake by Radish Cultivars. Sci. Total Environ. 2020, 702, 134887. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Miah, M.G.; Saha, S.R.; Karim, M.A.; Keya, S.S.; Akter, M.; Islam, M.; Tran, L.-S.P. Insight into Salt Tolerance Mechanisms of the Halophyte Achras Sapota: An Important Fruit Tree for Agriculture in Coastal Areas. Protoplasma 2019, 256, 181–191. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Clipson, N.J.W. Halophytes. Q. Rev. Biol. 1986, 61, 313–337. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-Induced Variation in Biochemical Markers Provides Insight into the Mechanisms of Salt Tolerance in Common (Phaseolus Vulgaris) and Runner (P. Coccineus) Beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef] [PubMed]
- Jangra, M.; Devi, S.; Satpal; Kumar, N.; Goyal, V.; Mehrotra, S. Amelioration Effect of Salicylic Acid Under Salt Stress in Sorghum bicolor L. Appl. Biochem. Biotechnol. 2022, 194, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Corn and Soybean Yield Response to Salinity Influenced by Soil Texture. Agron. J. 2018, 110, 1243–1253. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Jia, W. Salt-Stress-Induced ABA Accumulation Is More Sensitively Triggered in Roots than in Shoots. J. Exp. Bot. 2002, 53, 2201–2206. [Google Scholar] [CrossRef]
- Qiu, N.; Lu, Q.; Lu, C. Photosynthesis, Photosystem II Efficiency and the Xanthophyll Cycle in the Salt-adapted Halophyte Atriplex Centralasiatica. New Phytol. 2003, 159, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Rangani, J.; Kumari, A.; Parida, A.K. Efficient Regulation of Arsenic Translocation to Shoot Tissue and Modulation of Phytochelatin Levels and Antioxidative Defense System Confers Salinity and Arsenic Tolerance in the Halophyte Suaeda Maritima. Environ. Exp. Bot. 2017, 143, 149–171. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Y.; Li, X.; Zhang, L.; Fan, S. Transcriptomic Analysis Identifies Novel Genes and Pathways for Salt Stress Responses in Suaeda Salsa Leaves. Sci. Rep. 2020, 10, 4236. [Google Scholar] [CrossRef]
- Lu, C.; Yuan, F.; Guo, J.; Han, G.; Wang, C.; Chen, M.; Wang, B. Current Understanding of Role of Vesicular Transport in Salt Secretion by Salt Glands in Recretohalophytes. Int. J. Mol. Sci. 2021, 22, 2203. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Toma, C. Saline Environments. In Anatomical Adaptations of Halophytes; Springer International Publishing: Cham, Switzerland, 2017; pp. 29–37. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. CRC Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef] [PubMed]
- Gagneul, D.; Aìnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A Reassessment of the Function of the So-Called Compatible Solutes in the Halophytic Plumbaginaceae Limonium Latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling Salt Tolerance Mechanisms in Halophytes: A Comparative Study on Four Mediterranean Limonium Species with Different Geographic Distribution Patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W.; Donahue, R.L. Soils in Our Environment, 7th ed.; Prudence Hall: Englewood Cliffs, NJ, USA, 1995; p. 323. [Google Scholar]
- Panta, S.; Flowers, T.; Doyle, R.; Lane, P.; Haros, G.; Shabala, S. Growth Responses of Atriplex Lentiformis and Medicago Arborea in Three Soil Types Treated with Saline Water Irrigation. Environ. Exp. Bot. 2016, 128, 39–50. [Google Scholar] [CrossRef]
- Warrence, N.J.; Bauder, J.W.; Pearson, K.E. Basics of Salinity and Sodicity Effects on Soil Physical Properties. Dep. L. Resour. Environ. Sci. Mont. State Univ. MT 2002, 129, 1–29. [Google Scholar]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin Improves Drought Stress Tolerance of Tomato by Modulating Plant Growth, Root Architecture, Photosynthesis, and Antioxidant Defense System. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource Use Efficiency of Indoor Lettuce (Lactuca Sativa L.) Cultivation as Affected by Red:Blue Ratio Provided by LED Lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Campbell, C.R.; Plank, C.O. Preparation of Plant Tissue for Laboratory Analysis. In Handbook of Methods for Plant Analysis; Kalra, Y.P., Ed.; Taylor & Francis Group: New York, NY, USA, 1998; p. 287. [Google Scholar]
- West, S.G.; Finch, J.F.; Curran, P.J. Structural Equation Models with Nonnormal Variables: Problems and Remedies. In Structural Equation Modeling: Concepts, Issues, and Applications; Hoyle, R.H., Ed.; Sage Publications Inc.: Newbury Park, CA, USA, 1995; pp. 56–75. [Google Scholar]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Garson, G.D. Testing Statistical Assumptions; Statistical Associates Publishing: Asheboro, NC, USA, 2012. [Google Scholar]
- IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honfi, P.; Eisa, E.A.; Tilly-Mándy, A.; Kohut, I.; Ecseri, K.; Mosonyi, I.D. Salt Tolerance of Limonium gmelinii subsp. hungaricum as a Potential Ornamental Plant for Secondary Salinized Soils. Plants 2023, 12, 1807. https://doi.org/10.3390/plants12091807
Honfi P, Eisa EA, Tilly-Mándy A, Kohut I, Ecseri K, Mosonyi ID. Salt Tolerance of Limonium gmelinii subsp. hungaricum as a Potential Ornamental Plant for Secondary Salinized Soils. Plants. 2023; 12(9):1807. https://doi.org/10.3390/plants12091807
Chicago/Turabian StyleHonfi, Péter, Eman Abdelhakim Eisa, Andrea Tilly-Mándy, Ildikó Kohut, Károly Ecseri, and István Dániel Mosonyi. 2023. "Salt Tolerance of Limonium gmelinii subsp. hungaricum as a Potential Ornamental Plant for Secondary Salinized Soils" Plants 12, no. 9: 1807. https://doi.org/10.3390/plants12091807
APA StyleHonfi, P., Eisa, E. A., Tilly-Mándy, A., Kohut, I., Ecseri, K., & Mosonyi, I. D. (2023). Salt Tolerance of Limonium gmelinii subsp. hungaricum as a Potential Ornamental Plant for Secondary Salinized Soils. Plants, 12(9), 1807. https://doi.org/10.3390/plants12091807





