Retention of Mutations in Colchicine-Induced Ornamental Succulent Echeveria ‘Peerless’
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenotypic Traits
2.1.1. Plant Growth Parameters
2.1.2. Leaf Spectrophotometric Color Readings
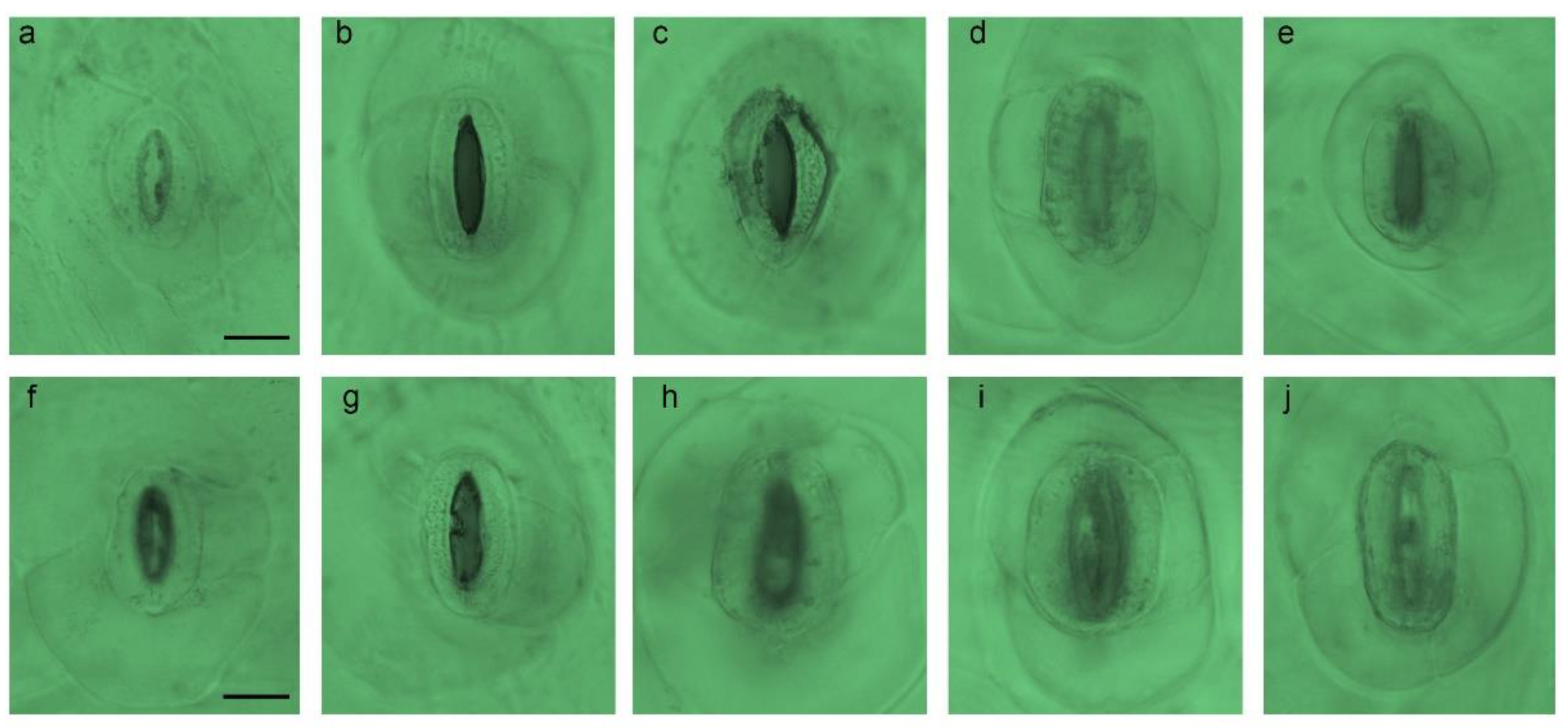
2.2. Stomata Characteristics
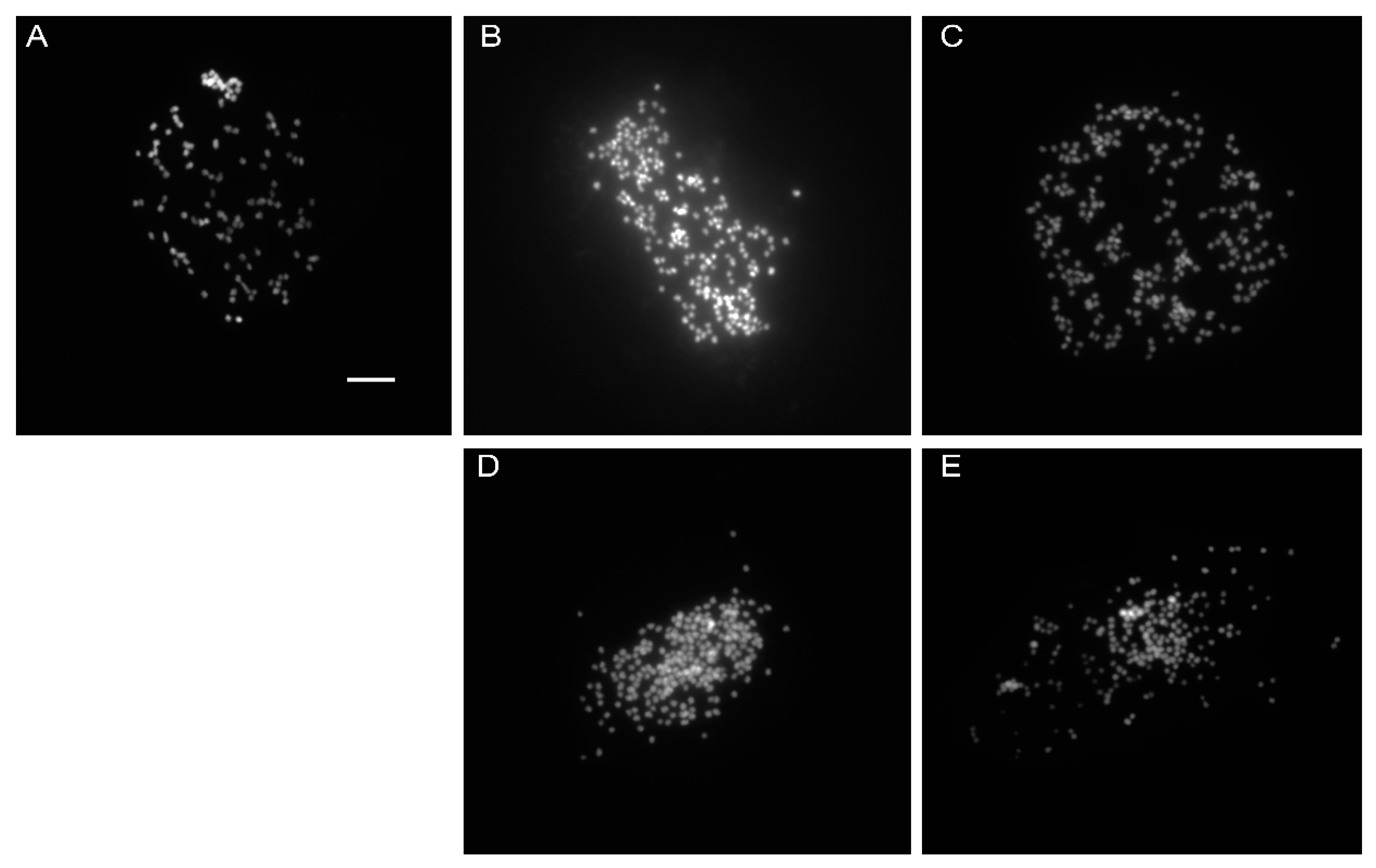
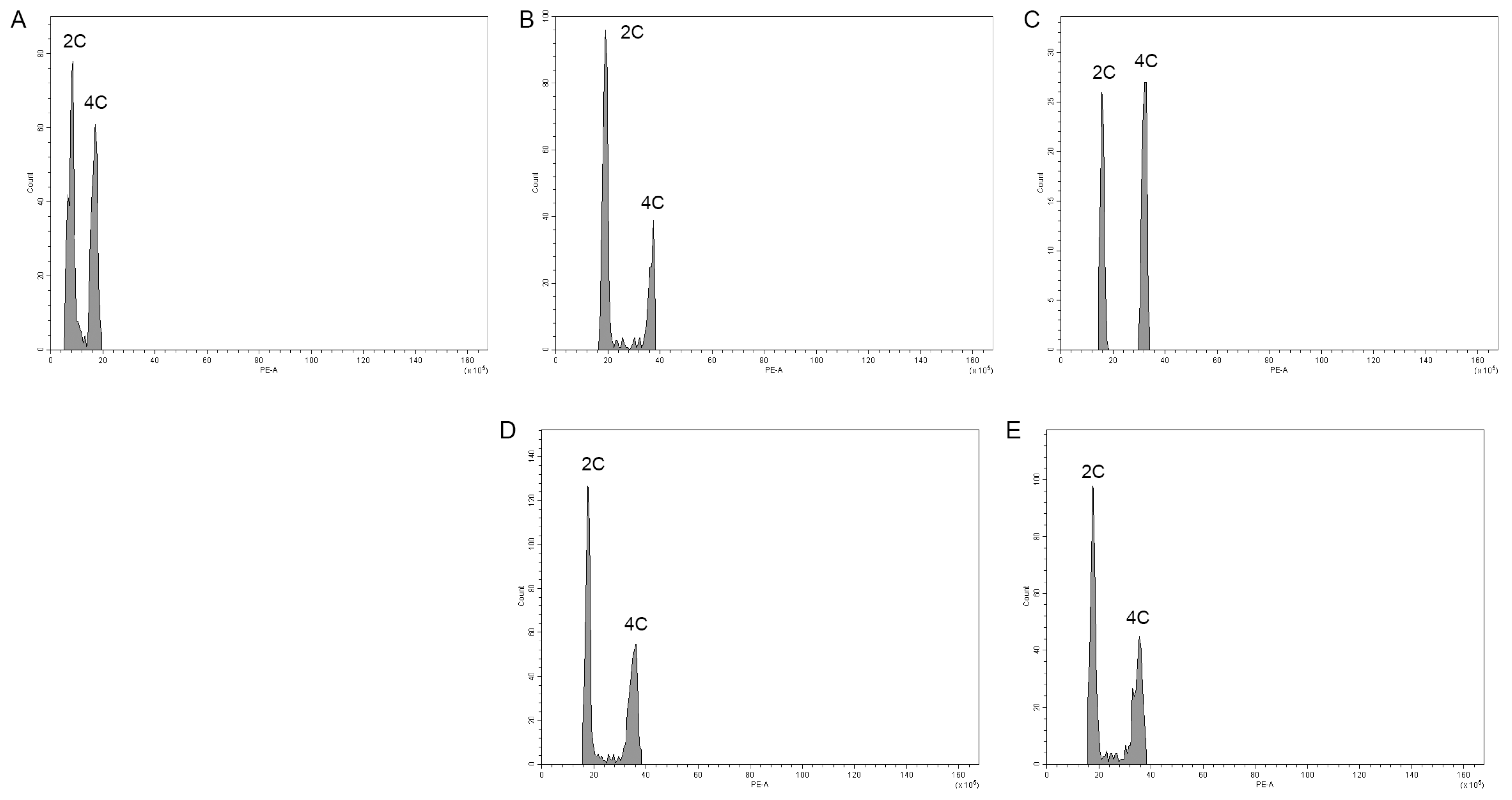
2.3. Chromosome Counting and Nuclear DNA Genome Size Estimation Using Flow Cytometry
3. Materials and Methods
3.1. Plant Materials
3.2. Plant Management
3.3. Data Collection
3.3.1. Phenotypic Evaluation
3.3.2. Stomatal Characteristics
3.3.3. Chromosome Counting
3.3.4. Flow Cytometry Analysis
3.3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, H.P. Landscape gardening for ecological and aesthetic gains. In Floriculture and Landscape Gardening; Malhotra, S.K., Ram, L., Eds.; Central Institute of Horticulture: Nagaland, India, 2017; pp. 1–10. [Google Scholar]
- Millennials & Succulents: What Is All the Hype behind These Plump Plants? Available online: https://extension.illinois.edu/blogs/good-growing/2019-06-19-millennials-succulents-what-all-hype-behind-these-plump-plants (accessed on 10 October 2021).
- Edwards, E.J.; Ogburn, R.M. Plant venation: From succulence to succulents. Curr. Biol. 2013, 23, 340–341. [Google Scholar]
- Sevik, H.; Karakas, H.; Karaca, U. Color—Chlorophyll relationship of some indoor ornamental plants. Int. J. Eng. Sci. Res. Technol. 2013, 2, 1706–1712. [Google Scholar]
- Cabahug, R.A.M.; Khanh, H.T.T.M.; Lim, K.B.; Hwang, J.J. Phenotype and ploidy evaluation of colchicine-induced Echeveria ‘Peerless’. Toxicol. Environ. Health Sci. 2021, 13, 17–24. [Google Scholar] [CrossRef]
- Foster, B.P.; Shu, Q.Y. Plant mutagenesis in crop improvement: Basic terms and applications. In Plant Mutation Breeding and Biotechnology; Shu, Q.Y., Foster, B.P., Nakagawa, H., Eds.; Food and Agriculture Organization: Rome, Italy, 2011; pp. 9–20. [Google Scholar]
- van Harten, A.M. Mutation Breeding: Theory and Practical Application. Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- International Atomic Energy Agency (IAEA). Available online: https://www.mvgs.iaea.org/LaboratoryProtocals.aspx (accessed on 9 August 2021).
- Pathirana, R. Plant mutation breeding in agriculture. CAB Rev. 2011, 32, 1–20. [Google Scholar] [CrossRef]
- He, M.; Gao, W.; Gao, Y.; Liu, Y.; Yang, X.; Jiao, H.; Zhou, Y. Polyploidy induced by colchicine in Dendranthema indicum var. aromaticum, a scented chrysanthemum. Eur. J. Hortic. Sci. 2016, 81, 219–226. [Google Scholar] [CrossRef]
- Suzuki, K.; Takatsu, Y.; Ginai, T.; Kasumi, M. Plant regeneration and chromosome doubling of wild gladiolus species. Acta Hortic. 2005, 673, 175–181. [Google Scholar] [CrossRef]
- Sadhukhan, R.; Ganguly, A.; Singh, P.K.; Sarkar, H.K. Study of induced polyploidy in African marigold (Tagetes ecrecta L.). Environ. Ecol. 2014, 32, 1219–1222. [Google Scholar]
- Vichiato, M.R.M.; Vichiato, M.; Pasqual, M.; Castro, D.M.D.; Dutra, L.F. Tetraploidy induction and identification in Dendrobium nobile Lindl (Orchidaceae). Rev. Cienc. Agron. 2007, 38, 385–390. [Google Scholar]
- Glazunova, A.; Hazieva, F.; Samatadze, T. Effect of Colchicine treatment on the cariology and morphology signs of Polemonium caeruleum L. BIO Web Conf. 2019, 17, 00210. [Google Scholar] [CrossRef][Green Version]
- El-Nashar, Y.I.; Ammar, M.H. Mutagenic influences of colchicine on phenological and molecular diversity of Calendula officinalis L. Genet. Mol. Res. 2016, 15, 15027745. [Google Scholar] [CrossRef]
- Kumar, M.K.; Rani, M.U. Colchiploidy in fruit breeding. A review. Horticulturae 2013, 2, 325–326. [Google Scholar]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Cabahug, R.A.M.; Khanh, H.T.T.M.; Lim, K.B.; Hwang, J.J. Phenotype and Ploidy Analysis of the Colchicine-induced M1 Generation of Echeveria Species. Hortic. Sci. Technol. 2020, 38, 522–537. [Google Scholar]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Baig, M.M.Q.; Quresh, A.A.; Shah, M.K.N.; Hafiz, I.A. Induction and identification of colchicine induced polyploidy in Gladiolrandiflorasrus ‘White Prosperity’. Folia Hortic. 2018, 30, 307–319. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Mishra, S.K. Effect of colchicine on mitotic polyploidization and morphological characteristics of Phlox drummondi. Afr. J. Biotechnol. 2012, 11, 9336–9342. [Google Scholar] [CrossRef]
- Eng, W.H.; Ho, W.S. Polyploidization using colchicine in horticultural plants: A review. Sci. Hortic. 2019, 246, 604–617. [Google Scholar] [CrossRef]
- Ning, G.G.; Shi, X.P.; Hu, H.R.; Yan, Y.; Bao, M.Z. Development of a range of polyploidy lines and Petunia hybrida and the relationship of ploidy with the single-/double-flower trait. HortScience 2009, 44, 250–255. [Google Scholar] [CrossRef]
- Kushwah, K.S.; Verma, R.C.; Patel, S.; Jain, N.K. Colchicine induced polyploidy in Chrysanthemum carinatum L. J. Phylogenet. Evol. Biol. 2018, 6, 193. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Van Huylenbroek, J.; De Schepper, S.; Van Labeke, M.C. Breeding for polyploidy in Belgian azalea (Rhododendron simsii hybrids). Acta Hortic. 2006, 714, 113–118. [Google Scholar] [CrossRef]
- Castro, C.M.; Oliveria, A.C.; Calvaho, F.I.F. Changes in allele frequencies in colchicine treated ryegrass population with APD marker. Agrociencia 2003, 9, 107–112. [Google Scholar]
- Blakeslee, A.F.; Avery, A.G. Method of inducing doubling of chromosomes in plants by treatment with colchicine. J. Hered. 1937, 28, 393–411. [Google Scholar] [CrossRef]
- Corneillie, S.; De Storme, N.; Van Acker, R.; Fangel, J.U.; De Brunye, M.; De Rycke, R.; Geelen, D.; Willats, W.G.T.; Vanholme, B.; Boerjan, W. Polyploidy affects plant growth and alters cell wall composition. Plant Physiol. 2019, 179, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Katoh, H.; Ikeuchi, M. Mutations in a putative chloride efflux transporter gene suppress the chloride requirement of photosystem II in the cytochrome c550-deficient mutant. Plant Cell Physiol. 2006, 47, 799–804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flowers, T.J. Chloride as a nutrient and as an osmoticum. In Advances in Plant Nutrition; Tinker, P.B., Laüchli, A., Eds.; CABI: New York, NY, USA, 1988; Volume 3, pp. 55–78. [Google Scholar]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Najeeb, U.; Naeem, M.S.; Daud, M.K.; Cao, J.S.; Gong, H.J. Induction of tetraploidy in Juncus effusus by colchicine. Biol. Plantarum 2010, 54, 659–663. [Google Scholar] [CrossRef]
- Zohary, D. Domestication of Crop Plants. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 217–227. [Google Scholar]
- Moghbel, N.; Borujeni, M.K.; Bernard, F. Colchicine effect on the DNA content and stomata size of Glycyrrhiza glabra var. glandulifera and Carthamus tinctorius L. cultured in vitro. J. Genet. Eng. Biotech. 2015, 13, 1–6. [Google Scholar] [CrossRef]
- Huang, H.; Gao, S.; Wang, D.; Huang, P.; Li, J. Autotetraploidy induced in Nianmaohuangqin (Radix Scutellariae viscidulae) with colchicine in vitro. J. Trad. Chin. Med. 2014, 34, 199–205. [Google Scholar] [CrossRef]
- Chen, Z.J.; Ni, Z. Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. Bioessays 2006, 28, 240–252. [Google Scholar] [CrossRef]
- Azizan, N.I.; Shamsiah, A.; Hasan, N.A.; Hussein, S. Morphological characterization of colchicine-induced mutants in Stevia rebaudiana. IOP Conf. 2020, 757, 012006. [Google Scholar] [CrossRef]
- Sajjad, Y.; Jaskani, M.J.; Mehmood, A.; Ahmad, I.; Abbas, H. Effect of colchicine on in vitro polyploidy induction in African marigold (Tagetes erecta). Pak. J. Bot. 2013, 45, 1255–1258. [Google Scholar]
- Low, J.E. The Echeverias, some facts about this genus, flowers, culture. Int. Crassulaceae Netw. 2007, 2, 1–2. [Google Scholar]
- Uhl, C.H. Chromosomes and hybrids of Echeveria X. South American species of Series Nudae. Haseltonia 2006, 12, 31–40. [Google Scholar] [CrossRef]
- Uhl, C.H. Chromosomes and hybrids of Echeveria. 4. Series Urceolatae E. Walther. Haseltonia 1996, 4, 66–88. [Google Scholar]
- Palomino, G.; Martínez-Ramón, J.; Cepeda-Cornejo, V.; Ladd-Otero, M.; Romero, P.; Reyes-Santiago, J. Chromosome number, ploidy level, and nuclear DNA content in 23 species of Echeveria (Crassulaceae). Genes 2021, 12, 1950. [Google Scholar] [CrossRef] [PubMed]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Yant, L.; Bomblies, K. Genome management and mismanagement—Cell-level opportunities and challenges of whole-genome duplication. Genes Dev. 2015, 29, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Pierre, P.M.; Sousa, S.M.; Davide, L.C.; Machado, M.A.; Viccini, L.F. Karyotype analysis, DNA content and molecular screening in Lippia alba (Verbenaceae). An. Acad. Bras. Cien. 2011, 83, 993–1006. [Google Scholar] [CrossRef]
- Zhou, K.; Fleet, P.; Nevo, E.; Zhang, X.; Sun, G. Transcriptome analysis reveals plant response to colchicine treatment during on chromosome doubling. Sci. Rep. 2017, 7, 8503. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Plants 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Bao, M. Colchicine-induced chromosome doubling in Platanus acerifolia and its effect on plant morphology. Euphytica 2007, 157, 145–154. [Google Scholar] [CrossRef]
- Tosca, A.; Pandolfi, R.; Citterio, S.; Fasoli, A.; Sgorbati, S. Determination by flow cytometry of the chromosome doubling capacity of colchicine and oryzalin in gynogenetic haploids of gerbera. Plant Cell Rep. 1995, 14, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.H.; Guo, X.; Wang, Q.; Pan, Q.F.; Tian, Y.; Liu, P.; Zhao, J.; Wang, G.; Sun, S.; Tang, K.X. Induction and flow cytometry identification of tetraploids from seed-derived explants through colchicine treatments in Catharanthus roseus (L.) G. Don. BioMed Res. Int. 2011, 2011, 793198. [Google Scholar]
- Gitz, G.C.; Baker, J.T. Methods for creating stomatal impressions directly onto achievable slides. Agron. J. 2009, 101, 232–236. [Google Scholar] [CrossRef]
- Kirov, I.; Divashuk, M.; Laere, K.V.; Soloviev, A.; Khrustaleva, L. An easy “SteamDrop” method for high quality plant chromosome preparation. Mol. Cytogenet. 2014, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Prot. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- Temsh, E.M.; Kouteckym, P.; Urfus, T.; Smarda, P.; Dolezel, J. Reference standards for flow cytometric estimation of absolute nuclear DNA content in plants. J. Quant. Cell Sci. 2021, 101, 710–724. [Google Scholar] [CrossRef]


| Treatment | MV1 | MV2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant (mm) | Leaf (mm) | Plant (mm) | Leaf (mm) | |||||||||
| Height | Diameter | No. of Leaves | Length | Width | Thickness | Height | Diameter | No. of Leaves | Length | Width | Thickness | |
| Control | 35.53 ± 4.92 1 | 97.75 ± 5.15 | 33.67 ± 0.88 | 39.04 ± 7.44 | 21.23 ± 3.95 | 5.21 ± 0.91 | 36.32 ± 1.24 | 65.15 ± 1.20 | 49.00 ± 0.84 | 45.05 ± 0.83 | 19.18 ± 0.10 | 4.98 ± 0.12 |
| 0.2% + 3 h | 33.58 ± 6.46 | 73.94 ± 3.53 | 18.50 ± 1.50 | 34.67 ± 1.05 | 31.38 ± 1.14 | 5.84 ± 4.21 | 48.45 ± 0.00 | 85.88 ± 0.00 | 30.00 ± 0.00 | 41.96 ± 0.00 | 29.97 ± 0.00 | 9.82 ± 0.00 |
| 0.4% + 3 h | 42.60 ± 9.25 | 85.43 ± 5.15 | 20.00 ± 2.08 | 37.98 ± 3.58 | 23.70 ± 4.96 | 7.55 ± 1.26 | 49.57 ± 1.20 | 83.81 ± 0.83 | 29.00 ± 1.41 | 33.96 ± 2.23 | 26.97 ± 1.36 | 9.59 ± 1.14 |
| 0.6% + 3 h | 48.92 ± 4.11 | 86.79 ± 2.68 | 19.67 ± 2.00 | 38.17 ± 3.59 | 23.98 ± 4.35 | 8.35 ± 2.13 | 55.95 ± 0.00 | 90.42 ± 0.00 | 39.00 ± 0.00 | 41.56 ± 0.00 | 26.27 ± 0.00 | 9.53 ± 0.00 |
| 0.8% + 3 h | 36.64 ± 6.00 | 65.21 ± 2.46 | 22.40 ± 1.87 | 30.43 ± 3.85 | 22.12 ± 1.39 | 23.1 ± 1.17 | 50.62 ± 0.71 | 73.34 ± 2.07 | 25.80 ± 1.38 | 36.26 ± 0.96 | 24.83 ± 0.74 | 8.80 ± 0.61 |
| Treatment | MV1 | MV2 | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| Control | 40.73 ± 1.60 1 | 6.48 ± 1.08 a 2 | 12.19 ± 2.35 | 39.13 ± 1.53 d | 5.52 ± 2.86 a | 13.19 ± 2.80 a |
| 0.2% + 3 h | 47.60 ± 1.48 | 2.15 ± 0.48 c | 6.43 ± 0.64 | 48.84 ± 2.66 c | 5.07 ± 0.92 a | 6.72 ± 0.58 b |
| 0.4% + 3 h | 42.92 ± 3.36 | 2.00 ± 0.72 c | 11.91 ± 1.59 | 49.23 ± 1.12 c | 3.28 ± 0.68 b | 7.90 ± 1.07 b |
| 0.6% + 3 h | 41.18 ± 2.02 | −0.81 ± 0.44 e | 11.79 ± 2.93 | 50.32 ± 0.54 b | 5.09 ± 0.62 a | 6.45 ± 1.12 b |
| 0.8% + 3 h | 45.58 ± 2.85 | 0.51 ± 1.29 d | 10.50 ± 1.27 | 53.60 ± 1.54 b | 2.87 ± 0.24 c | 7.01 ± 0.77 b |
| F-test 2 | NS | ** | NS | * | * | * |
| Treatment | MV1 | MV2 | ||
|---|---|---|---|---|
| Density | Size (µm) | Density | Size (µm) | |
| Control | 10.33 ± 0.33 1 a 2 | 20.58 ± 0.59 d | 10.54 ± 0.81 a | 19.26 ± 0.35 d |
| 0.2% + 3 h | 9.33 ± 0.88 b | 25.68 ± 0.74 b | 7.67 ± 0.67 b | 24.02 ± 0.14 c |
| 0.4% + 3 h | 6.33 ± 0.33 c | 25.92 ± 0.67 b | 5.67 ± 0.67 c | 27.31 ± 0.14 b |
| 0.6% + 3 h | 8.35 ± 0.89 b | 28.03 ± 0.25 a | 9.00 ± 0.00 b | 28.10 ± 0.03 a |
| 0.8% + 3 h | 8.55 ± 0.67 b | 22.75 ± 0.15 c | 8.33 ± 0.33 b | 28.47 ± 0.12 a |
| F-test 3 | ** | ** | ** | ** |
| Treatments | MV1 | MV2 |
|---|---|---|
| Control | 2 n = > 110 | |
| 0.2% + 3 h | 2 n = > 206 | 2 n = > 204 |
| 0.4% + 3 h | 2 n = > 215 | 2 n = > 184 |
| Treatments | 2 C (Mbps) 1 | CV (%) 2 | 1 C (Mbps) | 1 C (pg) 3 |
|---|---|---|---|---|
| Control | 2071.63 | 0.72 | 1035.82 | 1.06 |
| MV1 | ||||
| 0.2% + 3 h | 4949.46 | 1.04 | 2474.73 | 2.53 |
| 0.4% + 3 h | 4110.86 | 1.67 | 2055.43 | 2.10 |
| MV2 | ||||
| 0.2% + 3 h | 4604.74 | 0.48 | 2302.37 | 2.35 |
| 0.4% + 3 h | 4618.33 | 1.30 | 2309.17 | 2.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabahug, R.A.M.; Tran, M.K.T.H.; Ahn, Y.-J.; Hwang, Y.-J. Retention of Mutations in Colchicine-Induced Ornamental Succulent Echeveria ‘Peerless’. Plants 2022, 11, 3420. https://doi.org/10.3390/plants11243420
Cabahug RAM, Tran MKTH, Ahn Y-J, Hwang Y-J. Retention of Mutations in Colchicine-Induced Ornamental Succulent Echeveria ‘Peerless’. Plants. 2022; 11(24):3420. https://doi.org/10.3390/plants11243420
Chicago/Turabian StyleCabahug, Raisa Aone M., My Khanh Thi Ha Tran, Yun-Jae Ahn, and Yoon-Jung Hwang. 2022. "Retention of Mutations in Colchicine-Induced Ornamental Succulent Echeveria ‘Peerless’" Plants 11, no. 24: 3420. https://doi.org/10.3390/plants11243420
APA StyleCabahug, R. A. M., Tran, M. K. T. H., Ahn, Y.-J., & Hwang, Y.-J. (2022). Retention of Mutations in Colchicine-Induced Ornamental Succulent Echeveria ‘Peerless’. Plants, 11(24), 3420. https://doi.org/10.3390/plants11243420





