The Effect of Functionalized Multiwall Carbon Nanotubes with Fe and Mn Oxides on Lactuca sativa L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Plant Tissues
2.1.1. Characterization of Physiological Growth Parameters
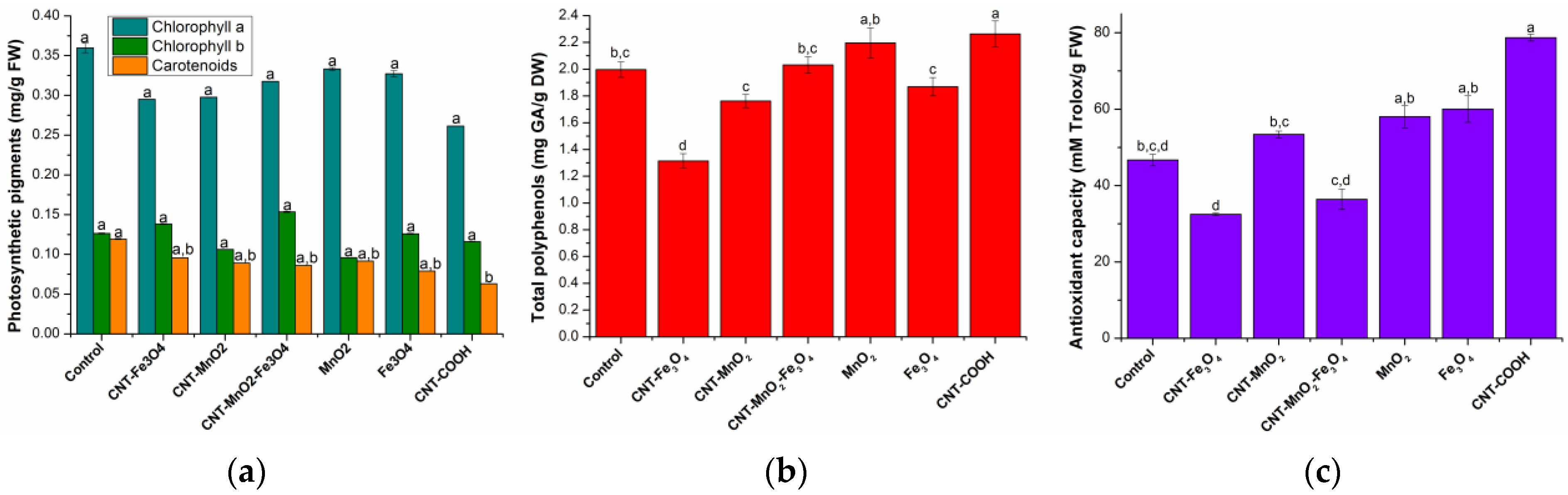
2.1.2. Assimilatory Pigments Determination
2.1.3. Total Polyphenols Determination
2.1.4. Antioxidant Activity Determination
2.1.5. TEM Analysis
2.1.6. Elemental Content in Lettuce Leaves
Microelements
Macroelements
Other Elements
2.2. Root-to-Leaves Transport
2.3. Relationship between Element Content and Bioactive Compounds and Water Content in Lettuce Leaves by Treatment
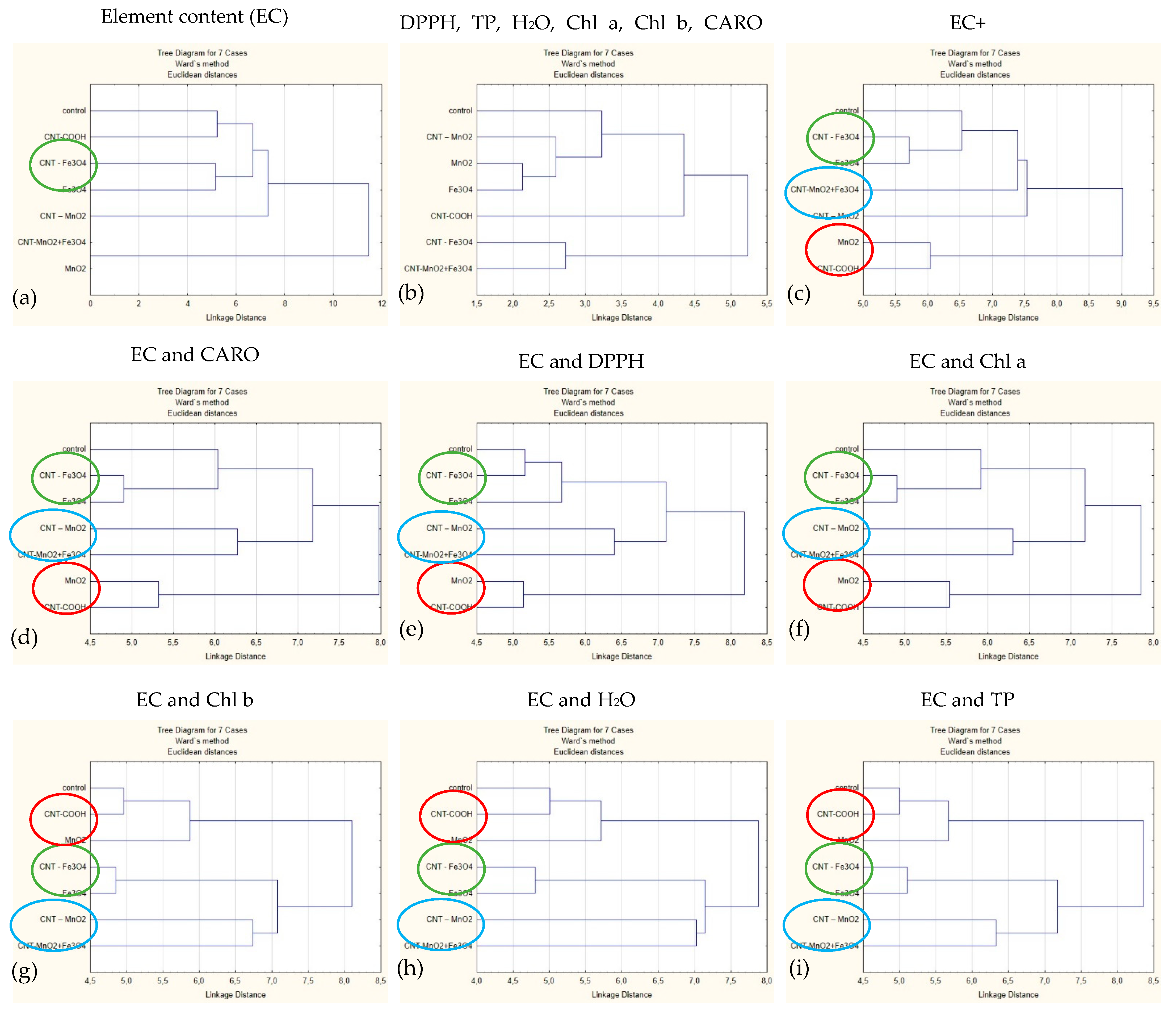
2.4. Cluster Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Growth Conditions
3.3. Determining the Effect of the Investigated Nanoparticles on the Plant Tissue
3.3.1. Determination of Physiological Growth Parameters
3.3.2. Extraction and Characterization of Assimilating Pigments
3.3.3. Preparation and Characterization of Alcoholic Extracts
3.3.4. TEM Analysis of Wheat Tissue
3.3.5. Elemental Content
3.3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrahari, S.; Dubey, A. Nanoparticles in plant growth and development. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Ghorbanpour, M., Bhargava, P., Varma, A., Choudhary, D., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Goswami, P.; Yadav, S.; Mathur, J. Positive and negative effects of nanoparticles on plants and their applications in agriculture. Plant Sci. Today 2019, 6, 232–242. [Google Scholar] [CrossRef]
- Tawfik, A.S. Insights into carbon nanotube-metal oxide composite: Embedding in membranes. Int. J. Sci. Res. Environ. Sci. Toxicol. 2017, 2, 1–4. [Google Scholar]
- Singla, R.; Kumari, A.; Yadav, S.K. Impact of nanomaterials on plant physiology and functions. In Nanomaterials and Plant Potential; Springer Science and Business Media LLC: Berlin, Germany, 2019; pp. 349–377. [Google Scholar]
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, H.; Lal, R. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) Seed germination: Nanotoxicants or nanonutrients? Water Air Soil Pollut. 2016, 227, 42. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of nanoparticles in plants. In Nanotechnology and Plant Sciences; Siddiqui, M., Al-Whaibi, M., Mohammad, F., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Bret, J.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef]
- Nhan, L.V.; Ma, C.; Rui, Y.; Liu, S.; Li, X.; Xing, B.; Liu, L. Phytotoxic mechanism of nanoparticles: Destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci. Rep. 2015, 5, 11618. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Soran, M.L.; Lung, I.; Opriș, O.; Culicov, O.; Ciorîță, A.; Stegarescu, A.; Zinicovscaia, I.; Yushin, N.; Vergel, K.; Kacso, I.; et al. The effect of TiO2 nanoparticles on the composition and ultrastructure of wheat. Nanomaterials 2021, 11, 3413. [Google Scholar] [CrossRef]
- Faisal, M.; Saquib, Q.; Alatar, A.A.; Al-Khedhairy, A.A.; Hegazy, A.K.; Musarrat, J. Phytotoxic hazards of NiO-nanoparticles in tomato: A study on mechanism of cell death. J. Hazard. Mater. 2013, 250–251, 318–332. [Google Scholar] [CrossRef]
- Burklew, C.E.; Ashlock, J.; Winfrey, W.B.; Zhang, B. Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). PLoS ONE 2012, 7, e34783. [Google Scholar] [CrossRef]
- Večeřová, K.; Večeřa, Z.; Dočekal, B.; Oravec, M.; Pompeiano, A.; Tříska, J.; Urban, O. Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. Pollut. 2016, 218, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lung, I.; Opris, O.; Soran, M.L.; Culicov, O.; Ciorîță, A.; Stegarescu, A.; Zinicovscaia, I.; Yushin, N.; Vergel, K.; Kacso, I.; et al. The impact assessment of CuO nanoparticles on the composition and ultrastructure of Triticum aestivum L. Int. J. Environ. Res. Public Health 2021, 18, 6739. [Google Scholar] [CrossRef] [PubMed]
- Iannone, M.F.; Groppa, M.D.; de Sousa, M.E.; van Raap, M.B.F.; Benavides, M.P. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ. Exp. Bot. 2016, 131, 77–88. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Ma, C.; Wang, Y.; Wu, C.; Huang, J.; Xing, B. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 2016, 159, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Pei, X.; Cui, L.; Zhu, H. Effect of CNT-COOH addition on the compressive strength, chloride resistance, and microstructure of cement mortar. Adv. Mater. Sci. Eng. 2022, 2022, 3345279. [Google Scholar] [CrossRef]
- Qiao, S.; Peng, X.; Wang, L.; Duan, S.; Chu, J.; Jia, P. Highly sensitive humidity sensor based on oblique carbon nanoplumes. Sensors 2018, 18, 3407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Z.; Chen, F. Surface modification of carbon nanotubes with an enhanced antifungal activity for the control of plant fungal pathogen. Materials 2017, 10, 1375. [Google Scholar] [CrossRef]
- Ali, M.H.; Sobze, J.-M.; Pham, T.H.; Nadeem, M.; Liu, C.; Galagedara, L.; Cheema, M.; Thomas, R. Carbon nanoparticles functionalized with carboxylic acid improved the germination and seedling vigor in upland boreal forest species. Nanomaterials 2020, 10, 176. [Google Scholar] [CrossRef]
- Magnabosco, G.; Pantano, M.F.; Rapino, S.; Di Giosia, M.; Valle, F.; Taxis, L.; Sparla, F.; Falini, G.; Pugno, N.M.; Calvaresi, M. A plant bioreactor for the synthesis of carbon nanotube bionic nanocomposites. Front. Bioeng. Biotechnol. 2020, 8, 560349. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Gutowski, S.M.; Walters, K.S.; Yan, B.; Schnoor, J.L. Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean. Environ. Sci. Technol. 2015, 49, 7380–7390. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ortiz, H.; Gaucin-Delgado, J.M.; Preciado-Rangel, P.; Fortis-Hernandez, M.; Hernandez-Montiel, L.G.; De La Cruz-Lazaro, E.; Lara-Capistran, L. Copper oxide nanoparticles biosynthetized improve germination and bioactive compounds in wheat sprouts. Not. Bot. Horti. Agrobot. Cluj Napoca 2022, 50, 12657. [Google Scholar] [CrossRef]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology applications and implications of agrochemicals towards sustainable agriculture and food systems. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, C.; Wen, J.; Wu, G.; Tao, M. Research on the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–171. [Google Scholar]
- Alkhatib, R.; Alkhatib, B.; Abdo, N.; Al-Eitan, L.; Creamer, R. Physio-biochemical and ultrastructural impact of (Fe3O4) nanoparticles on tobacco. BMC Plant Biol. 2019, 19, 253. [Google Scholar] [CrossRef]
- Morla, S.; Rao, C.R.; Chakrapani, R. Factors affecting seed germination and seedling growth of tomato plants cultured in vitro conditions. J. Chem. Biol. Phys. 2011, 1, 328–334. [Google Scholar]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutrition. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R. The role of antioxidants in human health. Andreescu and Hepel. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Armelin, M.J.A.; Trevizam, A.R.; Muraoka, T.; Silva, M.L.S.; Saiki, M.; Maihara, V.A. Instrumental Neutron Activation Analysis Applied to Multielement Determination in a Variety of Lettuce Grown in a Contaminated Soil and Treated with Phosphate. 3rd-INCC 2011, ID:58. Available online: http://repositorio.ipen.br/bitstream/handle/123456789/14193/17308.pdf?sequence=1&isAllowed=y (accessed on 27 March 2023).
- Kicińska, A.; Wikar, J. The efect of fertilizing soils degraded by the metallurgical industry on the content of elements in Lactuca sativa L. Sci. Rep. 2021, 11, 4072–4089. [Google Scholar] [CrossRef]
- Freitas, M.C.; Pacheco, A.M.G.; Bacchi, M.A.; Dionísio, I.; Landsberger, S.; Braisted, J.; Fernandes, E.A.N. Compton suppression instrumental neutron activation analysis performance in determining trace- and minor-element contents in foodstuff. J. Radioanal. Nucl. Chem. 2008, 276, 149–156. [Google Scholar] [CrossRef]
- Sussa, F.V.; Furlan, M.R.; Victorino, M.; Figueira, R.C.L.; Silva, P.S.C. Essential and non-essential elements in lettuce produced on a rooftop urban garden in São Paulo metropolitan region (Brazil) and assessment of human health risks. J. Radioanal. Nucl. Chem. 2022, 331, 5869–5879. [Google Scholar] [CrossRef]
- Pančevski, Z.; Staflov, T.; Bačeva, K. Distribution of heavy metals in lettuce and carrot grown in the vicinity of lead and zinc smelter plant. Int. J. Pure Appl. Chem. 2014, 9, 17–26. [Google Scholar]
- Ittipongse, A.; Fungklin, R. Determine of heavy metal contents in fresh vegetable by using nuclear activation analysis technique. SNRU J. Sci. Technol. 2016, 8, 187–191. [Google Scholar]
- Ríos, J.J.; Blasco, B.; Cervilla, L.M.; Rubio-Wilhelmi, M.M.; Ruiz, J.M.; Romero, L. Regulation of sulphur assimilation in lettuce plants in the presence of selenium. Plant Growth Regul. 2008, 56, 43–51. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rahman, M.k. Influence of different organic manures on growth, yield and mineral nutrient accumulation in lettuce (Lactuca sativa L.). Dhaka Univ. J. Biol. Sci. 2021, 30, 159–168. [Google Scholar] [CrossRef]
- Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I.; Leszczyńska, T. Chemical composition of lettuce (Lactuca sativa L.) biofortified with iodine by KIO3, 5-iodo-, and 3.5-diiodosalicylic acid in a hydroponic cultivation. Agronomy 2020, 10, 1022. [Google Scholar] [CrossRef]
- Pacheco, A.M.G.; Freitas, M.C.; Ventura, M.G.; Dionısio, I.; Ermakova, E. Chemical elements in common vegetable components of Portuguese diets, determined by k0-INAA. Nucl. Instrum. Methods Phys. Res. A 2006, 564, 721–728. [Google Scholar] [CrossRef]
- Scandium, S.I. 2004. Available online: https://www.researchgate.net/publication/303773841_Scandium (accessed on 27 March 2023).
- Bukar, P.H.; Onoja, M.A. Assessment of metal pollutants in lettuce (Lactuca sativa) cultivated via irrigation in Maiduguri, Nigeria. Int. Res. J. Pub. Environ. Health 2020, 7, 60–66. [Google Scholar]
- Itanna, F. Metals in leafy vegetables grown in Addis Ababa and toxicological implications. Ethiop. J. Health Develop. 2002, 16, 295–302. [Google Scholar] [CrossRef]
- Yañez, L.M.; Alfaro, J.A.; Avila Carreras, N.M.E.; Bovi Mitre, G. Arsenic accumulation in lettuce (Lactuca sativa L.) and broad bean (Vicia faba L.) crops and its potential risk for human consumption. Heliyon 2019, 5, e01152. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Song, W.-Y. Arsenic uptake and translocation in plants. Plant Cell Physiol. 2015, 57, 4–13. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Halka, M.; Sady, W. Biofortification of six varieties of lettuce (Lactuca sativa L.) with iodine and selenium in combination with the application of salicylic acid. Front. Plant Sci. 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Paramonova, T.A.; Kuzmenkova, N.V.; Godyaeva, M.M.; Belyaev, V.R.; Ivanov, M.M.; Agapkina, G.I. Cesium-137 root uptake by oat and lettuce test crops from radioactively contaminated chernozem under model experiment conditions. Mosc. Univ. Soil Sci. Bull. 2018, 73, 18–25. [Google Scholar] [CrossRef]
- Šuňovská, A.; Horník, M.; Marešová, J.; Pipíška, M.; Augustín, J. 137Cs uptake and translocation in leafy vegetable: A study with Lactuca sativa L. grown under hydroponic conditions. Nova Biotechnol. Chim. 2012, 11, 153–166. [Google Scholar] [CrossRef]
- McBride, M.B.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.G.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef]
- Soran, M.L.; Opriș, O.; Lung, I.; Kacso, I.; Porav, A.S.; Stan, M. The efficiency of the multi-walled carbon nanotubes used for antibiotics removal from wastewaters generated by animal farms. Environ. Sci. Pollut. Res. 2017, 24, 16396–16406. [Google Scholar] [CrossRef] [PubMed]
- Stegarescu, A.; Lung, I.; Leoștean, C.; Kacso, I.; Opriș, O.; Lazăr, M.D.; Copolovici, L.; Guțoiu, S.; Stan, M.; Popa, A.; et al. Green Synthesis, Characterization and Test of MnO2 Nanoparticles as Catalyst in Biofuel Production from Grape Residue and Seeds Oil. Waste Biomass Valorization 2020, 11, 5003–5013. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opriș, O.; Gutoiu, S.; Leoștean, C.; Lazăr, M.D.; Kacso, I.; Silipas, T.D.; Porav, A.S. Evaluation of CNT-COOH/MnO2/Fe3O4 nanocomposite for ibuprofen and paracetamol removal from aqueous solutions. J. Hazard. Mater. 2021, 403, 123528. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzimol. 1987, 148, 350–382. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]


| Control | CNT-Fe3O4 | CNT-MnO2 | CNT-Fe3O4-MnO2 | MnO2 | Fe3O4 | CNT-COOH | ||
|---|---|---|---|---|---|---|---|---|
| Na | LL | 3.14 ± 0.34 | 3.83 ± 0.24 | 4.13 ± 0.23 | 3.97 ± 0.23 | 3.36 ± 0.31 | 3.82 ± 0.25 | 3.80 ± 0.45 |
| LR | 1.75 ± 0.05 | 1.98 ± 0.25 | 1.90 ± 0.29 | 1.86 ± 0.43 | 1.80 ± 0.49 | 2.32 ± 0.24 | 1.80 ± 0.30 | |
| Mg * | LL | 2.69 ± 0.12 | 2.51 ± 0.15 | 2.93 ± 0.18 | 2.86 ± 0.17 | 2.10 ± 0.12 | 2.49 ± 0.09 | 2.52 ± 0.14 |
| Al ** | LL | 75.50 ± 16.70 | 75.90 ± 14.60 | 81.00 ± 20.60 | 79.70 ± 11.70 | 42.30 ± 3.90 | 45.20 ± 2.40 | 48.00 ± 13.50 |
| S * | LL | 5.25 ± 0.94 | 3.70 ± 0.58 | 3.93 ± 0.54 | 5.61 ± 1.76 | 3.73 ± 1.08 | 4.77 ± 1.24 | 5.53 ± 0.61 |
| Cl * | LL | 15.70 ± 1.55 | 13.90 ± 1.03 | 13.90 ± 0.54 | 20.10 ± 0.92 | 13.20 ± 2.87 | 15.00 ± 3.26 | 16.00 ± 0.80 |
| K * | LL | 89.70 ± 5.20 | 88.30 ± 5.67 | 85.70 ± 3.67 | 105.00 ± 3.28 | 90.00 ± 7.37 | 90.30 ± 9.87 | 86.00 ± 2.08 |
| LR | 68.50 ± 5.31 | 60.70 ± 6.79 | 55.70 ± 6.98 | 65.00 ± 11.50 | 57.70 ± 8.97 | 71.50 ± 11.80 | 63.70 ± 8.69 | |
| Ca * | LL | 9.15 ± 0.04 | 10.30 ± 0.64 | 12.20 ± 0.81 | 12.03 ± 0.32 | 8.27 ± 0.23 | 9.93 ± 0.48 | 9.30 ± 1.11 |
| Sc *** | LL | 35.70 ± 4.63 | 31.30 ± 6.17 | 30.30 ± 5.90 | 27.70 ± 3.28 | - | - | - |
| LR | 491.00 ± 187.00 | 183.00 ± 50.90 | 207.00 ± 53.70 | 136.00 ± 48.40 | 182.00 ± 54.40 | 272.00 ± 148.00 | 217.00 ± 69.20 | |
| Mn ** | LL | 41.20 ± 3.10 | 33.60 ± 1.20 | 61.70 ± 8.70 | 43.80 ± 4.30 | 51.30 ± 7.80 | 36.80 ± 2.90 | 36.70 ± 0.35 |
| Fe ** | LL | 190.00 ± 18.50 | 169.00 ± 22.30 | 152.00 ± 14.30 | 189.00 ± 29.60 | 60.70 ± 19.30 | 222.00 ± 125.00 | 71.00 ± 10.80 |
| LR | 1335.00 ± 470.00 | 453.00 ± 84.50 | 740.00 ± 187.00 | 478.00 ± 152.00 | 475.00 ± 107.00 | 915.00 ± 388.00 | 673.00 ± 211.00 | |
| Co *** | LL | 99.30 ± 17.40 | 81.70 ± 13.00 | 71.70 ± 7.42 | 79.70 ± 7.84 | 52.00 ± 5.57 | 94.00 ± 42.80 | 72.70 ± 15.90 |
| LR | 680.00 ± 130.00 | 313.00 ± 54.30 | 403.00 ± 18.60 | 316.00 ± 61.50 | 396.00 ± 69.20 | 700.00 ± 269.00 | 803.00 ± 103.00 | |
| Zn ** | LL | 73.00 ± 11.80 | 60.30 ± 4.80 | 71.70 ± 5.80 | 64.70 ± 4.40 | 69.70 ± 5.20 | 59.00 ± 4.00 | 56.70 ± 5.50 |
| LR | 70.00 ± 0.80 | 58.90 ± 20.30 | 46.70 ± 1.90 | 46.50 ± 12.60 | 43.80 ± 3.20 | 63.50 ± 7.80 | 49.70 ± 5.20 | |
| As ** | LL | 1.28 ± 0.11 | 2.04 ± 0.15 | 2.49 ± 0.36 | 1.49 ± 0.04 | 0.40 ± 0.05 | 0.55 ± 0.06 | 0.57 ± 0.07 |
| LR | 2.38 ± 0.04 | 1.81 ± 0.35 | 1.87 ± 0.13 | 1.87 ± 0.41 | 1.68 ± 0.23 | 2.16 ± 0.21 | 1.92 ± 0.13 | |
| Se *** | LL | 250.00 ± 20.00 | 307.00 ± 14.50 | 340.00 ± 11.50 | 223.00 ± 57.80 | - | - | 203.00 ± 3.33 |
| Br ** | LL | 1.47 ± 0.28 | 1.15 ± 0.05 | 1.17 ± 0.04 | 1.24 ± 0.09 | 1.12 ± 0.09 | 1.43 ± 0.07 | 1.57 ± 0.19 |
| LR | 4.70 ± 0.41 | 2.65 ± 0.31 | 3.27 ± 0.22 | 2.84 ± 0.74 | 2.70 ± 0.55 | 4.26 ± 1.78 | 4.30 ± 0.31 | |
| Rb ** | LL | 29.70 ± 1.45 | 30.30 ± 1.67 | 30.33 ± 1.33 | 36.00 ± 1.15 | 31.70 ± 2.73 | 32.70 ± 3.18 | 31.30 ± 1.20 |
| LR | 25.50 ± 0.41 | 21.00 ± 3.06 | 22.30 ± 1.20 | 23.70 ± 3.48 | 21.60 ± 3.51 | 26.50 ± 3.70 | 22.30 ± 1.86 | |
| Sr ** | LL | 22.60 ± 1.07 | 25.60 ± 3.78 | 26.90 ± 0.98 | 23.20 ± 0.92 | 24.20 ± 2.00 | 26.80 ± 0.61 | 24.50 ± 1.50 |
| LR | 40.50 ± 1.22 | 29.20 ± 2.91 | 30.80 ± 1.72 | 32.20 ± 5.14 | 27.40 ± 3.11 | 39.00 ± 4.08 | 30.00 ± 1.73 | |
| Mo ** | LL | 0.75 ± 0.19 | 0.46 ± 0.11 | 5.25 ± 2.50 | 4.25 ± 1.33 | 0.90 ± 0.27 | 0.59 ± 0.23 | 2.19 ± 0.94 |
| LR | 1.04 ± 0.01 | 0.87 ± 0.08 | 0.73 ± 0.09 | 0.92 ± 0.20 | 0.71 ± 0.10 | 1.11 ± 0.16 | 0.93 ± 0.09 | |
| Sb *** | LL | 25.70 ± 3.28 | 60.70 ± 32.00 | 24.70 ± 2.03 | 25.70 ± 3.84 | 11.90 ± 1.15 | 80.70 ± 63.20 | 16.20 ± 3.40 |
| LR | 365.00 ± 85.70 | 115.00 ± 32.50 | 204.00 ± 31.00 | 126.00 ± 39.00 | 190.00 ± 67.10 | 318.00 ± 173.00 | 231.00 ± 63.80 | |
| Cs ** | LL | 20.70 ± 2.33 | 20.30 ± 6.89 | 20.00 ± 6.56 | 20.80 ± 3.25 | 12.10 ± 3.77 | 40.00 ± 29.60 | 13.70 ± 4.10 |
| LR | 230.00 ± 82.90 | 62.30 ± 18.40 | 117.00 ± 30.50 | 74.00 ± 22.30 | 83.70 ± 23.50 | 132.00 ± 49.80 | 112.00 ± 31.80 | |
| Ba ** | LL | 9.87 ± 0.78 | 12.50 ± 1.50 | 11.00 ± 1.27 | 9.80 ± 1.71 | 7.10 ± 0.26 | 9.87 ± 2.78 | 6.90 ± 1.04 |
| LR | 50.00 ± 11.40 | 20.30 ± 1.86 | 27.30 ± 4.98 | 21.20 ± 4.42 | 23.50 ± 5.09 | 37.50 ± 9.39 | 38.70 ± 6.64 | |
| La ** | LR | 1.54 ± 0.62 | 0.38 ± 0.07 | 0.92 ± 0.21 | 0.38 ± 0.12 | 0.44 ± 0.12 | 0.79 ± 0.29 | 0.61 ± 0.21 |
| Sm *** | LL | 18.70 ± 3.53 | 16.60 ± 4.13 | 17.50 ± 3.62 | 15.40 ± 2.63 | 2.67 ± 1.12 | 22.10 ± 19.40 | - |
| LR | 260.00 ± 97.90 | 72.70 ± 11.85 | 141.00 ± 22.50 | 67.00 ± 26.70 | 71.00 ± 22.00 | 113.00 ± 44.10 | 88.30 ± 29.60 | |
| Ta *** | LR | 49.50 ± 20.00 | 12.60 ± 3.60 | 22.00 ± 5.13 | 15.40 ± 6.97 | 15.60 ± 4.21 | 31.00 ± 18.80 | 24.60 ± 7.21 |
| W ** | LR | - | - | - | - | - | - | 0.28 ± 0.02 |
| Th *** | LL | 21.80 ± 4.23 | 24.70 ± 7.88 | 22.7 ± 6.36 | 16.70 ± 0.88 | - | - | - |
| LR | 416.00 ± 165.00 | 104.00 ± 17.40 | 239 ± 23.5 | 110.00 ± 38.50 | 129.00 ± 38.70 | 217.00 ± 64.90 | 171.00 ± 55.80 |
| Control | CNT-Fe3O4 | CNT-MnO2 | CNT-Fe3O4- MnO2 | MnO2 | Fe3O4 | CNT-COOH | |
|---|---|---|---|---|---|---|---|
| Na | 1.79 | 1.94 | 2.17 | 2.14 | 1.86 | 1.65 | 2.11 |
| K | 1.31 | 1.46 | 1.54 | 1.61 | 1.56 | 1.26 | 1.35 |
| Sc | 0.07 | 0.17 | 0.15 | 0.20 | − | − | − |
| Fe | 0.14 | 0.37 | 0.21 | 0.39 | 0.13 | 0.24 | 0.11 |
| Co | 0.15 | 0.26 | 0.18 | 0.25 | 0.13 | 0.13 | 0.09 |
| Zn | 1.04 | 1.02 | 1.54 | 1.39 | 1.59 | 0.93 | 1.14 |
| As | 0.54 | 1.13 | 1.33 | 0.80 | 0.23 | 0.26 | 0.30 |
| Br | 0.31 | 0.43 | 0.36 | 0.44 | 0.41 | 0.33 | 0.37 |
| Rb | 1.16 | 1.44 | 1.36 | 1.52 | 1.46 | 1.23 | 1.40 |
| Sr | 0.56 | 0.88 | 0.87 | 0.72 | 0.88 | 0.69 | 0.82 |
| Mo | 0.72 | 0.53 | 7.22 | 4.63 | 1.28 | 0.53 | 2.34 |
| Sb | 0.07 | 0.53 | 0.12 | 0.20 | 0.06 | 0.25 | 0.07 |
| Cs | 0.09 | 0.33 | 0.17 | 0.28 | 0.14 | 0.30 | 0.12 |
| Ba | 0.20 | 0.61 | 0.40 | 0.46 | 0.30 | 0.26 | 0.18 |
| Sm | 0.07 | 0.23 | 0.12 | 0.23 | 0.04 | 0.23 | − |
| Th | 0.05 | 0.24 | 0.09 | 0.15 | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podar, D.; Boza, C.-L.; Lung, I.; Soran, M.-L.; Culicov, O.; Stegarescu, A.; Opriş, O.; Ciorîță, A.; Nekhoroshkov, P. The Effect of Functionalized Multiwall Carbon Nanotubes with Fe and Mn Oxides on Lactuca sativa L. Plants 2023, 12, 1959. https://doi.org/10.3390/plants12101959
Podar D, Boza C-L, Lung I, Soran M-L, Culicov O, Stegarescu A, Opriş O, Ciorîță A, Nekhoroshkov P. The Effect of Functionalized Multiwall Carbon Nanotubes with Fe and Mn Oxides on Lactuca sativa L. Plants. 2023; 12(10):1959. https://doi.org/10.3390/plants12101959
Chicago/Turabian StylePodar, Dorina, Camelia-Loredana Boza, Ildiko Lung, Maria-Loredana Soran, Otilia Culicov, Adina Stegarescu, Ocsana Opriş, Alexandra Ciorîță, and Pavel Nekhoroshkov. 2023. "The Effect of Functionalized Multiwall Carbon Nanotubes with Fe and Mn Oxides on Lactuca sativa L." Plants 12, no. 10: 1959. https://doi.org/10.3390/plants12101959
APA StylePodar, D., Boza, C.-L., Lung, I., Soran, M.-L., Culicov, O., Stegarescu, A., Opriş, O., Ciorîță, A., & Nekhoroshkov, P. (2023). The Effect of Functionalized Multiwall Carbon Nanotubes with Fe and Mn Oxides on Lactuca sativa L. Plants, 12(10), 1959. https://doi.org/10.3390/plants12101959







