eYGFPuv-Assisted Transgenic Selection in Populus deltoides WV94 and Multiplex Genome Editing in Protoplasts of P. trichocarpa × P. deltoides Clone ‘52-225’
Abstract
1. Introduction
2. Results
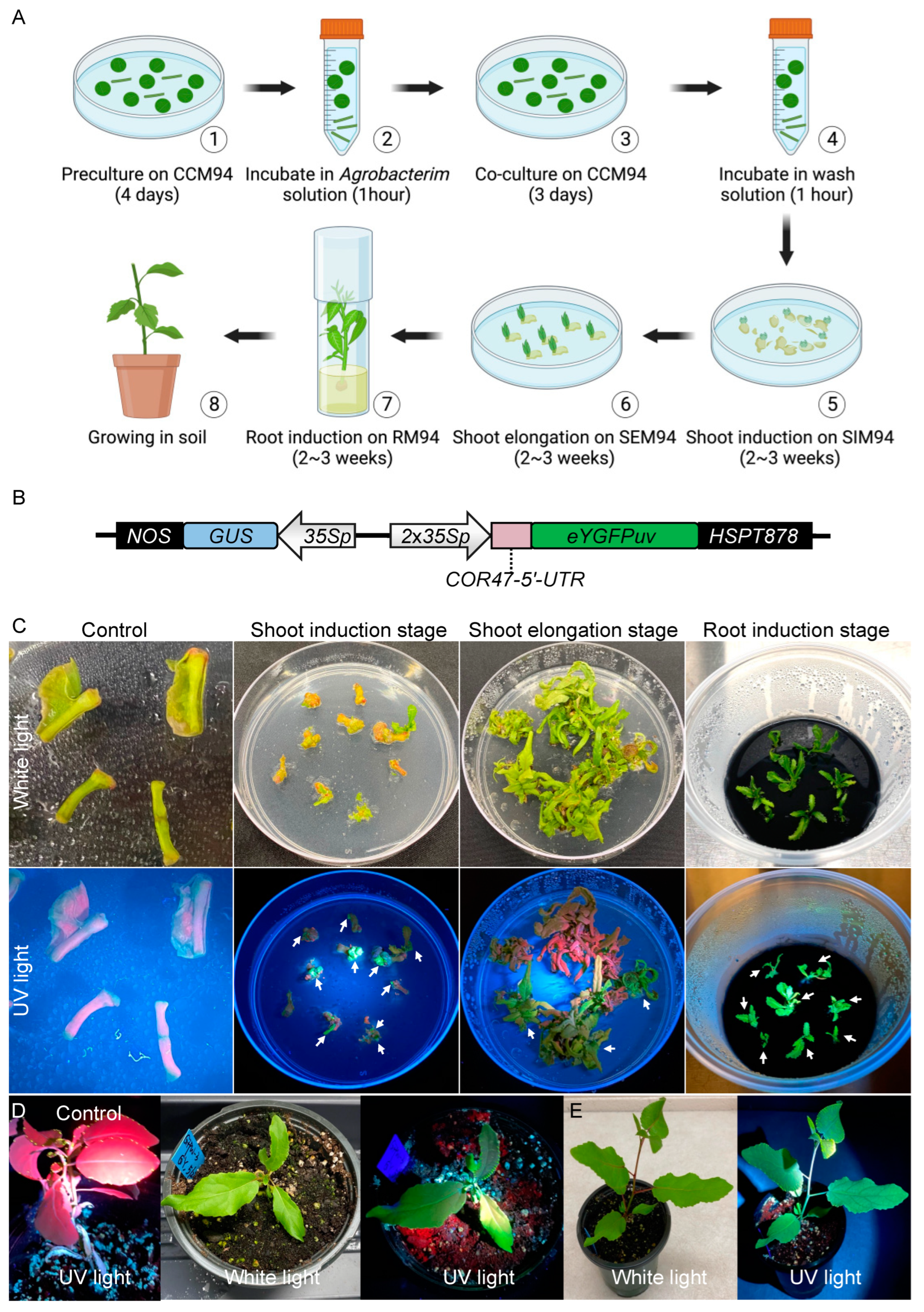
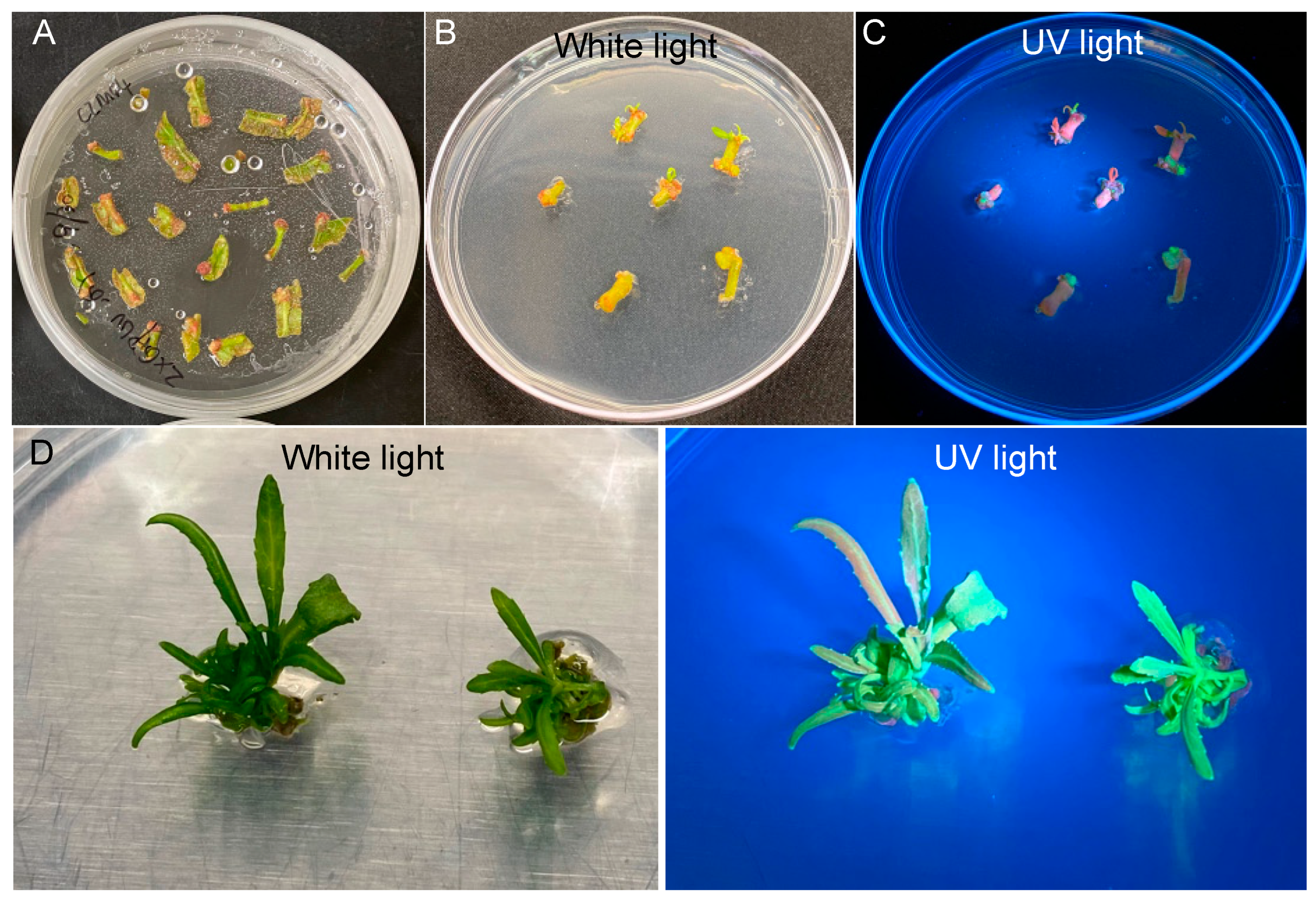
2.1. eYGFPuv-Assisted Plant Transformation in Populus Deltoides
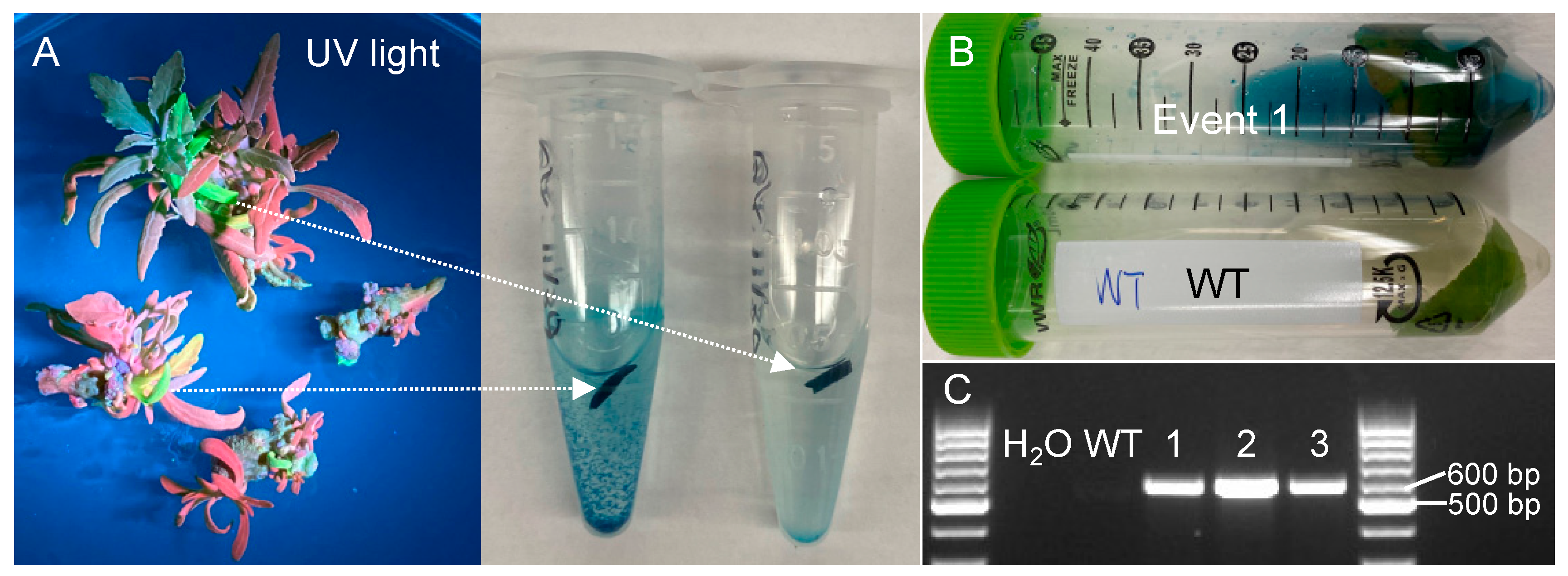
2.2. Verification of Transgenic Events
2.3. Quantification of Transformation Efficiency
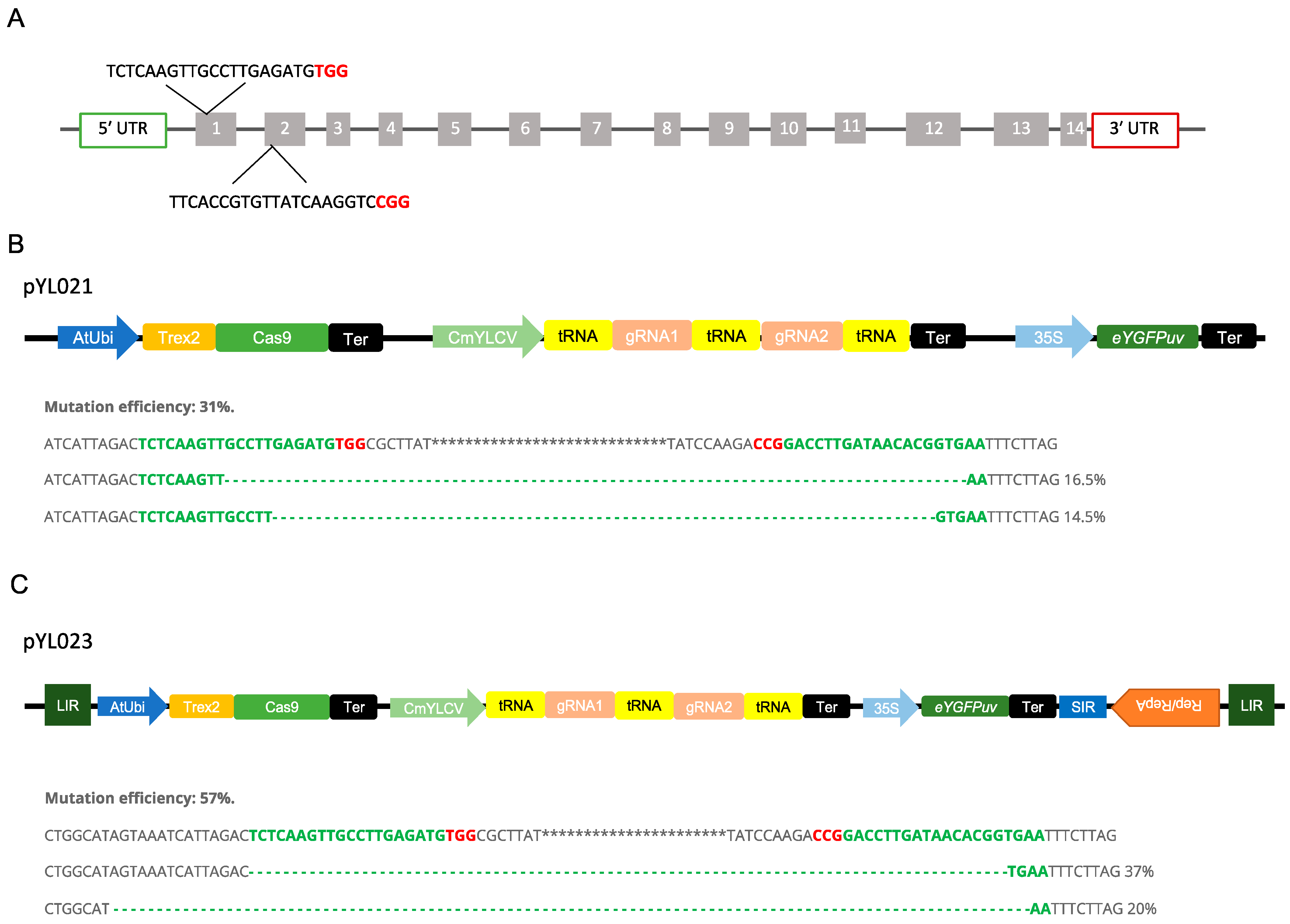
2.4. Development of Multiplexed Gene Editing Using Poplar Protoplasts
3. Discussion
4. Material and Methods
4.1. Plant Materials
4.2. Vector Construction
4.3. Genotyping of Transgenic Plants
4.4. GUS Staining
4.5. Protoplast Transformation
4.6. Mutation Profiling
4.7. Visualization of Transgenic Events
4.8. Poplar Transformation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, G. Populus: Arabidopsis for forestry. Do we need a model tree? Ann. Bot. 2002, 90, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.D.; Pu, Y.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Kalluri, U.C.; Yoo, C.G.; Ragauskas, A.J. Transgenic poplar designed for biofuels. Trends Plant Sci. 2020, 25, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, P.; Ragauskas, A.J.; Tuskan, G.A. Poplar as a feedstock for biofuels: A review of compositional characteristics. Biofuels Bioprod. Biorefining 2010, 4, 209–226. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Zhou, G.; Yue, Z.; Hu, Q.; Chen, Y.; Liu, B.; Qiu, Q.; Wang, Z.; Zhang, J.; et al. Genomic insights into salt adaptation in a desert poplar. Nat. Commun. 2013, 4, 2797. [Google Scholar] [CrossRef]
- Yang, W.; Wang, K.; Zhang, J.; Ma, J.; Liu, J.; Ma, T. The draft genome sequence of a desert tree Populus pruinosa. GigaScience 2017, 6, 9. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Wang, X.-R.; Zeng, Q.-Y. De novo assembly of white poplar genome and genetic diversity of white poplar population in Irtysh River basin in China. Sci. China Life Sci. 2019, 62, 609–618. [Google Scholar] [CrossRef]
- Ma, J.; Wan, D.; Duan, B.; Bai, X.; Bai, Q.; Chen, N.; Ma, T. Genome sequence and genetic transformation of a widely distributed and cultivated poplar. Plant Biotechnol. J. 2019, 17, 451–460. [Google Scholar] [CrossRef]
- Wu, H.; Yao, D.; Chen, Y.; Yang, W.; Zhao, W.; Gao, H.; Tong, C. De novo genome assembly of Populus simonii further supports that Populus simonii and populus trichocarpa belong to different sections. G3 Genes|Genomes|Genet. 2020, 10, 455–466. [Google Scholar] [CrossRef]
- Bai, S.; Wu, H.; Zhang, J.; Pan, Z.; Zhao, W.; Li, Z.; Tong, C. Genome assembly of salicaceae populus deltoides (eastern cottonwood) I-69 based on nanopore sequencing and Hi-C technologies. J. Hered. 2021, 112, 303–310. [Google Scholar] [CrossRef]
- Qiu, D.; Bai, S.; Ma, J.; Zhang, L.; Shao, F.; Zhang, K.; Yang, Y.; Sun, T.; Huang, J.; Zhou, Y.; et al. The genome of Populus alba x Populus tremula var. glandulosa clone 84K. DNA Res. 2019, 26, 423–431. [Google Scholar] [CrossRef]
- Coleman, H.D.; Cánovas, F.M.; Man, H.; Kirby, E.G.; Mansfield, S.D. Enhanced expression of glutamine synthetase (GS1a) confers altered fibre and wood chemistry in field grown hybrid poplar (Populus tremula X alba) (717–1B4). Plant Biotechnol. J. 2012, 10, 883–889. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Q.; Wang, W.; Wang, X.; Lu, M. Salt tolerance conferred by over-expression ofOsNHX1 gene in Poplar 84K. Chin. Sci. Bull. 2005, 50, 225–229. [Google Scholar] [CrossRef]
- Maloney, V.J.; Mansfield, S.D. Characterization and varied expression of a membrane-bound endo-β-1,4-glucanase in hybrid poplar. Plant Biotechnol. J. 2010, 8, 294–307. [Google Scholar] [CrossRef]
- Cho, J.S.; Nguyen, V.P.; Jeon, H.W.; Kim, M.H.; Eom, S.H.; Lim, Y.J.; Kim, W.C.; Park, E.J.; Choi, Y.I.; Ko, J.H. Overexpression of PtrMYB119, a R2R3-MYB transcription factor from Populus trichocarpa, promotes anthocyanin production in hybrid poplar. Tree Physiol. 2016, 36, 1162–1176. [Google Scholar] [CrossRef]
- Fahrenkrog, A.M.; Neves, L.G.; Resende, M.F., Jr.; Dervinis, C.; Davenport, R.; Barbazuk, W.B.; Kirst, M. Population genomics of the eastern cottonwood (Populus deltoides). Ecol. Evol. 2017, 7, 9426–9440. [Google Scholar] [CrossRef]
- Chen, C.; Chu, Y.; Ding, C.; Su, X.; Huang, Q. Genetic diversity and population structure of black cottonwood (Populus deltoides) revealed using simple sequence repeat markers. BMC Genet. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Novaes, E.; Osorio, L.; Drost, D.R.; Miles, B.L.; Boaventura-Novaes, C.R.D.; Benedict, C.; Dervinis, C.; Yu, Q.; Sykes, R.; Davis, M.; et al. Quantitative genetic analysis of biomass and wood chemistry of Populus under different nitrogen levels. New Phytol. 2009, 182, 878–890. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhang, X.S. Performance of 14 hybrid poplar clones grown in Beijing, China. Biomass Bioenergy 2010, 34, 906–911. [Google Scholar] [CrossRef]
- Kaczmarek, D.J.; Coyle, D.R.; Coleman, M.D. Survival and growth of a range of Populus clones in central South Carolina USA through age ten: Do early assessments reflect longer-term survival and growth trends? Biomass Bioenergy 2013, 49, 260–272. [Google Scholar] [CrossRef]
- Coyle, D.R.; Coleman, M.D.; Durant, J.A.; Newman, L.A. Survival and growth of 31 Populus clones in South Carolina. Biomass Bioenergy 2006, 30, 750–758. [Google Scholar] [CrossRef]
- Karve, A.A.; Jawdy, S.S.; Gunter, L.E.; Allen, S.M.; Yang, X.; Tuskan, G.A.; Wullschleger, S.D.; Weston, D.J. Initial characterization of shade avoidance response suggests functional diversity between Populus phytochrome B genes. New Phytol. 2012, 196, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Macaya-Sanz, D.; Chen, J.G.; Kalluri, U.C.; Muchero, W.; Tschaplinski, T.J.; Gunter, L.E.; Simon, S.J.; Biswal, A.K.; Bryan, A.C.; Payyavula, R.; et al. Agronomic performance of Populus deltoides trees engineered for biofuel production. Biotechnol. Biofuels 2017, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Muchero, W.; Bryan, A.C.; Yee, K.; Guo, H.-B.; Zhang, J.; Tschaplinski, T.J.; Singan, V.R.; Lindquist, E.; Payyavula, R.S.; et al. A 5-Enolpyruvylshikimate 3-Phosphate Synthase functions as a transcriptional repressor in Populus. Plant Cell 2018, 30, 1645–1660. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.; Qiu, J.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Yuan, G.; Martin, S.; Hassan, M.M.; Tuskan, G.A.; Yang, X. PARA: A new platform for the rapid assembly of gRNA arrays for multiplexed CRISPR technologies. Cells 2022, 11, 2467. [Google Scholar] [CrossRef]
- Liu, Y.; Merrick, P.; Zhang, Z.Z.; Ji, C.H.; Yang, B.; Fei, S.Z. Targeted mutagenesis in tetraploid switchgrass (Panicum virgatum L.) using CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 381–393. [Google Scholar] [CrossRef]
- Li, G.; Sretenovic, S.; Eisenstein, E.; Coleman, G.; Qi, Y.P. Highly efficient C-to-T and A-to-G base editing in a Populus hybrid. Plant Biotechnol. J. 2021, 19, 1086–1088. [Google Scholar] [CrossRef]
- Pan, C.; Li, G.; Malzahn, A.A.; Cheng, Y.; Leyson, B.; Sretenovic, S.; Gurel, F.; Coleman, G.D.; Qi, Y. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 2022, 8, 513–525. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.T.; Chen, Y.N.; Yin, T.M. Efficient CRISPR/Cas9-mediated gene editing in an interspecific hybrid poplar with a highly heterozygous genome. Front. Plant Sci 2020, 11, 996. [Google Scholar] [CrossRef]
- Fan, D.; Liu, T.; Li, C.; Jiao, B.; Li, S.; Hou, Y.; Luo, K. Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Sci. Rep. 2015, 5, 12217. [Google Scholar] [CrossRef]
- Weiss, T.; Wang, C.; Kang, X.; Zhao, H.; Elena Gamo, M.; Starker, C.G.; Crisp, P.A.; Zhou, P.; Springer, N.M.; Voytas, D.F. Optimization of multiplexed CRISPR/Cas9 system for highly efficient genome editing in Setaria viridis. Plant J. 2020, 104, 828–838. [Google Scholar] [CrossRef]
- Cermak, T.; Curtin, S.J.; Gil-Humanes, J.; Cegan, R.; Kono, T.J.Y.; Konecna, E.; Belanto, J.J.; Starker, C.G.; Mathre, J.W.; Greenstein, R.L.; et al. A multipurpose toolkit to enable advanced genome engineering in plants. Plant Cell 2017, 29, 1196–1217. [Google Scholar] [CrossRef]
- Yuan, G.; Lu, H.; Tang, D.; Hassan, M.M.; Li, Y.; Chen, J.-G.; Tuskan, G.A.; Yang, X. Expanding the application of a UV-visible reporter for transient gene expression and stable transformation in plants. Hortic. Res. 2021, 8, 234. [Google Scholar] [CrossRef]
- Yuan, G.; Lu, H.; Weston, D.J.; Jawdy, S.; Tschaplinski, T.J.; Tuskan, G.A.; Yang, X. Reporter genes confer new-to-nature ornamental traits in plants. Hortic. Res. 2022, 9, uhac077. [Google Scholar] [CrossRef]
- Stachel, S.E.; Messens, E.; Van Montagu, M.; Zambryski, P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 1985, 318, 624–629. [Google Scholar] [CrossRef]
- Ali, M.E.; Waliullah, S. A core35s promoter of cauliflower mosaic virus drives more efficient replication of turnip crinkle virus. Plants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Zhou, X.; Jacobs, T.B.; Xue, L.-J.; Harding, S.A.; Tsai, C.-J. Exploiting SNPs for biallelic CRISPR mutations in the outcrossing woody perennial Populus reveals 4-coumarate:CoA ligase specificity and redundancy. New Phytol. 2015, 208, 298–301. [Google Scholar] [CrossRef]
- Rottmann, W.H.; Meilan, R.; Sheppard, L.A.; Brunner, A.M.; Skinner, J.S.; Ma, C.; Cheng, S.; Jouanin, L.; Pilate, G.; Strauss, S.H. Diverse effects of overexpression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. Plant J. 2000, 22, 235–245. [Google Scholar] [CrossRef]
- Goralogia, G.S.; Howe, G.T.; Brunner, A.M.; Helliwell, E.; Nagle, M.F.; Ma, C.; Lu, H.; Goddard, A.L.; Magnuson, A.C.; Klocko, A.L.; et al. Overexpression of SHORT VEGETATIVE PHASE-LIKE (SVL) in Populus delays onset and reduces abundance of flowering in field-grown trees. Hortic. Res. 2021, 8, 167. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Hamill, J.D.; Rounsley, S.; Spencer, A.; Todd, G.; Rhodes, M.J.C. The use of the polymerase chain reaction in plant transformation studies. Plant Cell Rep. 1991, 10, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, J.; Lardet, L.; Martin, F.; Chapuset, T.; Oliver, G.; Montoro, P. The green fluorescent protein as an efficient selection marker for Agrobacterium tumefaciens-mediated transformation in Hevea brasiliensis (Müll. Arg). Plant Cell Rep. 2010, 29, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, L.; Bi, D.; Tian, X.; Li, L.; Xu, Z.; Wang, L.; Zou, X.; Gao, X.; Yang, H. Generation of marker-free transgenic rice resistant to rice blast disease using Ac/Ds transposon-mediated transgene reintegration system. Front. Plant Sci 2021, 12, 644437. [Google Scholar] [CrossRef]
- Jung, M.; Shin, S.H.; Park, J.M.; Lee, S.N.; Lee, M.Y.; Ryu, K.H.; Paek, K.Y.; Harn, C.H. Detection of transgene in early developmental stage by GFP monitoring enhances the efficiency of genetic transformation of pepper. Plant Biotechnol. Rep. 2011, 5, 157–167. [Google Scholar] [CrossRef]
- Schmülling, T.; Schell, J. Transgenic tobacco plants regenerated from leaf disks can be periclinal chimeras. Plant Mol. Biol. 1993, 21, 705–708. [Google Scholar] [CrossRef]
- Padilla, I.M.G.; Burgos, L. Aminoglycoside antibiotics: Structure, functions and effects on in vitro plant culture and genetic transformation protocols. Plant Cell Rep. 2010, 29, 1203–1213. [Google Scholar] [CrossRef]
- Cseke, L.J.; Cseke, S.B.; Podila, G.K. High efficiency poplar transformation. Plant Cell Rep. 2007, 26, 1529–1538. [Google Scholar] [CrossRef]
- Chetty, V.J.; Ceballos, N.; Garcia, D.; Narváez-Vásquez, J.; Lopez, W.; Orozco-Cárdenas, M.L. Evaluation of four Agrobacterium tumefaciens strains for the genetic transformation of tomato (Solanum lycopersicum L.) cultivar Micro-Tom. Plant Cell Rep. 2013, 32, 239–247. [Google Scholar] [CrossRef]
- Hwang, H.-H.; Yu, M.; Lai, E.-M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 15, e0186. [Google Scholar] [CrossRef]
- Khodadadi, F.; Tohidfar, M.; Vahdati, K.; Dandekar, A.M.; Leslie, C.A. Functional analysis of walnut polyphenol oxidase gene (JrPPO1) in transgenic tobacco plants and PPO induction in response to walnut bacterial blight. Plant Pathol. 2020, 69, 756–764. [Google Scholar] [CrossRef]
- Yang, S.; Liu, R.; Li, W.; Jing, Y.; Pak, S.; Li, C. Rapid and efficient regeneration of Populus ussuriensis kom. from root explants through direct de novo shoot organogenesis. Forests 2022, 13, 806. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M. Plant regeneration through the direct induction of shoot buds from petiole explants of Jatropha curcas: A biofuel plant. Ann. Appl. Biol. 2010, 156, 367–375. [Google Scholar] [CrossRef]
- Lazzarotto, C.R.; Malinin, N.L.; Li, Y.C.; Zhang, R.C.; Yang, Y.; Lee, G.; Cowley, E.; He, Y.H.; Lan, X.; Jividen, K.; et al. CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat. Biotechnol. 2020, 38, 1317–1327. [Google Scholar] [CrossRef]
- Allen, F.; Crepaldi, L.; Alsinet, C.; Strong, A.J.; Kleshchevnikov, V.; De Angeli, P.; Palenikova, P.; Khodak, A.; Kiselev, V.; Kosicki, M.; et al. Predicting the mutations generated by repair of Cas9-induced double-strand breaks. Nat. Biotechnol. 2019, 37, 64–72. [Google Scholar] [CrossRef]
- Liu, G.W.; Yin, K.Q.; Zhang, Q.W.; Gao, C.X.; Qiu, J.L. Modulating chromatin accessibility by transactivation and targeting proximal dsgRNAs enhances Cas9 editing efficiency in vivo. Genome Biol. 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Teng, F.; Li, T.; Zhou, Q. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 684–686. [Google Scholar] [CrossRef]
- Bortesi, L.; Zhu, C.F.; Zischewski, J.; Perez, L.; Bassie, L.; Nadi, R.; Forni, G.; Lade, S.B.; Soto, E.; Jin, X.; et al. Patterns of CRISPR/Cas9 activity in plants, animals and microbes. Plant Biotechnol. J. 2016, 14, 2203–2216. [Google Scholar] [CrossRef]
- Uusi-Makela, M.I.E.; Barker, H.R.; Bauerlein, C.A.; Hakkinen, T.; Nykter, M.; Ramet, M. Chromatin accessibility is associated with CRISPR-Cas9 efficiency in the zebrafish (Danio rerio). PLoS ONE 2018, 13, e0196238. [Google Scholar] [CrossRef]
- Daer, R.M.; Cutts, J.P.; Brafman, D.A.; Haynes, K.A. The impact of chromatin dynamics on Cas9-mediated genome editing in human cells. Acs Synth. Biol. 2017, 6, 428–438. [Google Scholar] [CrossRef]
- Weiss, T.; Crisp, P.A.; Rai, K.M.; Song, M.R.; Springer, N.M.; Zhang, F. Epigenetic features drastically impact CRISPR-Cas9 efficacy in plants. Plant Physiol. 2022, 190, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.P.; Shiratori, I.; Shimizu, A.; Kato, K.; Mii, M.; Waga, I. Generation of brilliant green fluorescent petunia plants by using a new and potent fluorescent protein transgene. Sci. Rep. 2018, 8, 16556. [Google Scholar] [CrossRef] [PubMed]
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Glazebrook, J. Quick miniprep for plant DNA isolation. Cold Spring Harb. Protoc. 2009, 2009, pdb.prot5179. [Google Scholar] [CrossRef]
- Guo, J.; Morrell-Falvey, J.L.; Labbé, J.L.; Muchero, W.; Kalluri, U.C.; Tuskan, G.A.; Chen, J.-G. Highly efficient isolation of populus mesophyll protoplasts and its application in transient expression assays. PLoS ONE 2012, 7, e44908. [Google Scholar] [CrossRef]
- Vickers, C.E.; Schenk, P.M.; Li, D.; Mullineaux, P.M.; Gresshoff, P.M. pGFPGUSPlus, a new binary vector for gene expression studies and optimising transformation systems in plants. Biotechnol. Lett. 2007, 29, 1793–1796. [Google Scholar] [CrossRef]
- Ahmed, I.; Islam, M.; Arshad, W.; Mannan, A.; Ahmad, W.; Mirza, B. High-quality plant DNA extraction for PCR: An easy approach. J. Appl. Genet. 2009, 50, 105–107. [Google Scholar] [CrossRef]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2017, 33, 286–288. [Google Scholar] [CrossRef]
- Biswal, A.K.; Hao, Z.; Pattathil, S.; Yang, X.; Winkeler, K.; Collins, C.; Mohanty, S.S.; Richardson, E.A.; Gelineo-Albersheim, I.; Hunt, K.; et al. Downregulation of GAUT12 in populus deltoides by RNA silencing results in reduced recalcitrance, increased growth and reduced xylan and pectin in a woody biofuel feedstock. Biotechnol. Biofuels 2015, 8, 41. [Google Scholar] [CrossRef]
| Name | Quantity of Explants | Efficiency |
|---|---|---|
| Total explants | 150 | N/A |
| Survived explants | 143 | 95.3% |
| Explants with at least one shoot | 80 | 53.3% |
| Explants with at least one GFP callus | 23 | 15.3% |
| Explants with at least one GFP shoot | 13 | 8.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.; Liu, Y.; Yao, T.; Muchero, W.; Chen, J.-G.; Tuskan, G.A.; Yang, X. eYGFPuv-Assisted Transgenic Selection in Populus deltoides WV94 and Multiplex Genome Editing in Protoplasts of P. trichocarpa × P. deltoides Clone ‘52-225’. Plants 2023, 12, 1657. https://doi.org/10.3390/plants12081657
Yuan G, Liu Y, Yao T, Muchero W, Chen J-G, Tuskan GA, Yang X. eYGFPuv-Assisted Transgenic Selection in Populus deltoides WV94 and Multiplex Genome Editing in Protoplasts of P. trichocarpa × P. deltoides Clone ‘52-225’. Plants. 2023; 12(8):1657. https://doi.org/10.3390/plants12081657
Chicago/Turabian StyleYuan, Guoliang, Yang Liu, Tao Yao, Wellington Muchero, Jin-Gui Chen, Gerald A. Tuskan, and Xiaohan Yang. 2023. "eYGFPuv-Assisted Transgenic Selection in Populus deltoides WV94 and Multiplex Genome Editing in Protoplasts of P. trichocarpa × P. deltoides Clone ‘52-225’" Plants 12, no. 8: 1657. https://doi.org/10.3390/plants12081657
APA StyleYuan, G., Liu, Y., Yao, T., Muchero, W., Chen, J.-G., Tuskan, G. A., & Yang, X. (2023). eYGFPuv-Assisted Transgenic Selection in Populus deltoides WV94 and Multiplex Genome Editing in Protoplasts of P. trichocarpa × P. deltoides Clone ‘52-225’. Plants, 12(8), 1657. https://doi.org/10.3390/plants12081657







