Ecophysiological Differentiation among Two Resurrection Ferns and Their Allopolyploid Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Species and Study Populations
2.2. Leaf Structure and Water Content
2.3. Gas Exchange and Water Potential
2.4. Desiccation Tolerance
2.5. Antioxidant Capacity and Elemental Composition
2.6. Statistical Analyses
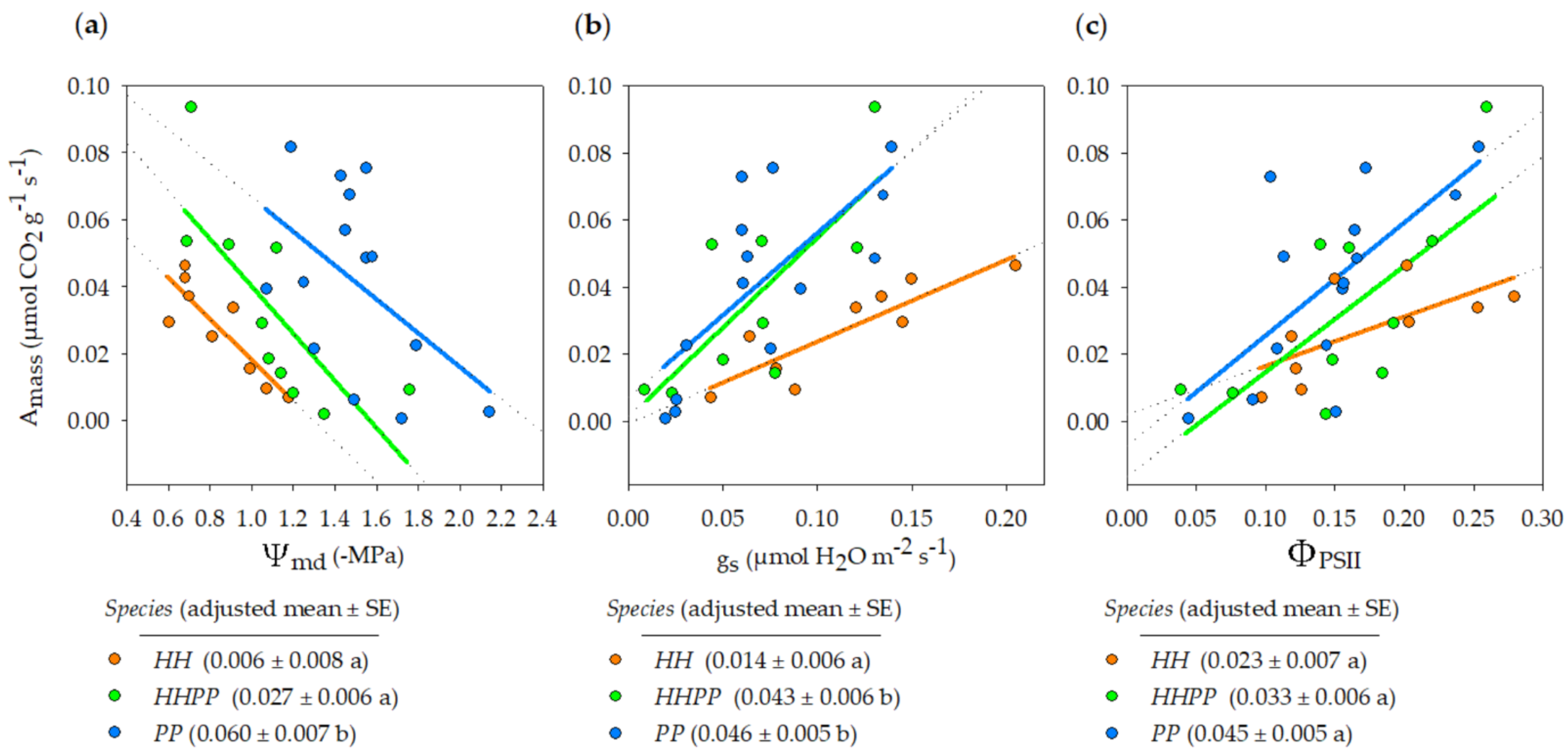
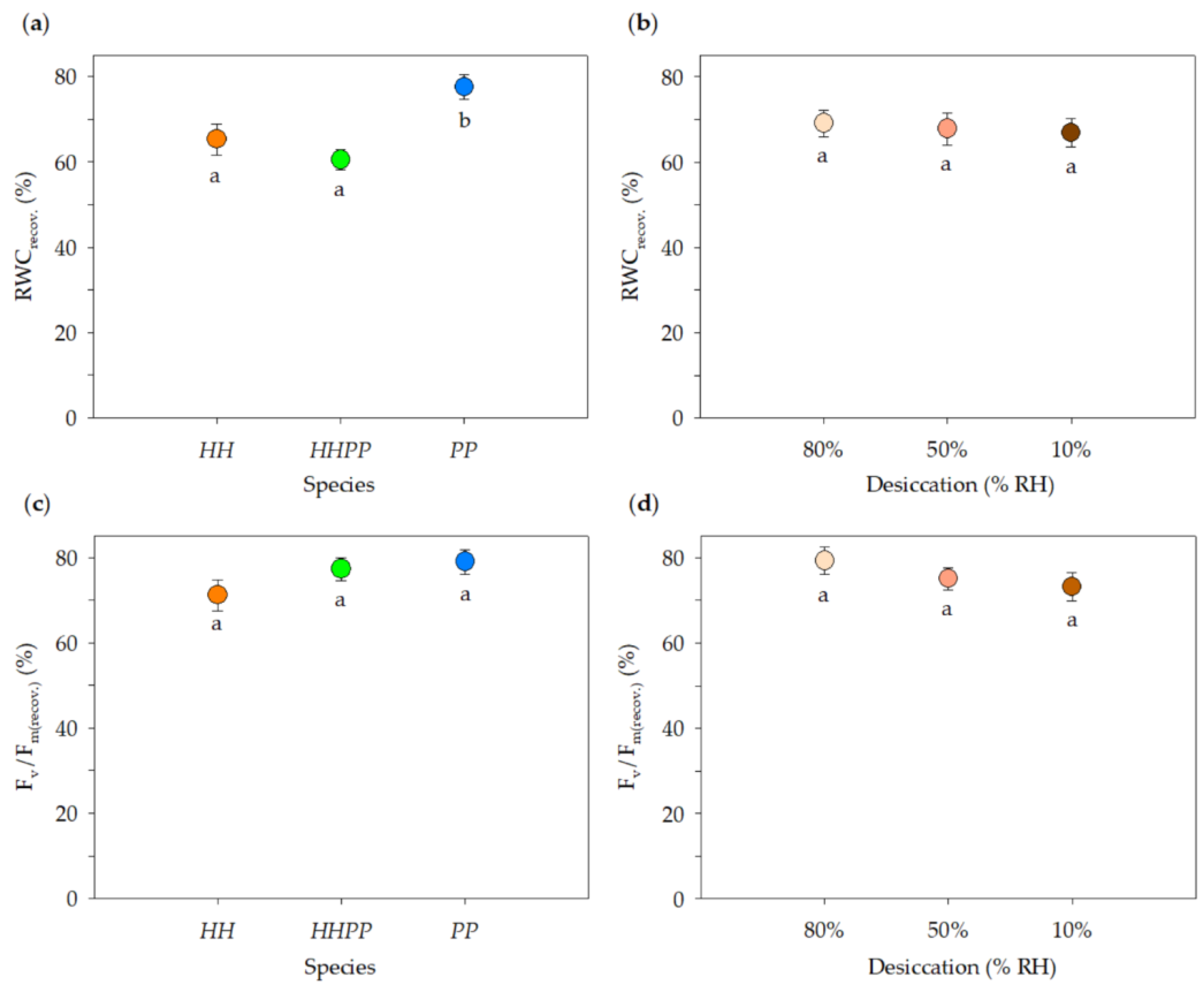
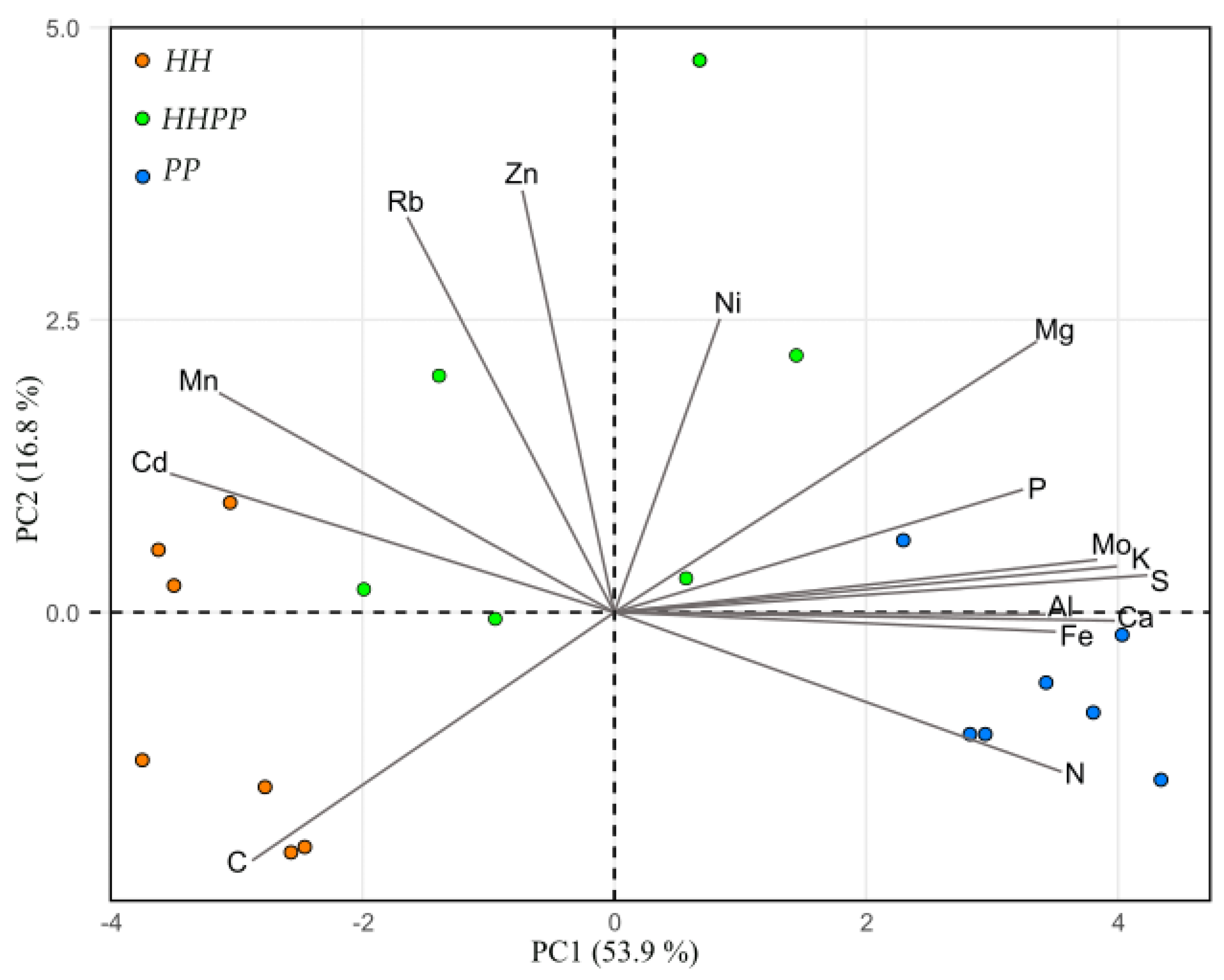
3. Results
3.1. Leaf Structure and Water Content
3.2. Gas Exchange and Water Potential
3.3. Desiccation Tolerance
3.4. Antioxidant Capacity and Elemental Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levin, D.A. Minority cytotype exclusion in local plant populations. Taxon 1975, 24, 35–43. [Google Scholar] [CrossRef]
- Wood, T.E.; Takebayashi, N.; Barker, M.S.; Mayrose, I.; Greenspoon, P.B.; Rieseberg, L. The frequency of polyploid speciation in vascular plants. Proc. Natl. Acad. Sci. USA 2009, 106, 13875–13979. [Google Scholar] [CrossRef] [PubMed]
- Husband, B.C.; Baldwin, S.J.; Suda, J. The incidence of polyploidy in natural plant populations: Major patterns and evolutionary processes. In Plant Genome Diversity; Greilhuber, J., Dolezel, J., Wendel, J.F., Eds.; Springer: Vienna, Austria, 2013; Volume 2, pp. 255–276. [Google Scholar] [CrossRef]
- Felber, F. Establishment of a tetraploid cytotype in a diploid population: Effect of relative fitness of the cytotypes. J. Evol. Biol. 1991, 4, 195–207. [Google Scholar] [CrossRef]
- Fowler, N.L.; Levin, D.A. Critical factors in the establishment of allopolyploids. Am. J. Bot. 2016, 103, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.J. A model for the establishment of polyploidy in plants. Am. Nat. 1996, 147, 33–46. [Google Scholar] [CrossRef]
- Ding, M.; Chen, Z.J. Epigenetic perspectives on the evolution and domestication of polyploid plant and crops. Curr. Opin. Plant Biol. 2018, 42, 37–48. [Google Scholar] [CrossRef]
- Akiyama, R.; Sun, J.; Hatakeyama, M.; Lischer, H.E.L.; Briskine, R.; Hay, A.; Shimizu Inatsugi, R. Fine-scale empirical data on niche divergence and homeolog expression patterns in an allopolyploid and its diploid progenitor species. New Phytol. 2021, 229, 3587–3601. [Google Scholar] [CrossRef]
- Rünk, K.; Moora, M.; Zobel, M. Do different competitive abilities of three fern species explain their different regional abundances? J. Veg. Sci. 2004, 15, 351–356. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Ellstrand, N.C. What can molecular and morphological markers tell us about plant hybridization? Crit. Rev. Pl. Sci. 1993, 12, 213–241. [Google Scholar] [CrossRef]
- Sessa, E.B.; Givnish, T.J. Leaf form and photosynthetic physiology of Dryopteris species distributed along light gradients in eastern North America. Funct. Ecol. 2014, 28, 108–123. [Google Scholar] [CrossRef]
- Buggs, R.J.A.; Wendel, J.F.; Doyle, J.J.; Soltis, D.E.; Soltis, P.S.; Coate, J.E. The legacy of diploid progenitors in allopolyploid gene expression patterns. Philos. Trans. R. Soc. B 2014, 369, 20130354. [Google Scholar] [CrossRef] [PubMed]
- Madlung, A. Polyploidy and its effect on evolutionary success: Old questions revisited with new tools. Heredity 2013, 110, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.M.; Fernández-Ocaña, A.; Rey, P.J.; Salido, T.; Amil-Ruiz, F.; Manzaneda, A.J. Variation in functional responses to water stress and differentiation between natural allopolyploid populations in the Brachypodium distachyon species complex. Ann. Bot. 2018, 121, 1369–1382. [Google Scholar] [CrossRef]
- Ehrendorfer, F. Polyploidy and distribution. In Polyploidy; Lewis, L.H., Ed.; Plenum Press: New York, NY, USA, 1980; Volume 13, pp. 45–60. [Google Scholar] [CrossRef]
- Glennon, K.L.; Ritchie, M.E.; Segraves, K.A. Evidence for shared broad-scale climatic niches of diploid and polyploid plants. Ecol. Lett. 2014, 17, 574–582. [Google Scholar] [CrossRef]
- Marchant, B.D.; Soltis, D.E.; Soltis, P.S. Patterns of abiotic niche shifts in allopolyploids relative to their progenitors. New Phytol. 2016, 212, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Baniaga, A.E.; Marx, H.E.; Arrigo, N.; Barker, M.S. Polyploid plants have faster rates of multivariate niche differentiation than their diploid relatives. Ecol. Lett. 2020, 23, 68–78. [Google Scholar] [CrossRef]
- Wang, D.; Xu, X.; Zhang, H.; Xi, Z.; Abbott, R.J.; Fu, J.; Liu, J. Abiotic niche divergence of hybrid species from their progenitors. Am. Nat. 2022, 200, 635–645. [Google Scholar] [CrossRef]
- Li, W.L.; Berlyn, G.P.; Ashton, P.M.S. Polyploids and their structural and physiological characteristics relative to water deficit in Betula papyrifera (Betulaceae). Am. J. Bot. 1996, 83, 15–20. [Google Scholar] [CrossRef]
- Manzaneda, A.J.; Rey, P.J.; Anderson, J.T.; Raskin, E.; Weiss-Lehman, C.; Mitchell-Olds, T. Natural variation, differentiation, and genetic trade-offs of ecophysiological traits in response to water limitation in Brachypodium distachyon and its descendent allotetraploid B. hybridum (Poaceae). Evolution 2015, 69, 2689–2704. [Google Scholar] [CrossRef]
- Bretagnolle, F.; Thompson, J.; Lumaret, R. The influence of seed size variation on seed germination and seedling vigour in diploid and tetraploid Dactylis glomerata L. Ann. Bot. 1995, 76, 607–615. [Google Scholar] [CrossRef]
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubesová, M.; Pysek, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.E.; Martínez Tosar, L.J.; Laino, L.E.; Iannicelli, J.; Regalado, J.J.; Escandón, A.S.; Baroli, I.; Causin, H.F.; Pitta-Álvarez, S.I. Impact of polyploidy on plant tolerance to abiotic and biotic stresses. Front. Plant Sci. 2022, 13, 869423. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, E.M.; Ihinger, J.; Jost, M.; Gamboa, V.; Marchant, B.; Bradford, D.; Doerge, R.W.; Tyagi, A.; Replogle, A.; Madlung, A. Environmental regulation of heterosis in the allopolyploid. Arab. Suec. Plant Physiol. 2016, 170, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Liu, X.; Marchant, D.B.; Visger, C.J.; Soltis, D.E. Polyploidy and novelty: Gottlieb’s legacy. Phil. Trans. R. Soc. B 2014, 369, 20130351. [Google Scholar] [CrossRef]
- Hao, G.Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef]
- Li, W.D.; Biswas, D.K.; Xu, H.; Xu, C.Q.; Wang, X.Z.; Liu, J.K.; Jiang, G.M. Photosynthetic responses to chromosome doubling in relation to leaf anatomy in Lonicera japonica subjected to water stress. Funct. Plant Biol. 2009, 36, 783–792. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Drouthe, C.; Dreyerc, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Madani, H.; Escrich, A.; Hosseini, B.; Sanchez-Muñoz, R.; Khojasteh, A.; Palazon, J. Effect of polyploidy induction on natural metabolite production in medicinal plants. Biomolecules 2021, 11, 899. [Google Scholar] [CrossRef]
- Lavania, U.C.; Srivastava, S.; Lavania, S.; Basu, S.; Misra, N.K.; Mukai, Y. Autopolyploidy differentially influences body size in plants, but facilitates enhanced accumulation of secondary metabolites, causing increased cytosine methylation. Plant J. 2012, 71, 539–549. [Google Scholar] [CrossRef]
- Rodríguez, M.C.S.; Edsgärd, D.; Sarfraz, H.S.; Alquezar, D.; Rasmussen, M.; Gilbert, T.; Nielsen, B.H.; Bartels, D.; Mundy, J. Transcriptomes of the desiccation tolerant resurrection plant Craterostigma plantagineum. Plant J. 2010, 63, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.; Lyall, R.; Petrov, V.; Bartels, D. Systems biology of resurrection plants. Cell. Mol. Life Sci. 2021, 78, 6365–6394. [Google Scholar] [CrossRef]
- Gaff, D.F.; Oliver, M. The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Funct. Plant Biol. 2013, 40, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Rakić, T.; Gajić, G.; Lazarević, M.; Stevanović, B. Effects of different light intensities, CO2 concentrations, temperatures and drought stress on photosynthetic activity in two paleoendemic resurrection plant species Ramonda serbica and R. nathaliae. Environ. Exp. Bot. 2015, 109, 63–72. [Google Scholar] [CrossRef]
- Chen, P.; Jung, N.U.; Giarola, V.; Bartels, D. The dynamic responses of cell walls in resurrection plants during dehydration and rehydration. Front. Plant Sci. 2020, 10, 1698. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carriquí, M.; Nadal, M.; Clemente-Moreno, M.J.; Coopman, R.E.; Fernie, A.R.; Flexas, J. Photosynthesis optimized across land plant phylogeny. Trends Plant Sci. 2019, 24, 947–958. [Google Scholar] [CrossRef]
- Nadal, M.; Perera-Castro, A.V.; Gulias, J.; Farrant, J.M.; Flexas, J. Resurrection plants optimize photosynthesis despite very thick cell walls by means of chloroplast distribution. J. Exp. Bot. 2021, 72, 2600–2610. [Google Scholar] [CrossRef]
- Pangua, E.; Pajarón, S.; Quintanilla, L.G. Fitness of an allopolyploid rupicolous fern compared with its diploid progenitors: From sporogenesis to sporophyte formation. Amer. J. Bot. 2019, 106, 984–995. [Google Scholar] [CrossRef]
- Jermy, A.C.; Paul, A.M. Cheilanthes Swartz. In Flora Europaea, 2nd ed.; Tutin, T.G., Burges, N.A., Chater, A.O., Eds.; University Press: Cambridge, UK, 1993; Volume 1, p. 12. [Google Scholar]
- Bañares, Á.; Blanca, G.; Güemes, J.; Moreno, J.C.; Ortiz, S. Atlas y Libro Rojo de la Flora Vascular Amenazada de España; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2008; 1069p. [Google Scholar]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. 2004. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- López-Pozo, M.; Flexas, J.; Gulías, J.; Carriquí, M.; Nadal, M.; Perera-Castro, A.V.; Clemente-Moreno, M.J.; Gago, J.; Núñez-Olivera, E.; Martínez-Abaigar, J.; et al. A field portable method for the semi-quantitative estimation of dehydration tolerance of photosynthetic tissues across distantly related land plants. Physiol. Plant. 2019, 167, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Teow, C.C.; Truong, V.-D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 2 November 2022).
- Tosens, T.; Nishida, K.; Gago, J.; Coopman, R.E.; Cabrera, H.M.; Carriquí, M.; Laanisto, L.; Morales, L.; Nadal, M.; Rojas, R.; et al. The photosynthetic capacity in 35 ferns and fern allies: Mesophyll CO2 diffusion as a key trait. New Phytol. 2016, 209, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Vasheka, O.; Gratani, L.; Puglielli, G. Frond physiological and structural plasticity of two Asplenium (Aspleniaceae) species coexisting in sun and shade conditions. Plant Ecol. Evol. 2019, 152, 426–436. [Google Scholar] [CrossRef]
- Nadal, M.; Brodribb, T.J.; Fernández-Marín, B.; García-Plazaola, J.I.; Arzac, M.I.; López-Pozo, M.; Perera-Castro, A.; Gulías, J.; Flexas, J.; Farrant, J.M. Differences in biochemical, gas exchange and hydraulic response to water stress in desiccation tolerant and sensitive fronds of the fern Anemia Caffrorum. New Phytol. 2021, 231, 1415–1430. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M.F.; Rossi, G. Photosynthetic performance and water use efficiency of the fern Cheilanthes persica. Photosynthetica 1998, 35, 507–516. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A. Passive origins of stomatal control in vascular plants. Science 2011, 331, 582–585. [Google Scholar] [CrossRef]
- Baer, A.; Wheeler, J.K.; Pittermann, J. Not dead yet: The seasonal water relations of two perennial ferns during California’s exceptional drought. New Phytol. 2016, 210, 122–132. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.; Evans, J.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- Siljak-Yakovlev, S.; Stevanovic, V.; Tomasevic, M.; Brown, S.C.; Stevanovic, B. Genome size variation and polyploidy in the resurrection plant genus Ramonda: Cytogeography of living fossils. Environ. Exp. Bot. 2008, 62, 101–112. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Omranian, N.; Sáez, P.; Figueroa, C.M.; Del-Saz, N.; Elso, M.; Poblete, L.; Orf, I.; Cuadros-Inostroza, A.; Cavieres, L.; et al. Low temperature tolerance of the Antarctic species Deschampsia antarctica: A complex metabolic response associated to nutrient remobilization. Plant Cell Environ. 2020, 43, 1376–1393. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Moreno, M.J.; Omranian, N.; Sáez, P.; Figueroa, C.M.; Del-Saz, N.; Elso, M.; Poblete, L.; Orf, I.; Cuadros-Inostroza, A.; Cavieres, L.; et al. Cytochrome respiration pathway and sulphur metabolism sustain stress tolerance to low temperature in the Antarctic species Colobanthus quitensis. New Phytol. 2020, 225, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, B.; Holzinger, A.; García-Plazaola, J.I. Photosynthetic strategies of desiccation-tolerant organisms. In Handbook of photosynthesis, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 663–681. [Google Scholar]
- Farrant, J. Mechanisms of desiccation tolerance in angiosperm resurrection plants. In Plant Desiccation Tolerance; Jenks, M.A., Wood, A.J., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 51–90. [Google Scholar]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 2004, 18, 419–425. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Lei, Y.-B.; Wang, R.-F.; Callaway, R.M.; Valiente-Banuet, A.; Inderjit; Li, Y.-P.; Zheng, Y.-L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA. 2009, 106, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Sardans, J.; Ogaya, R.; Estiarte, M. Nutrient stoichiometric relations and biogeochemical niche in coexisting plant species: Effect of simulated climate change. Pol. J. Ecol. 2008, 56, 613–622. [Google Scholar]
- Sardans, J.; Vallicrosa, H.; Zuccarini, P.; Farré-Armengol, G.; Fernández-Martínez, M.; Peguero, G.; Gargallo-Garriga, A.; Ciais, P.; Janssens, I.A.; Obersteiner, M.; et al. Empirical support for the biogeochemical niche hypothesis in forest trees. Nat. Ecol. Evol. 2021, 5, 184–194. [Google Scholar] [CrossRef]
- Oliver, J.M.; Farrant, M.J.; Hilhorst, W.M.H.; Mundree, S.; Williams, B.; Bewley, D.J. Desiccation tolerance: Avoiding cellular damage during drying and rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Van Dyke, M.N.; Levine, J.M.; Kraft, N.J.B. Small rainfall changes drive substantial changes in plant coexistence. Nature 2022, 611, 507–511. [Google Scholar] [CrossRef]

| Source of Variation | df 1 | SS | F | p |
|---|---|---|---|---|
| Species | 2 | 0.008 | 9.91 | 0.0005 |
| Ψmd | 1 | 0.008 | 19.80 | 0.0001 |
| Residual | 29 | 0.011 | ||
| Species | 2 | 0.005 | 8.10 | 0.0020 |
| gs | 1 | 0.009 | 29.5 | <0.0001 |
| Residual | 28 | 0.009 | ||
| Species | 2 | 0.002 | 3.26 | 0.0530 |
| ΦPSII | 1 | 0.008 | 21.44 | <0.0001 |
| Residual | 29 | 0.011 |
| Variable | Source of Variation | df 1 | SS | F | p |
|---|---|---|---|---|---|
| RWCrecov. | Species | 2 | 1289.7 | 8.13 | 0.0011 |
| Desiccation | 2 | 16.8 | 0.11 | 0.8997 | |
| Sp. × Desic. | 4 | 89.0 | 0.28 | 0.8889 | |
| Residual | 41 | 3252.8 | |||
| Fv/Fm(recov.) | Species | 2 | 228.3 | 1.64 | 0.2075 |
| Desiccation | 2 | 175.4 | 1.26 | 0.2956 | |
| Sp. × Desic. | 4 | 307.9 | 1.10 | 0.3683 | |
| Residual | 39 | 2718.5 |
| Species | |||
|---|---|---|---|
| Variable | HH | HHPP | PP |
| Ctotal | 51.4 ± 0.3 a | 47.9 ± 0.2 b | 48.7 ± 0.2 b |
| Ntotal | 1.77 ± 0.06 a | 1.86 ± 0.07 a | 2.27 ± 0.10 b |
| C/Ntotal | 29.2 ± 1.0 a | 26.0 ± 1.1 a | 21.7 ± 0.9 b |
| CCW | 45.6 ± 0.2 a | 44.6 ± 0.6 a,b | 43.3 ± 0.4 b |
| NCW | 2.83 ± 0.16 a | 3.12 ± 0.22 a | 2.80 ± 0.28 a |
| C/NCW | 16.4 ± 1.1 a | 14.7 ± 1.0 a | 16.2 ± 1.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla, L.G.; Aranda, I.; Clemente-Moreno, M.J.; Pons-Perpinyà, J.; Gago, J. Ecophysiological Differentiation among Two Resurrection Ferns and Their Allopolyploid Derivative. Plants 2023, 12, 1529. https://doi.org/10.3390/plants12071529
Quintanilla LG, Aranda I, Clemente-Moreno MJ, Pons-Perpinyà J, Gago J. Ecophysiological Differentiation among Two Resurrection Ferns and Their Allopolyploid Derivative. Plants. 2023; 12(7):1529. https://doi.org/10.3390/plants12071529
Chicago/Turabian StyleQuintanilla, Luis G., Ismael Aranda, María José Clemente-Moreno, Joan Pons-Perpinyà, and Jorge Gago. 2023. "Ecophysiological Differentiation among Two Resurrection Ferns and Their Allopolyploid Derivative" Plants 12, no. 7: 1529. https://doi.org/10.3390/plants12071529
APA StyleQuintanilla, L. G., Aranda, I., Clemente-Moreno, M. J., Pons-Perpinyà, J., & Gago, J. (2023). Ecophysiological Differentiation among Two Resurrection Ferns and Their Allopolyploid Derivative. Plants, 12(7), 1529. https://doi.org/10.3390/plants12071529





.png)


