1. Introduction
Nanotechnologies enable new strategical opportunities in many industries involving agriculture by means of several novel and sometimes underexplored mechanisms [
1,
2,
3]. Generally, nanoparticles (NPs) are defined as entities where at least 1 of the 3 dimensions does not exceed 100 nanometers. This dimension range provides a highly precise and sustainable tool for agriculture [
3,
4]. The dimensions, crystallinity, morphology, and high surface-to-volume ratio of NPs introduce unique chemical, physical, and biological properties, such as tunable reactivity and bioavailability, compared to their corresponding bulk or ionic counterparts [
5]. Due to exclusive physiochemical properties, NPs can be effectively applied for stress mitigation strategies, e.g., heavy metal contamination of the soil [
6,
7].
The sunflower plant of the Asteraceae family is one of the most broadly cultivated oil bearing crops worldwide [
8]. The quality of sunflower oil is highly dependent on the content of oleic and linoleic acids as the human body is unable to naturally produce these essential acids [
9]. Additionally, the common sunflower is highly suitable for foliar application of NP due to their broad leaves acquisition [
10]; diverse leave structures; surface morphology with stomata; trichomes diversity [
11,
12]; and suitable ability to take up, transport, transform, or accumulate various metals to different parts of the plants [
13]. Additionally, the sunflower cultivation environment can be adequately utilized for an ecological insect diversity estimation [
14,
15] in which the metallic NPs may be able to penetrate with varying degrees of harmful effects on terrestrial organisms [
16].
Newly applied NPs, present in low range-concentrations, have the advantage of gradual nutrient release and more targeted delivery, which is in contrast to commercially available fertilizers [
17]. Additionally, spray dispersion research is increasingly focused on the green synthesis of metal-based nanoparticles. Bio-synthetically prepared metal and metal oxide NPs can have various positive effects on crop production, including improving environmental stress tolerance against drought [
3,
18,
19]. Appropriate physiological response of plants could be accelerated synergically due to combination of metal-related nanofertilizes with organic residual compounds from biological synthesis [
3].
Nanoparticles primarily enter plant bodies through their leaf, stem, and root systems. Liu and Lal [
17] categorized Fe, a Zn-based NP, into micronutrient groups of fertilizers with relative essential responses in plants. However, there is limited information available on the combined effect of Au-NPs and SiO
2 composite materials. Elemental zinc is a plant micronutrient required for proper metabolism and acts as an essential part of more than 300 enzymes. It supports photosynthesis functions, regulates auxin and hydrocarbon metabolism, encourages cell membrane integrity, DNA transcription, and performs roles in other biological processes [
20]. Iron deficiency results in leaf chlorosis [
21], negatively influences the morphology and physiology of roots in the soil environment [
22], and is an integral part of proteins and transporters [
23]. Silica is not essential for plants, but it is bioavailable in the form of soluble silicic acid. Higher content of silica showed enhancement of plant vitality, exogenous stress responses, and provided mechanical strength to the arial parts of plant [
24]. Elemental gold does not classify as an essential element. Cations of Au
3+ can inhibit plant growth and development, and change plant physiology, biochemistry, and molecular structures to a certain level [
25]. In this context, in their review, Siddiqi and Husen [
25] present the role of Au NPs with several positive effects on plants which were observed at concentration corresponding to 10 μg mL
−1. Our previous study has shown that ZnO-NP application positively correlated with sunflower head diameter and weight of dry seeds [
26]. Spray application of Fe
3O
4-NPs increased dry and fresh weights of
Nicotiana benthamiana Domin plant [
27]. Similar effects on plant height, stem diameter, and seed yield were observed for SiO
2-NPs application to
Vetiveria zizanioides L. Nash [
28] and for Au-NPs to
Brassica juncea L. Czern. [
29]. Content of sunflower oil was higher after application of TiO
2-NPs. ZnO-NPs were associated with improved sunflower or foxtail millet physiology despite the seasonal climatic variability [
26,
30]. Moreover, the quality of sunflower fully ripe seeds can be affected by mineral nutrients, such as copper, phosphorus, or zinc [
9,
31]. However, the mechanisms of potential uptake and translocation of NPs and their metal derived residues to fully ripe seeds or impact on the profile of macro, micronutrients, and trace elements are still unknown.
The use of photocatalytically active NPs is an attractive platform for enhancing plant physiological reactions [
32]. Grimme et al. [
33] hypothesized that metal NPs can accelerate the energy transfer leading to an increase in plant photosynthesis reactions and resulting in higher quantitative parameters and yield. ZnO-NPs belong to a II-VI semiconductor [
34] and mesoporous silica (SiO
2) provides uniform pore size architecture with huge surface-to-volume ratio, allowing for anchoring of Au-NPs with their photocatalytic properties that can be activated by quantum yield effect. Gold NPs induced by sunlight wavelengths generate enable easier electron transfer which may help in photosynthesis [
35]. In addition to its semiconductor properties, iron oxide (Fe
3O
4) also has either photocatalytic or superparamagnetic properties [
36].
Current research of NPs spray deposition to plants focuses mainly to laboratory studies or greenhouse conditions [
37,
38]. Field experiments and applications in agronomy are still mostly absent. Due to aforementioned facts, our complex study investigated (i) the agronomical aspects of sunflower physiology, yield related parameters, and surface leaf morphology reactions; (ii) the final quality assessment with potential translocation of NPs or their metal residues, ratio of essential acids in sunflower oil, and anti-nutrients content; and (iii) agroecological impact on insect biodiversity based on the determination of epigeic insect groups.
2. Materials and Methods
2.1. Origin and Characterization of Sprayed Nanoparticles Applied on Sunflower
The foliar application of colloidal solution of zinc oxide NPs to sunflower had concentration of 15.99 mg L
−1, and the NPs were commercially acquired from Sigma-Aldrich (Saint Louis, MO, USA). Detailed characterization of the NPs morphology, crystallinity, dimensions, and structure parameters was published by Kolenčík et al. [
30] and colloidal characteristics, such as hydrodynamic diameter and zeta potential, are present in the work of Kolenčík et al. [
39].
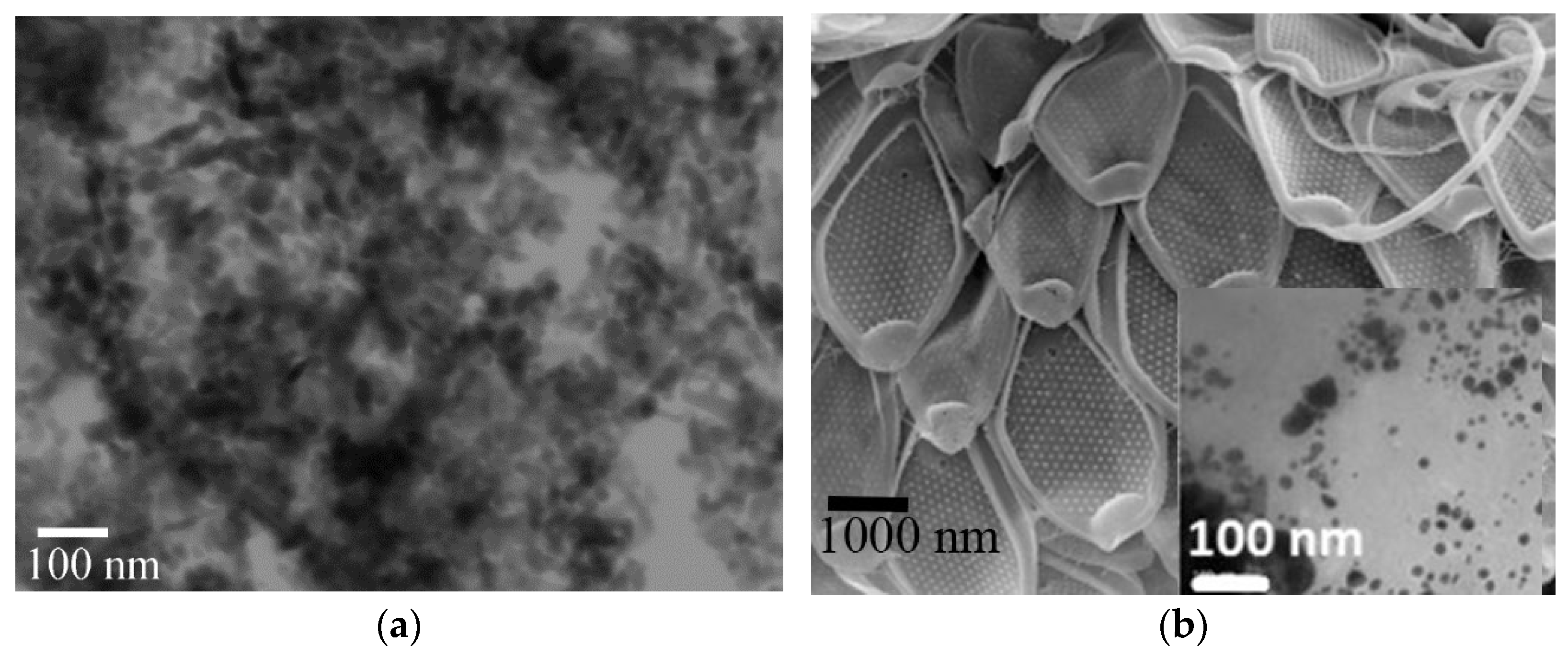
Another treatment used the mesoporous algae cells of
Mallomonas kalinae sp. nov. with silica-based composition (SiO
2) with anchored well-crystalline gold NPs with three types of morphology and approximate dimension of 10 nm. Special photocatalytic properties of this composite material have been already confirmed by observing nerve agent degradation [
40]. For our experimental purposes, we applied 0.1 mg L
−1 of Au-NPs with 10 mg L
−1 of SiO
2 corresponding to mesoporous algae cells of
Mallomonas kalinae sp. nov.
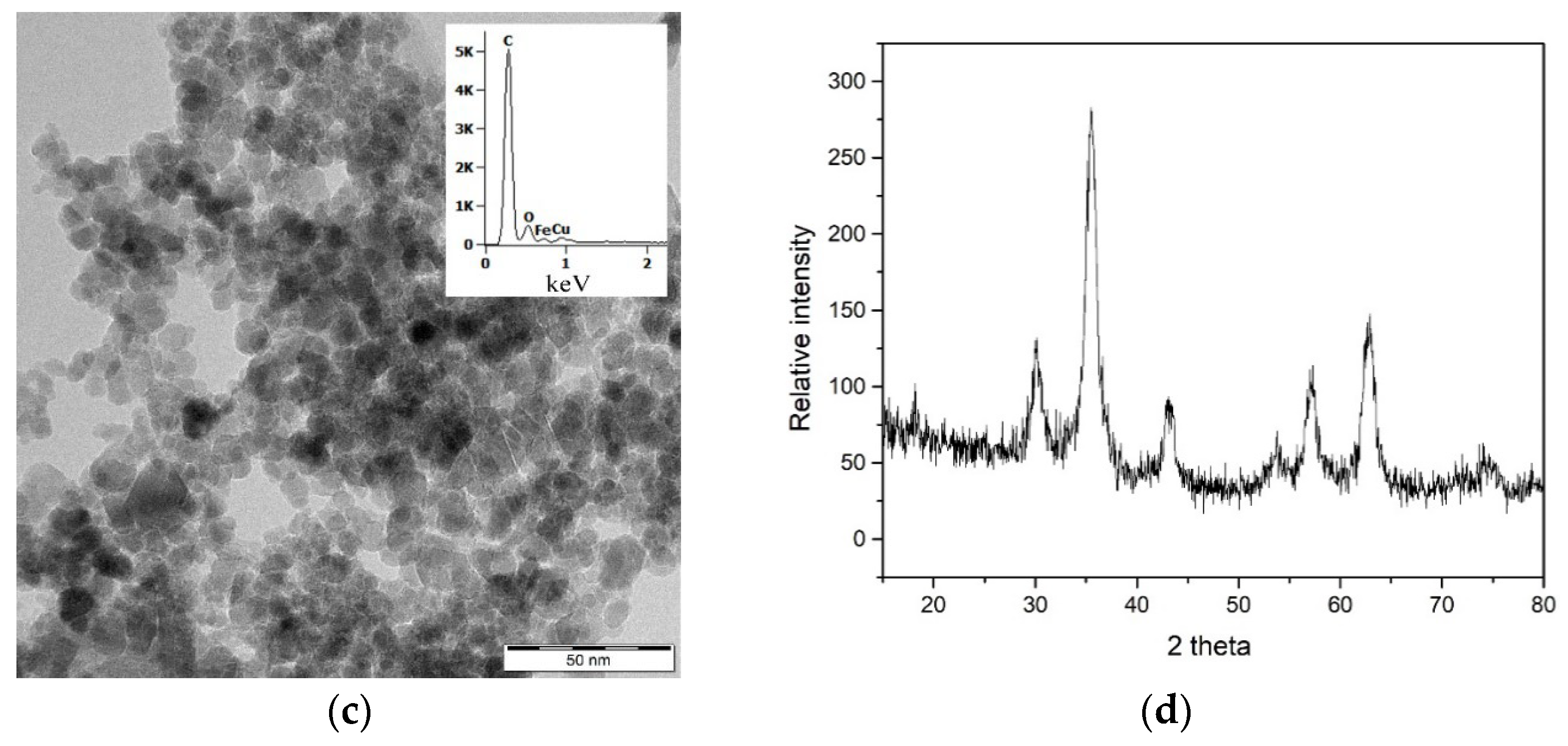
Iron oxide NPs (Fe
3O
4-NPs) were prepared according to Domingo et al. [
41] and we used it at the concentration of 76 mg L
−1. Citrate buffer was used for colloidal stabilization of Fe
3O
4-NP. Crystal structure characteristics and dimension calculation was carried out by X-ray diffraction analysis (XRD). XRD measurement was performed by BRUKER X8 DISCOVER diffractometer (Bruker, MA, USA) at the following conditions: 12 kW (40 kV, 300 mA) with Cu-anode. Gained data were used for calculation of unit cell parameters with TOPAS 3.0 software (Bruker, MA, USA) (
Table S1, Supplementary Materials). Fe
3O
4-NP
s morphological interpretation was studied by transmission electron microscopy (TEM) on JEOL JEM 2100 (JEOL, Tokyo, Japan) at 200 kV of accelerating voltage. Chemical composition of samples was analyzed by energy dispersive X-ray spectroscopy (EDS), spectra were acquired by scanning electron microscope (SEM) using Thermo Noran System 7 (Thermo Scientific, Waltham, MA, USA) with Si (Li) detector, accelerating voltage was 15 kV and acquisition time was 300 s.
2.2. Plant Material
Common sunflower (Helianthus annuus L.) hybrid SY Neostar (Syngenta, Basel, Switzerland) was used in our field experiment. This hybrid belongs to the two-line imidazoline resistant hybrid preferentially utilizable for ClearField Plus® system of production. SY Neostar hybrid has a short to medium height, medium to early growth and development, with a wide adaptability against inadequate environmental and growth conditions, and no specific agricultural requirements. Additionally, this hybrid contains around 47% of oil on average, shows high resistance to Sclerotinia sclerotiorum Lib. de Bary, Diaporte helianthi Munt.-Cvetk., Mihaljč., and M. Petrov, and is tolerant to Plasmopara halstedii Farl. Berl. and De Toni.
2.3. Experimental Location Description
The field study was carried out in the experimental fields of Slovak University of Agriculture in Nitra (SUA in Nitra), at Dolná Malanta close to Nitra (48°19′25.41″ N 18°09′2.89″ E), Slovak Republic, central Europe. The location is situated in the north-east part of Panonian Lowland, south of the Tribeč mountains at an elevation of 250 m above sea level. Basic petrological composition of the rock corresponds to granite-based and Mesozoic carbonate rocks, Neogene, Quarter development with eluvial sediments [
42]. Soil was classified as silt loam haplic Luvisol [
43]. X-ray powdered diffraction analysis (XRD) of soil was conducted by diffractometer PW1710 (Philips, Amsterdam, The Netherlands). The soil minerals composition according to the X-ray diffractogram corresponds to the quartz, muscovite, and anorthite (
Figure S1, Supplementary Materials). The research locality is situated in a typical maize crop region with intensive soil farming practices and the current sunflower was cultivated in 7-plot crop rotation.
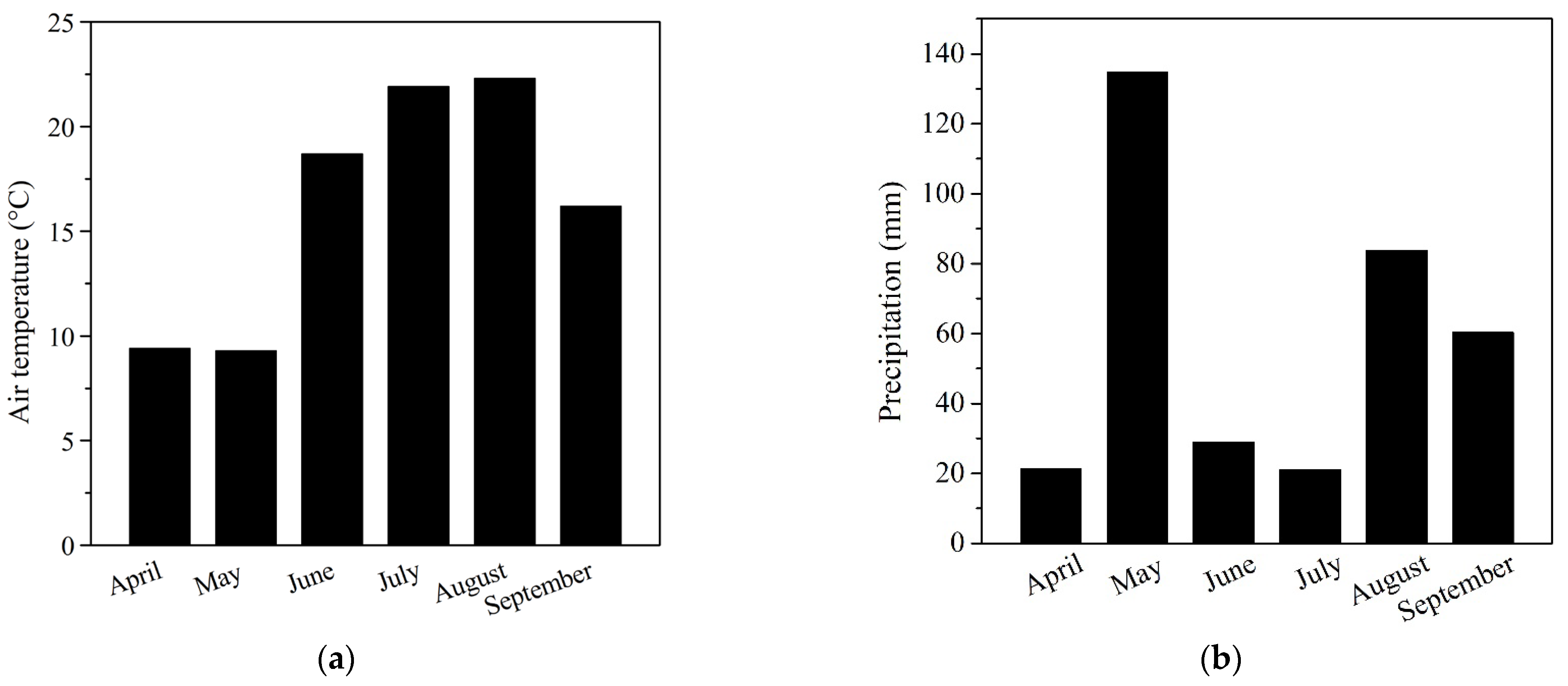
2.4. Climatic Seasonal Variations
Variability of the precipitation accumulation and average daily temperature during vegetation season in 2019 was monitored at meteorological station of SUA in Nitra (
Figure 1).
2.5. Field Experiment
The field experiment was conducted by a method of four randomly organized treatments in perpendicularly selected blocks according to Kolenčík et al. [
26]. Each treatment consisted of three replications. The plot area of one replication was 60 m
2.
The field was deeply ploughed with a Zetor tractor 6211 (Zetor Tractors, a.s., Brno, Czech Republic) in autumn 2018. The forecrop was winter wheat (
Triticum aestivum L.). Soil characteristics were analyzed before sowing of sunflower in spring 2019 and are shown in
Table 1.
Urea fertilizer was applied by machinery, i.e., by tractor, during pre-sowing soil cultivation using a Ferti fertilizer applicator (Agromehanica, Boljerac, Serbia). The fertilizer was applied on the basis of agrochemical analyses before sunflower sowing at a dose of 215 kg ha−1.
Soil sampling for nitrogen fertilization was carried out during pre-sowing soil cultivation in spring 2019. The results of soil sampling for nitrogen fertilization are presented in
Table 2.
Sunflower was sown in the rows to a depth of 60 mm in 220 mm seed distance and 700 mm inter-row spacing using a Monosem NG Plus 3 planter (Monosem, Largeasse, France) [
44]. Then, 4 L ha
−1 of Wing
® herbicide (BASF, Ludwigshafen am Rhein, Germany) was applied on the third day after sowing. All treatments, including the control, were sprayed with standard herbicide and fungicide by AGT 865T/S sprayer (Agromehanica, Boljerac, Serbia).
Colloidal solutions of NPs with a concentration of 15.99 mg L
−1 ZnO, 76 mg L
−1 Fe
3O
4 and 0.1 mg L
−1 Au anchored in mesoporous SiO
2 at a concentration of 10 mg L
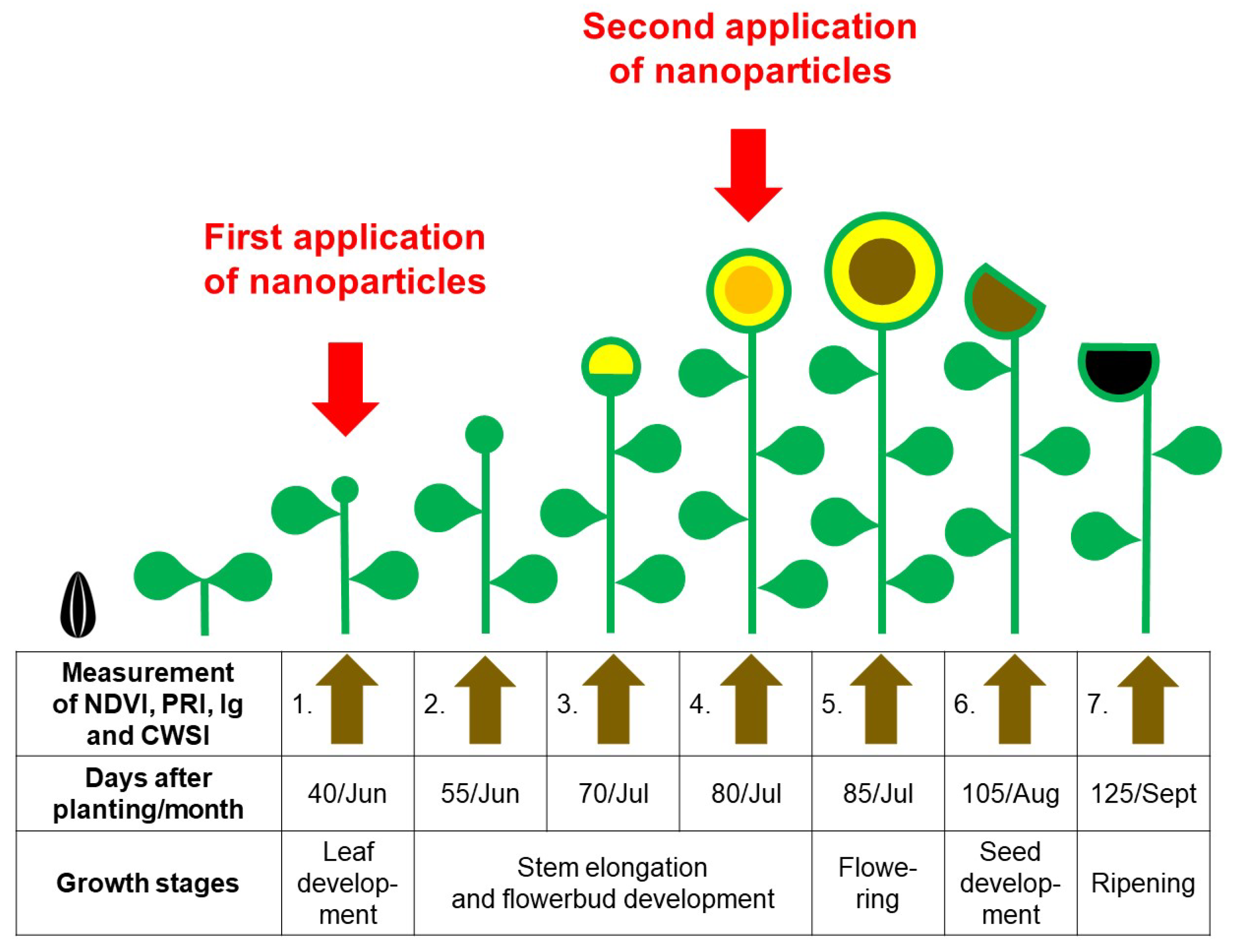
−1 were prepared in water and foliarly applied onto the plants using a hand-held sprayer (Mythos Di Martino, Mussolente, Italy). Application was performed early in the morning in windless conditions until the sunflower leaves were completely wet. The first spray application was made at day 40 at the leaf development stage, and the second spray at day 80 at the stem elongation stage at flower bud formation (
Figure 2). In control treatment, only water was used for preparation. Additionally, the same water was used for colloidal NPs solutions and subsequently applied to the sunflower leaves [
26].
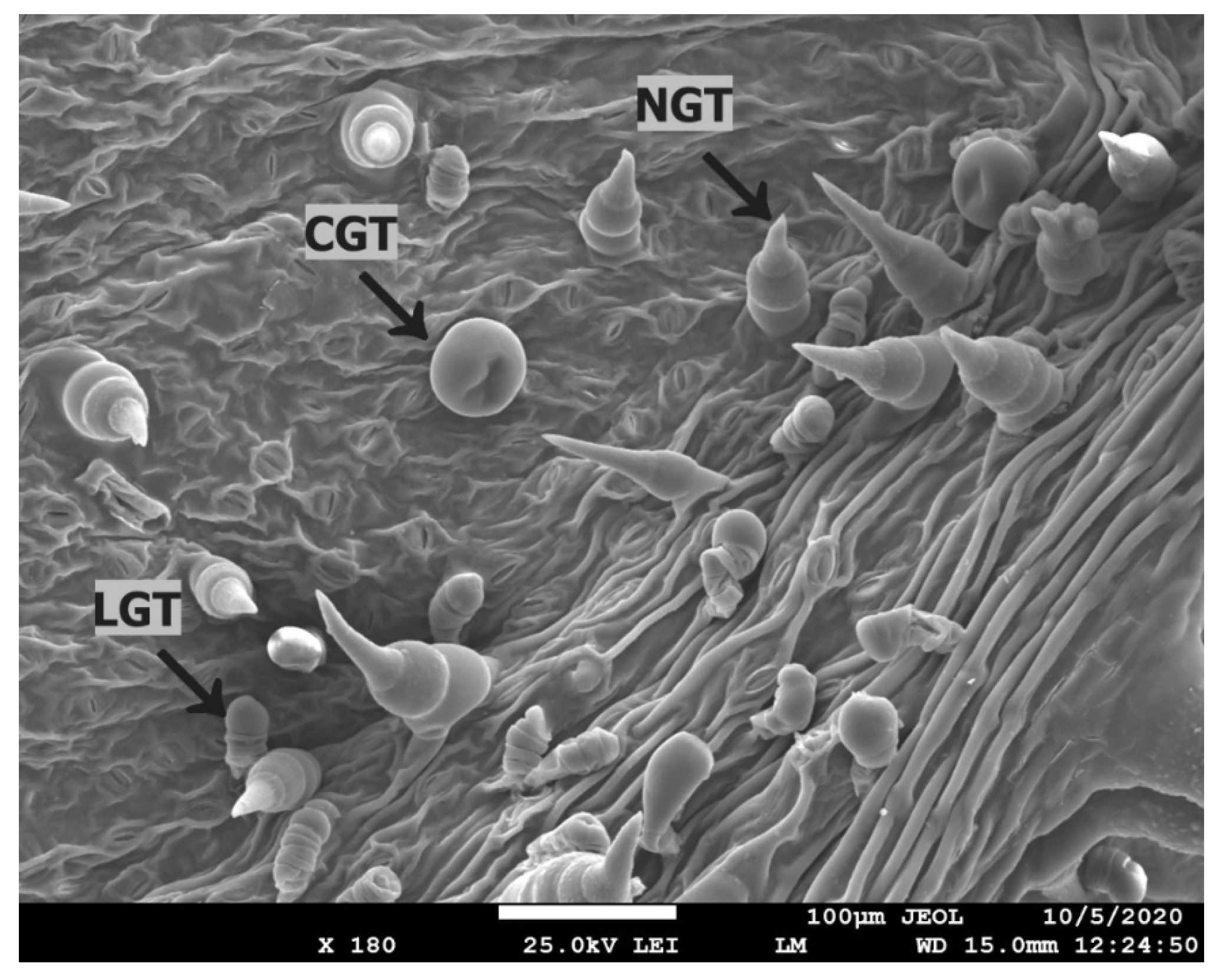
2.6. Microscopical Analysis of Trichomes and Stomata of Sunflower Leaves after Various Nanoparticles Treatments
Sunflower leaves were collected for surface structure investigation one week after the second spray deposition at a stage of flower bud formation. For precise determination of changes of surface structures and morphology analysis, the visualization by scanning electron microscopy with Quanta 450 (FEG, FEI, Hillsboro, OR, USA) was used according to studies conducted by Aschenbrenner and Horakh [
45]. Trichome distribution and their basic features were approximately estimated from leaf area of 1 mm
2 (=1,000,000 µm
2). Samples for stomata observation were prepared by microrelief (replica) method. Sunflower leaves were covered with colorless nail polish diluted with acetone. Replicas of stomata made with duct tape were placed on a glass slide [
46]. Stomata were observed by a light microscope Olympus BX 41, only on the abaxial side of the leaf. Photographs of stomata were made by Olympus E 520. Overall, four repetitions were performed per treatment (including control). In each repetition, 10 random photographs of leaf epidermis were made. The following list contains parameters that were evaluated: length and width of stomata, length and width of stomatal pores, and number of stomata per 100,000 µm
2.
2.7. Evaluation of Quantitative and Nutritive Parameters of Sunflower
After reaching full seed maturity, sunflowers were harvested by small pot combine harvester Claas Dominator 38 (CLAAS GmbH & Co. KGaA, Harsewinkel, Germany). The seed moisture level was analyzed by He Lite (Pfeuffer GmbH, Kitzingen, Germany). Yield was calculated in tons per hectare (t ha−1). Parameters, such as manual count of plants with heads (pcs), head diameter (mm) (Texi 4007 laboratory equipment; Texi GmbH, Berlin, Germany), head weight (g), and thousand seed weight (g) (TSW) (Kern PCB3500-2 lab scale; KERN & Sohn GmbH, Balingen, Germany and Numirex seed counter MEZOS spol. s r.o., Hradec Králové, Czech Republic), were examined for each treatment.
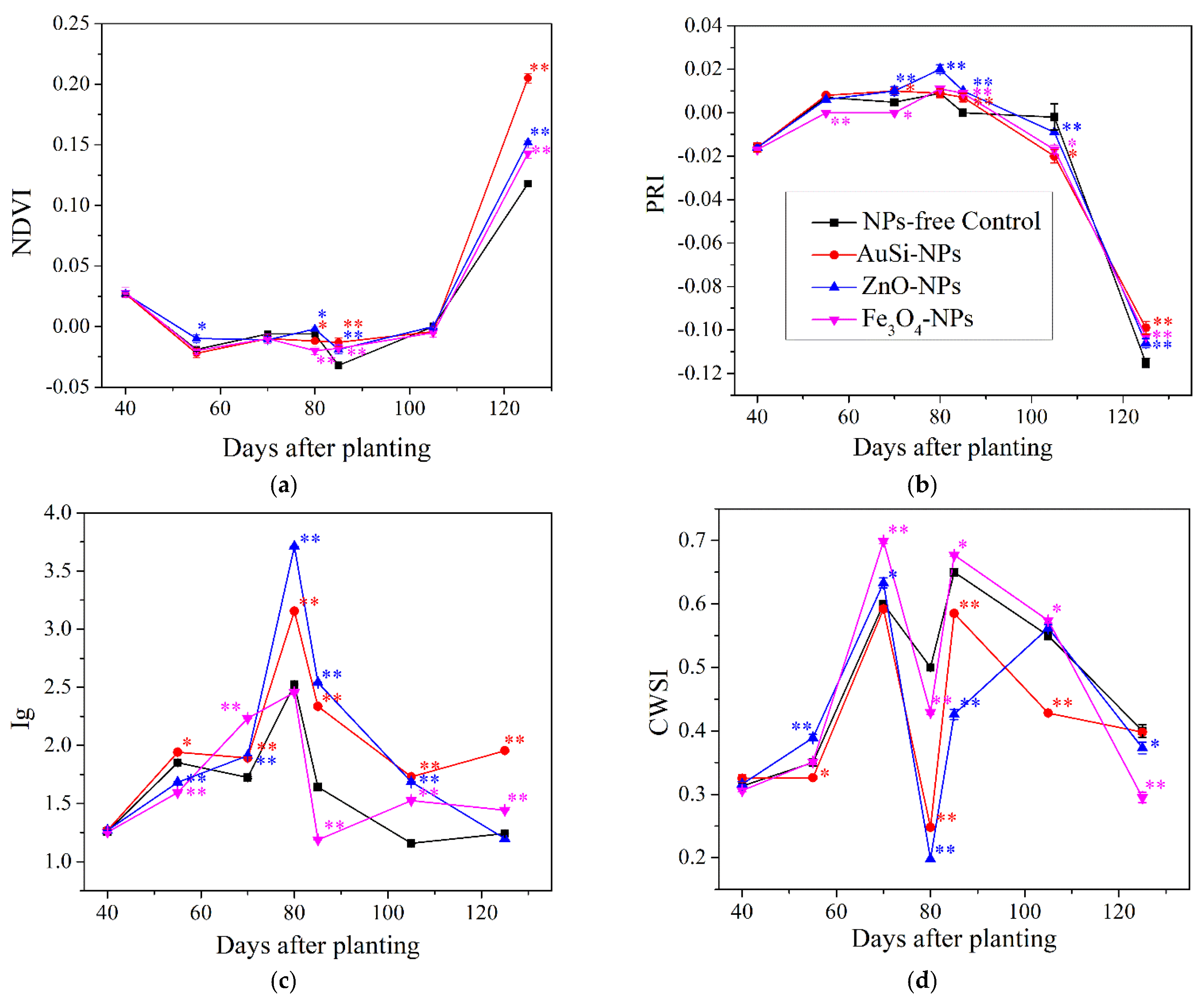
2.8. Analysis of the Course of Sunflower Physiology Based on Selected Physiological Indices
PlantPens NDVI 300 and PRI 200 (Photon Systems Instruments, Brno, Czech Republic) devices were used to evaluate the normalized difference vegetation index (NDVI) and photochemical reflectance index (PRI). The principle of the analysis is based on the absorption of reflected wavelengths from the leaf surface (at 740 nm and 660 nm for NDVI, and at 531 and 570 nm for PRI, respectively). The indices for NDVI and PRI were calculated according to the following Equations (1) and (2):
where R
531, R
570, R
660, and R
740 correspond to the reflecting wavelengths from the leaf surface, respectively. The above altered non-destructive methods were performed on the same day, on the same plants, on fully developed leaves, at the same developmental stage between 11.00 a.m. to 1.00 p.m. To include leaf heterogeneity, minimum of 10 point perpendicular-oriented measurements of leaf per index were made according to Gamon et al. [
47].
The EasIR-4 thermo-camera (Bibus AG, Fehraltorf, Switzerland) was used to analyze the stomatal conductance index (Ig) and the crop water stress index (CWSI) according to Jones et al. [
48], where leaf temperature parameters (T
leaf), dry (T
dry), and wet (T
wet) leaf surface and atmospheric moisture were included. Temperature images were taken diagonally on sunflower leaves from a distance of 2 m, at a height of 1.5 m, and at a resolution of 20.6° × 15.5° in auto-focus mode. Ig and CWSI were calculated according to Equations (3) and (4):
2.9. Sunflower Quality Assessment with Potential Translocation of Nanoparticles, Evaluation of Essential Acids in Sunflower Oil, and Anti-Nutrients Content
For quantifying the amount of sunflower oil, Soxhlet extraction was used [
49]. Analysis and profile of free fatty acids was carried out with a few sequential steps. First, the separation based on carbon numbers and saturation levels were conducted. Second, extraction was utilized on the shortest carbon chains with the lowest boiling points. Then, individual free fatty acids were detected by flame ionization detector (FID) integrated to the gas chromatograph Agilent 6890A GC (Agilent Technologies, Santa Clara, CA, USA). Profile of free fatty acids were evaluated as percentage of crude fat and other more complex methodological specifications are provided by Kolláthová et al. [
50].
Translocation and accumulation of applied NPs (zinc, gold–silica, and iron) and their potential transfer residues to kernel and hulls in full ripe seeds were analyzed using ICP-MS (Thermo Scientific iCap-Q, Bremen, Germany) according to methodologies published by several authors [
26,
51,
52,
53]. The calcium, phosphorus, and magnesium corresponding to mineral anti-nutrients of sunflower seeds were detected by ICP-OES (Varian Vista MPX, Mulgrave, Victoria, Australia) with yttrium as an internal standard. Potassium and manganese were detected by F-AAS (Perkin-Elmer Model 1100, Waltham, MA, USA). The colorimetric Kjeldahl method was used to define the total content of nitrogen and sulfur. All aforementioned elements were analyzed after digestion procedures of 0.15–0.30 g sample with Anton Paar Multiwave 3000 microwave digestion system (Graz, Austria) in PTFE pressure vessel. The mixture of concentrated HNO
3 and H
2O
2 at the pressure of 60 bars was used for dilution.
2.10. Examination of Nanoparticles Spray Application on Sunflowers on the Agro-Ecological Biodiversity Refection
The insects with predominant epigeic components were collected by the earth traps method. The method was based on 1 L jars, which were placed directly into soil and covered for protection against rainfall and rodents in all NPs-treatments and control (NPs-free). Periodically, in monthly intervals, jars were checked and refilled up with mixture of 5% of formaldehyde and 1/3 of fixative solution. For biological material determination, the typical insect features, especially impacted to Carabidae family were observed and systematically classified according to the work of Pokorný [
54]. The agro-ecological biodiversity was estimated in accordance with the presents epigeic population (abundance) and determination of
Coleoptera and
Carabidae obtained families.
2.11. Statistical Analysis
The observed data from trichomes and stomata analysis were statistically examined with a Tukey HSD test at α = 0.01 significance. The statistical analysis for all other treatments was performed with Statistica 10 software (StatSoft, Inc., Tulsa, OK, USA) [
55]. Prior to the evaluation of the multifactorial analysis of variance (ANOVA), the normality of experimental data was tested at α = 0.05 and α = 0.01 significance by the Student t-test, Shapiro–Wilk test for trials, and Fisher’s least significant difference (LSD).
5. Conclusions
Our spray applied nanoparticles to sunflowers under field conditions resulted in beneficial responses within various quantitative, physiological and nutritional parameters in contrast to NPs-free control. Unique effects were observed for each of the applications, commercially available ZnO-NPs, biosynthetically formed Au-NPs fixed into mesoporous algae Mallomonas kalinae sp. nov. (AuSi-treatment), and synthetically prepared Fe3O4-NPs, all at relatively low concentration levels were used. We observed unexpected and beneficial responses in sunflower physiology, oil content, and even epigeic insect diversity.
The most surprising effects on sunflowers were observed in the AuSi-treatment where the number of leaf trichomes and the concentration of linoleic acid has increased. At the same time, an improvement was observed in quantitative parameters seasonal physiology, agronomically relevant oil yield content, and potential silica translocation to full ripe seeds. Most surprisingly, we observed a significant increase in agro-biological diversity based on epigeic insect groups, even though gold and silica are not essential nutrients for plants or insects.
Our previously published empirical findings conducted on several crops have shown that ZnO-NPs performed important roles in many agronomical aspects. This observation was also confirmed in the current experimental study in the case of quantitative parameters, yield, and plant physiology. This response was promptly stimulated after the second spray application, the most likely due to higher number of present leaves trichomes and stomata. From nutritional quality point of view, ZnO-NPs treatment induced the low content of human antinutrient—phosphorus—and the higher content of beneficial linoleic acid, but there was no beneficial translocation of zinc. The hypothesis about the expected wider agro-ecological diversity was not confirmed. On the contrary, in this treatment, the number of epigeic insect groups and species populations were the lowest, including NPs-free control.
Even the other micronutrient-based nanoparticles treatment with Fe3O4 did not show significant trends in trichome distribution at the lower number of leaf stomata, the quantitative and yield-related parameters, and oil content. Only full ripe seeds provided nutritional values of the lower antinutrient content, without iron translocation, but with beneficial concentration of linoleic acid. Regarding the physiological responses, only stomatal conductance reacted adequately at the statistically significant level, mostly at the ripening development stage. Additionally, agrobiological diversity of epigeic groups showed low abundance. The overall unconvincing role of Fe3O4-NPs treatment could be associated with very low dimension of iron nanoparticles around 5 nm, with great solubility and reactivity.
In case of control (NPs-free variant), we observed the significant number of stomata, nutritional content of essential fatty acid, such as oleic acid, and relatively abundant agroecological diversity, but with negative seasonal physiological response.