Green Synthesis of Iron Oxide (Hematite) Nanoparticles and Their Influence on Sorghum bicolor Growth under Drought Stress
Abstract
1. Introduction
2. Results
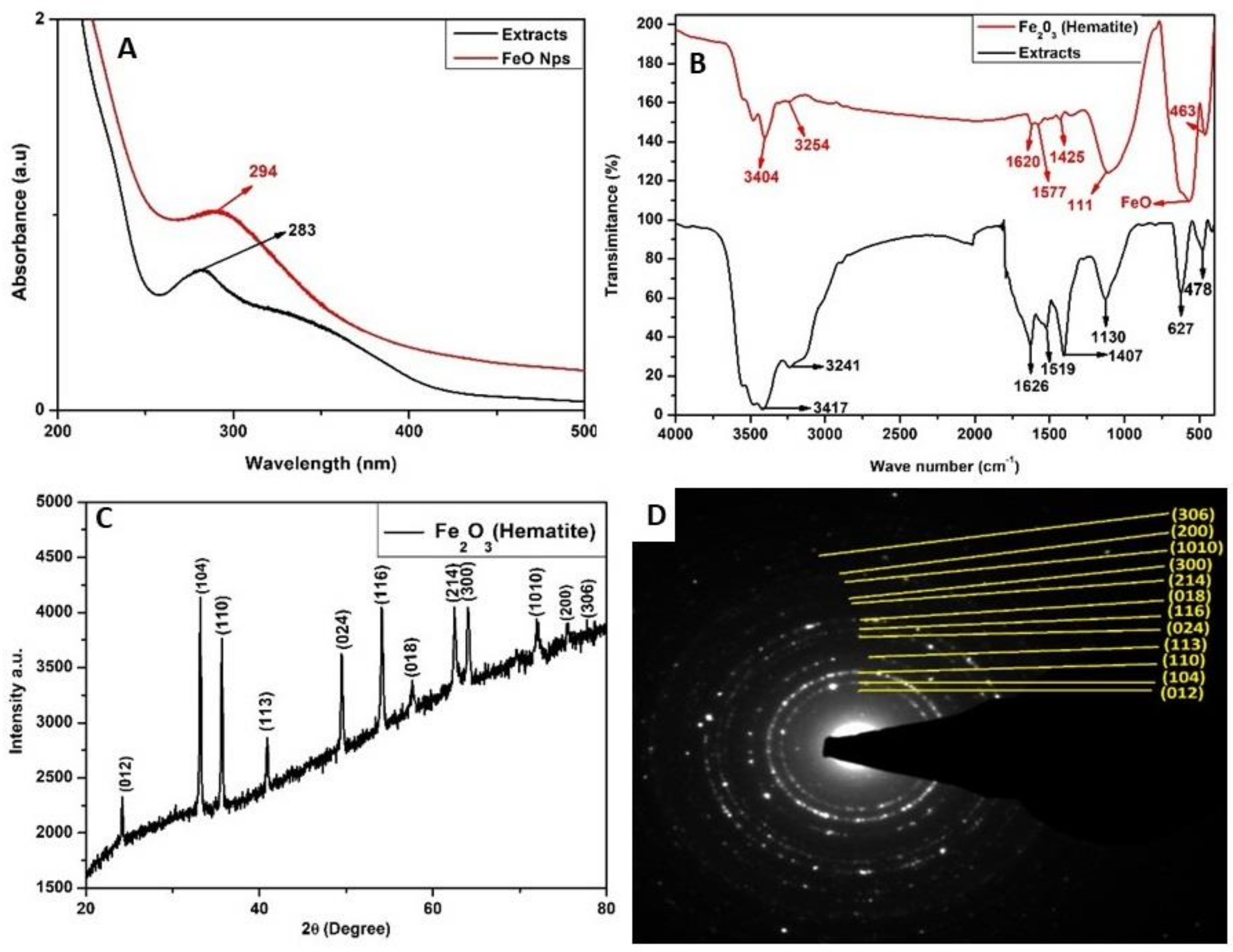
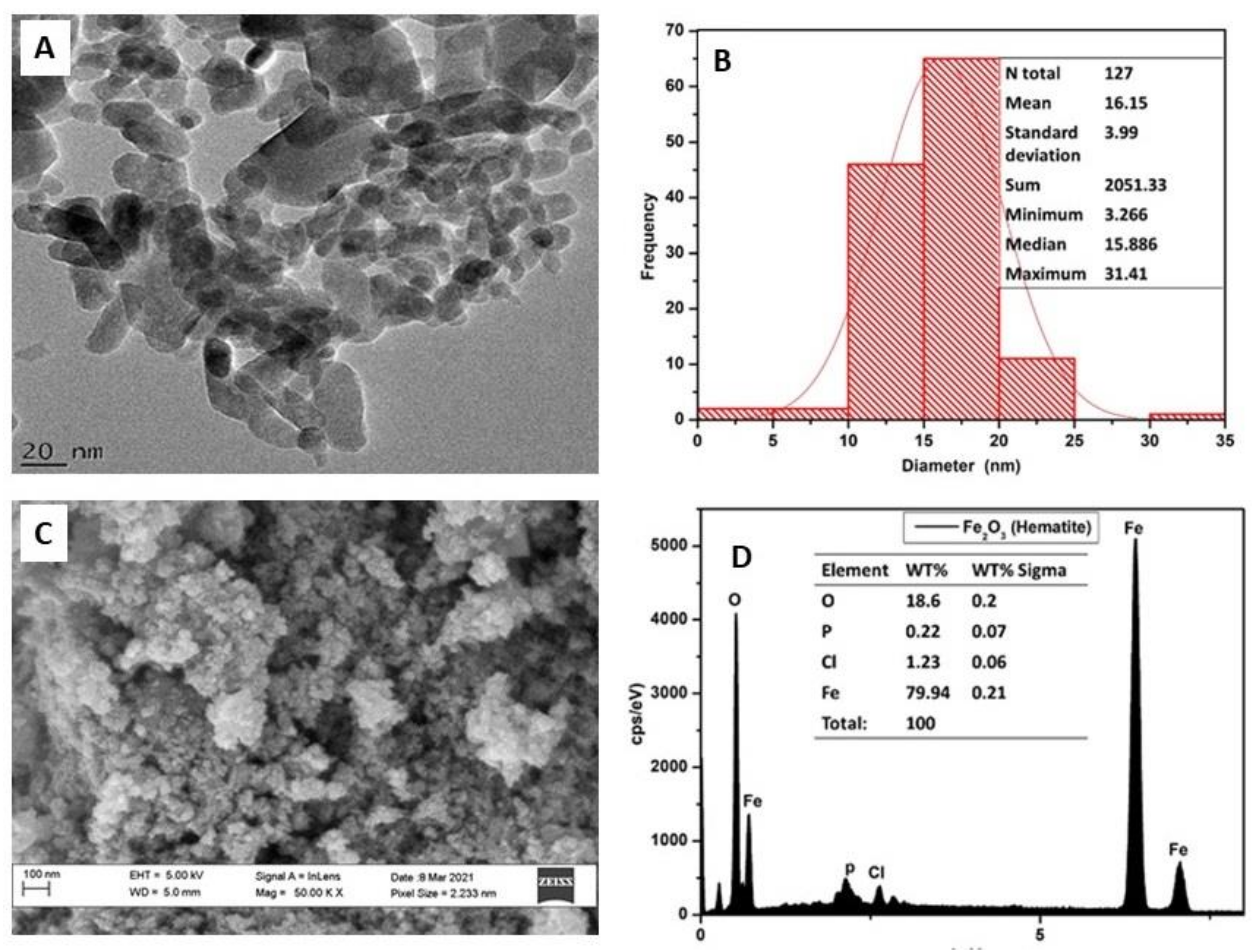
2.1. Characterization of Iron Oxide Nanoparticles
2.2. The Effect of αFe2O3 NP Priming on the Growth Attributes of S. bicolor under Drought Stress
2.2.1. Biomass
2.2.2. Photosynthetic Pigment and Growth Parameters
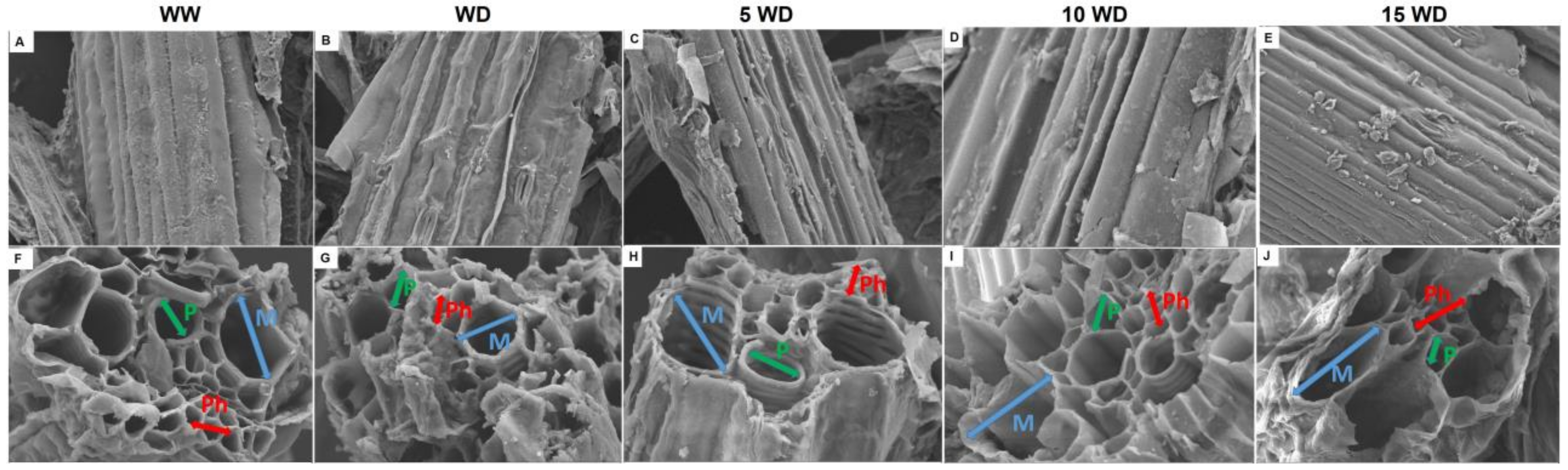
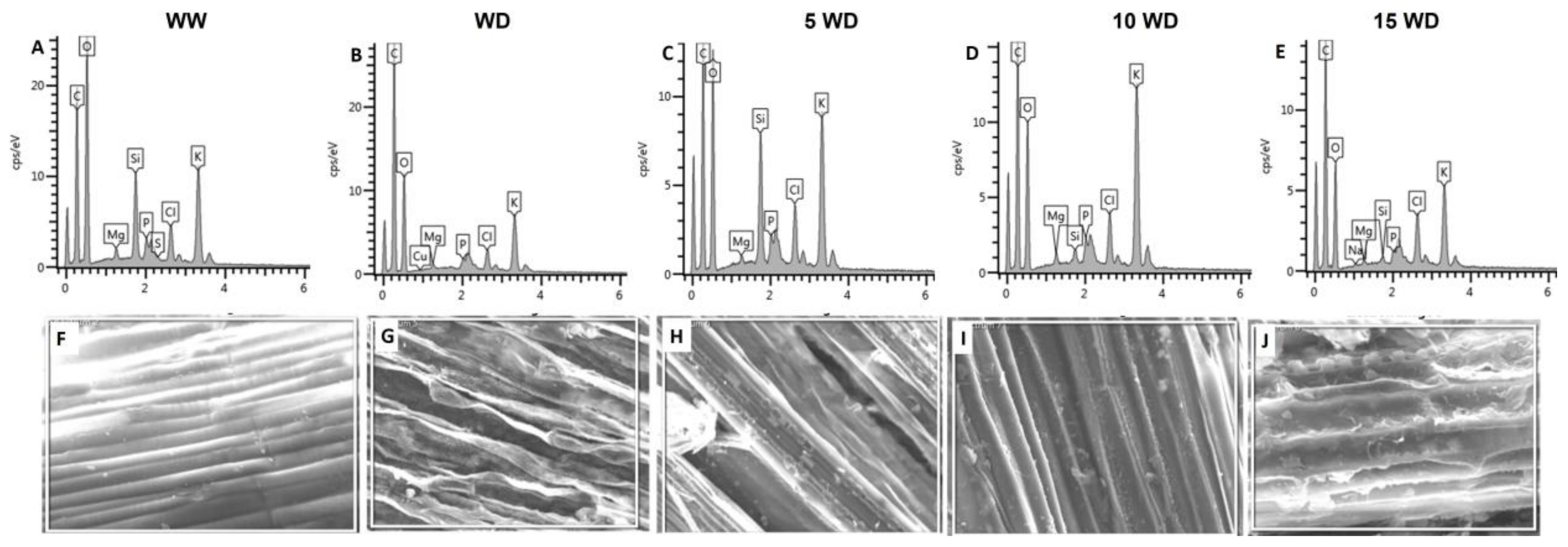
2.2.3. Effect of αFe2O3 NP Priming on the Anatomic Structure of Sorghum Plants
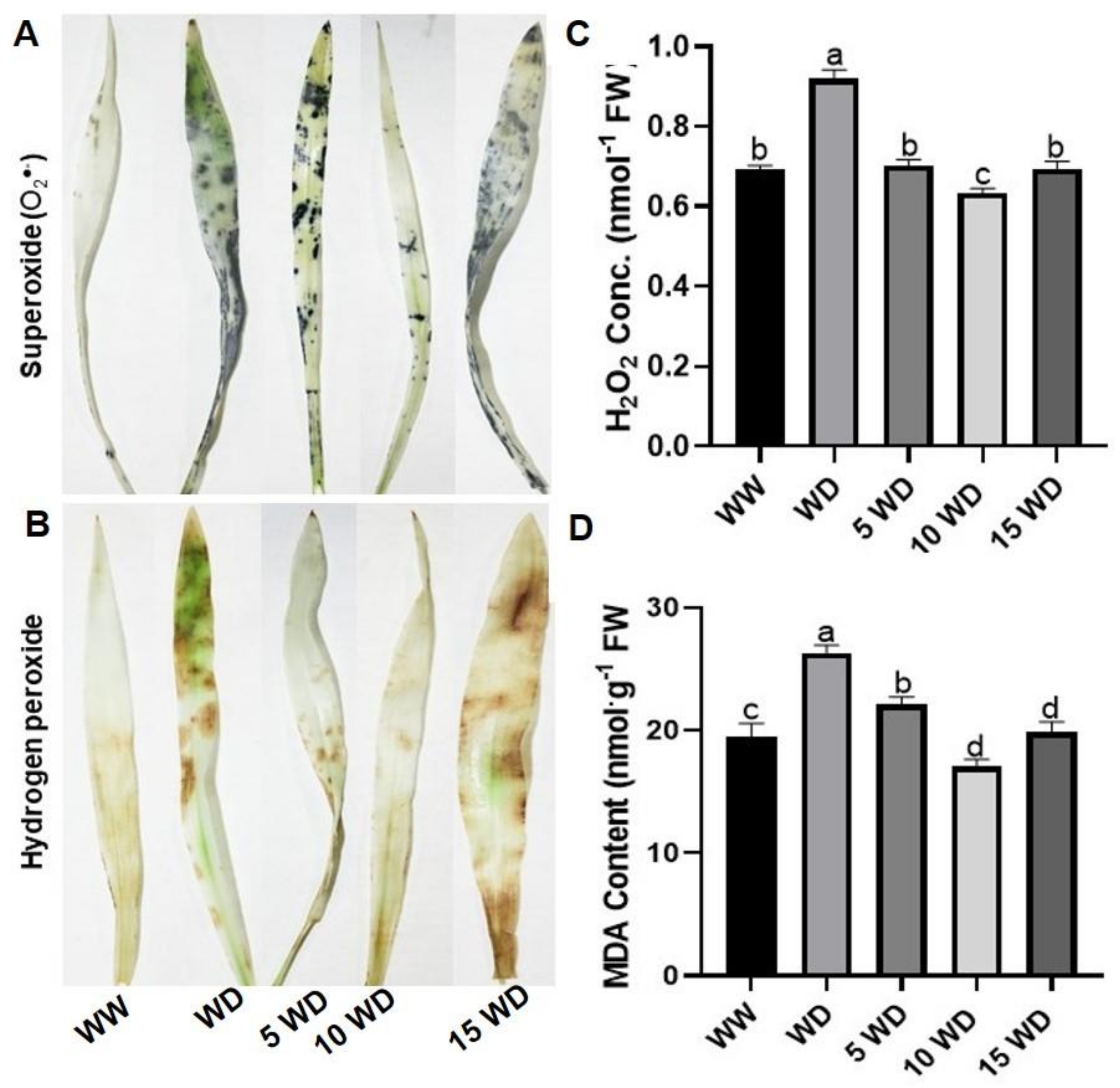
2.3. The Effect of Drought and αFe2O3 NP Priming on Oxidative Damage in Sorghum
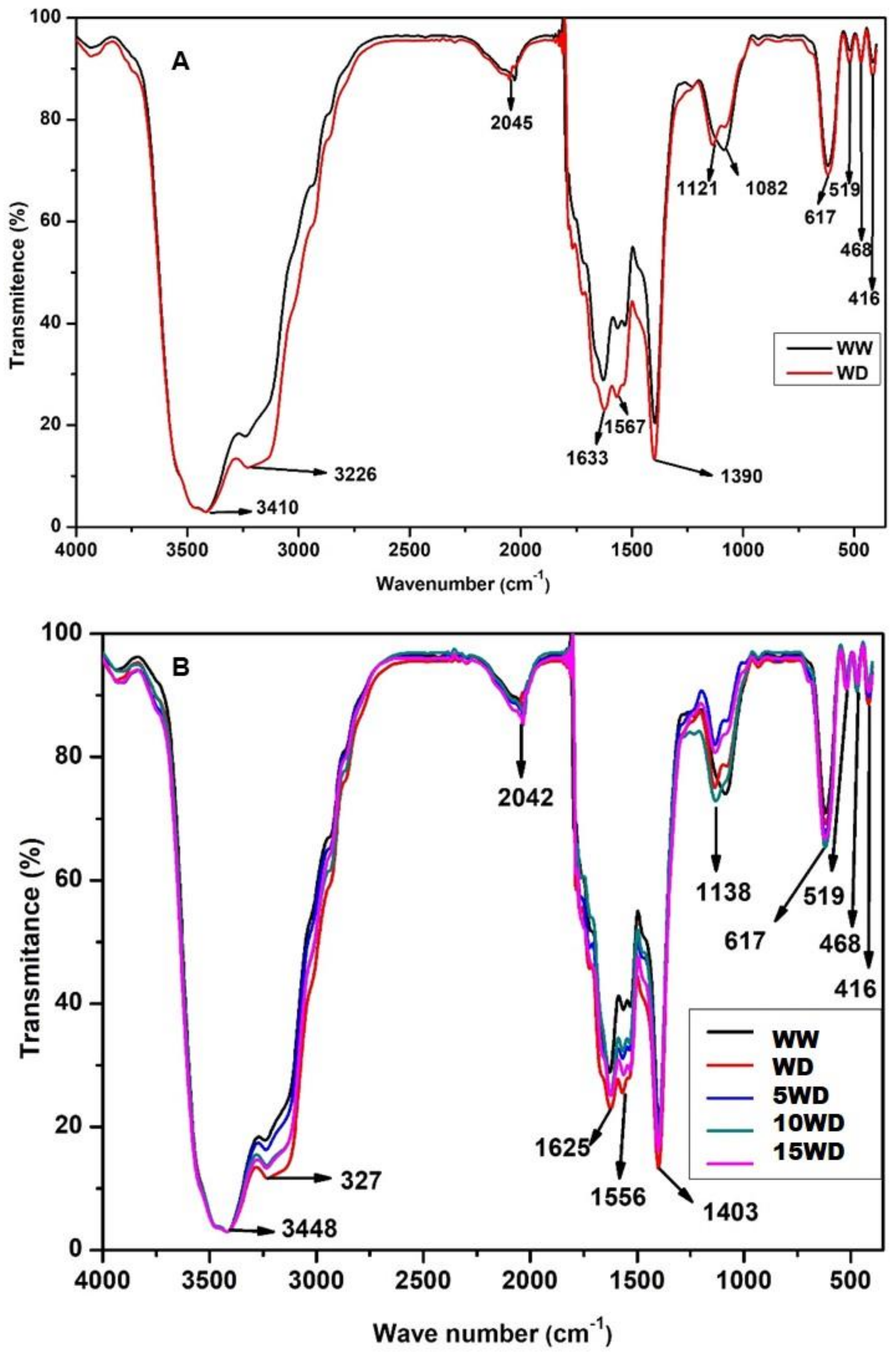
2.4. The Effect of Drought and αFe2O3 NP Priming on Biomolecules Analyzed by Fourier-Transform InfraRed Spectroscopic (FTIR)
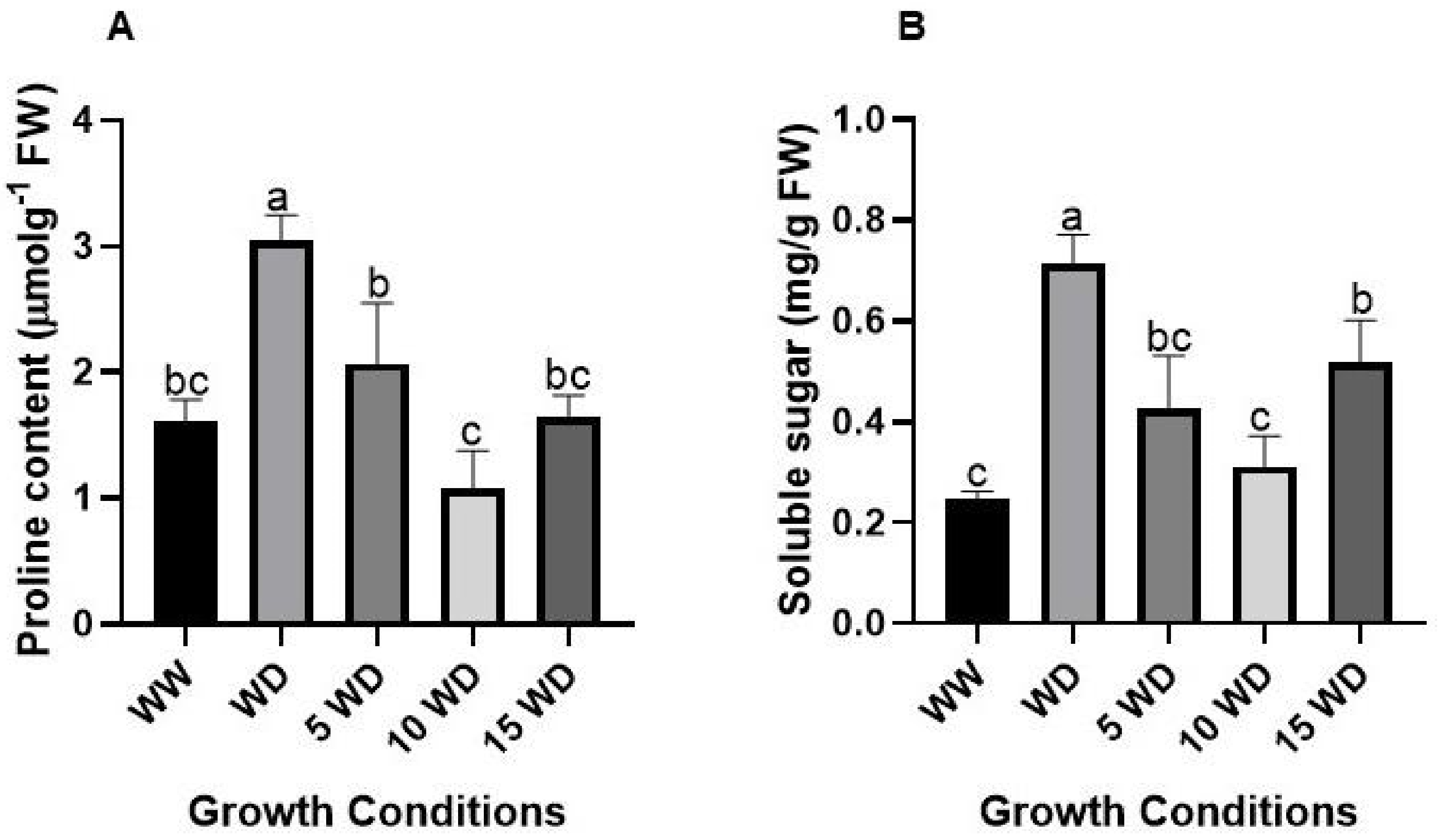
2.5. Effect of Drought and αFe2O3 NP Priming on Osmoregulation in Sorghum
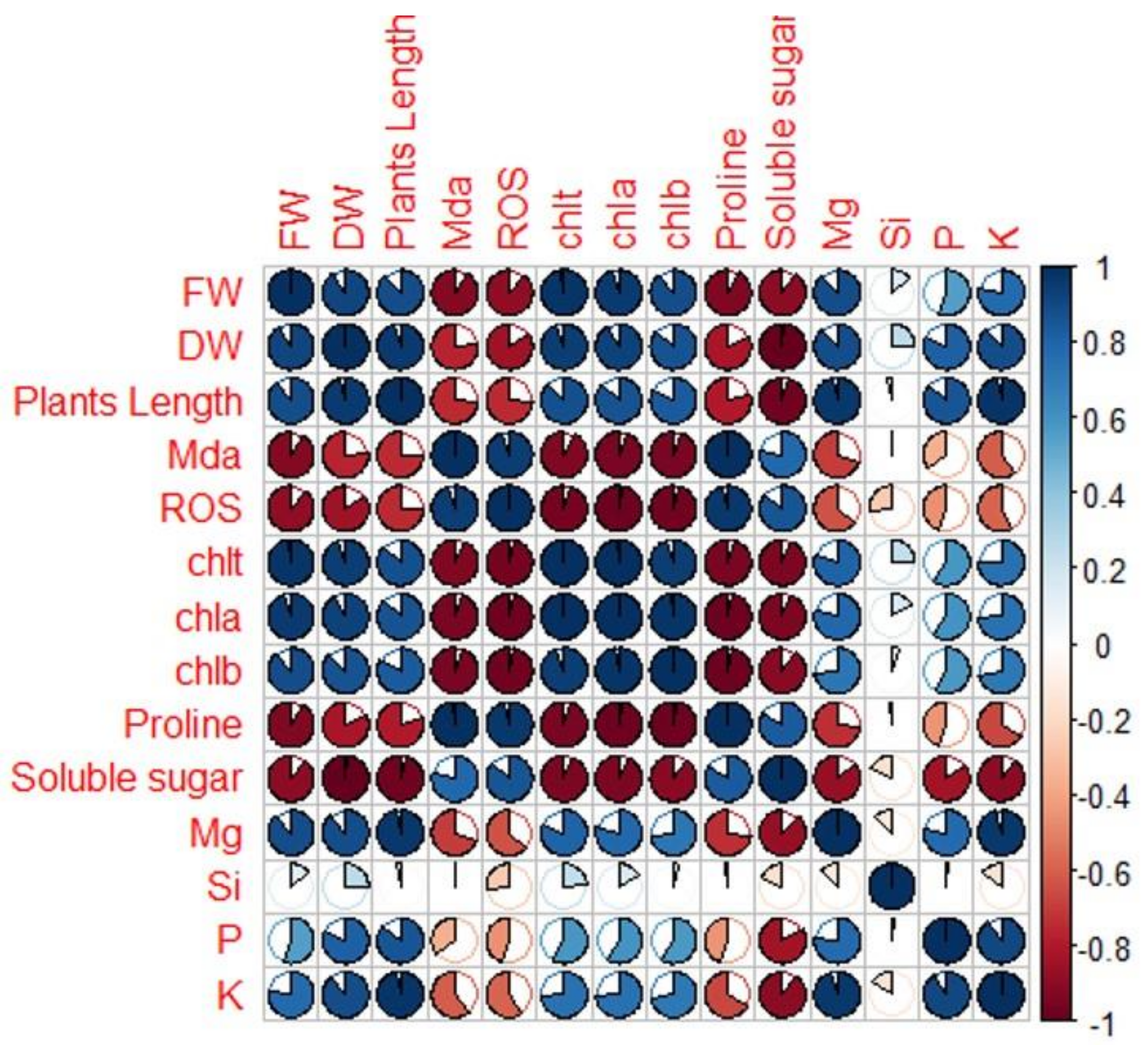
2.6. Pearson’s Traits Correlations
3. Discussion
4. Materials and Methods
4.1. Green Synthesis of Iron Oxide Nanoparticles
4.1.1. Extracts Preparations
4.1.2. Synthesis of αFe2O3 Nanoparticles
4.2. Characterization of Iron Oxide Nanoparticles
4.3. Sorghum Seeds Preparation and Growth Conditions
4.4. Growth Parameters
4.5. Determination of Photosynthetic Pigments
Chlb = [22.9 (OD645) − 4.68 (OD663)] × (V/1000) × W
Chlt = [20.2 (OD645) + 8.02 (OD663)] × (V/100) × W
4.6. Analysis of Oxidative Stress Markers
4.6.1. Histochemical Detection of Reactive Oxygen Species
4.6.2. Hydrogen Peroxide Content
4.6.3. Malondialdehyde Content
4.6.4. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of Biomolecules
4.7. Determination of Osmolyte Content
4.7.1. Proline Content
4.7.2. Soluble Sugars Content
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satish, L.; Shilpha, J.; Pandian, S.; Rency, A.S.; Rathinapriya, P.; Ceasar, S.A.; Largia, M.J.; Kumar, A.A.; Ramesh, M. Analysis of genetic variation in sorghum (Sorghum bicolor (L.) Moench) genotypes with various agronomical traits using SPAR methods. Gene 2016, 576, 581–585. [Google Scholar] [CrossRef]
- Krishnamurthy, V.; Shukla, J. Intraseasonal and seasonally persisting patterns of Indian monsoon rainfall. J. Clim. 2007, 20, 3–20. [Google Scholar] [CrossRef]
- Costa, P.H.; Neto, A.D.; Bezerra, M.A.; Prisco, J.T.; Gomes-Filho, E. Antioxidant-enzymatic system of two sorghum genotypes differing in salt tolerance. Braz. J. Plant Physiol. 2005, 17, 353–362. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Mpandeli, S.; Nesamvuni, E.; Maponya, P. Adapting to the impacts of drought by smallholder farmers in Sekhukhune District in Limpopo Province, South Africa. J. Agric. Sci. 2015, 7, 115. [Google Scholar] [CrossRef]
- Lin, J.; Shen, Q.; Wu, J.; Zhao, W.; Liu, L. Assessing the Potential of Downscaled Far Red Solar-Induced Chlorophyll Fluorescence from the Canopy to Leaf Level for Drought Monitoring in Winter Wheat. Remote Sens. 2022, 14, 1357. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, X.; Fan, W. Remote sensing inversion of leaf maximum carboxylation rate based on a mechanistic photosynthetic model. IEEE Trans. Geosci. Remote Sens. 2021, 60, 4400712. [Google Scholar] [CrossRef]
- Khan, M.T.; Ahmed, S.; Shah, A.A.; Noor Shah, A.; Tanveer, M.; El-Sheikh, M.A.; Siddiqui, M.H. Influence of Zinc Oxide Nanoparticles to Regulate the Antioxidants Enzymes, Some Osmolytes and Agronomic Attributes in Coriandrum sativum L. Grown under Water Stress. Agronomy 2021, 11, 2004. [Google Scholar] [CrossRef]
- Malcheska, F.; Ahmad, A.; Batool, S.; Müller, H.M.; Ludwig-Müller, J.; Kreuzwieser, J.; Randewig, D.; Hänsch, R.; Mendel, R.R.; Hell, R.; et al. Drought-enhanced xylem sap sulfate closes stomata by affecting ALMT12 and guard cell ABA synthesis. Plant Physiol. 2017, 174, 798–814. [Google Scholar] [CrossRef]
- Ramachandran, P.; Wang, G.; Augstein, F.; de Vries, J.; Carlsbecker, A. Continuous root xylem formation and vascular acclimation to water deficit involves endodermal ABA signalling via miR165. Development 2018, 145, dev159202. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Dubey, A.; Kumar, A.; Malla, M.A.; Chowdhary, K.; Singh, G.; Gudasalamani, R.H.; Sharma, S.; Saati-Santamaria, Z.; Menéndez, E.; Dames, J.F. Approaches for the amelioration of adverse effects of drought stress on crop plants. Front. Biosci. 2021, 26, 928. [Google Scholar]
- Grgac, R.; Rozsypal, J.; Des Marteaux, L.; Štětina, T.; Koštál, V. Stabilization of insect cell membranes and soluble enzymes by accumulated cryoprotectants during freezing stress. Proc. Natl. Acad. Sci. USA 2022, 119, e2211744119. [Google Scholar] [CrossRef] [PubMed]
- Askary, M.; Talebi, S.M.; Amini, F.; Bangan, A.D. Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 2017, 63. [Google Scholar] [CrossRef]
- Ghernaout, D.; Alghamdi, A.; Touahmia, M.; Aichouni, M.; Ait Messaoudene, N. Nanotechnology phenomena in the light of the solar energy. J. Energy Environ. Chem. Eng. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Jatav, G.; Nirmal, D. Application of nanotechnology in soil-plant system. Asian J. Soil Sci. 2013, 8, 176–184. [Google Scholar]
- Kamalizadeh, M.; Bihamta, M.; Zarei, A. Drought stress and TiO2 nanoparticles affect the composition of different active compounds in the Moldavian dragonhead plant. Acta Physiol. Plant. 2019, 41, 21. [Google Scholar] [CrossRef]
- Sreelakshmi, B.; Induja, S.; Adarsh, P.P.; Rahul, H.L.; Arya, S.M.; Aswana, S.; Haripriya, R.; Aswathy, B.R.; Manoj, P.K.; Vishnudasan, D. Drought stress amelioration in plants using green synthesised iron oxide nanoparticles. Mater. Today Proc. 2021, 41, 723–727. [Google Scholar] [CrossRef]
- Rakgotho, T.; Ndou, N.; Mulaudzi, T.; Iwuoha, E.; Mayedwa, N.; Ajayi, R.F. Green-synthesized zinc oxide nanoparticles mitigate salt stress in Sorghum bicolor. Agriculture 2022, 12, 597. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Černík, M.; Padil, V.V. Metal Oxide Nanoparticles for Environmental Remediation. Nanotechnol. Environ. Remediat. 2022, 183–213. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021, 269, 116134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, F.; Guo, W.; Huang, J.; Dai, Z.; Yi, L.; Wang, Y. Influence of α-and γ-Fe2O3 nanoparticles on watermelon (Citrullus lanatus) physiology and fruit quality. Water Air Soil Pollut. 2020, 231, 143. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Sharma, V.; Kumari, N. Biosynthesized Hematite Nanoparticles Mitigating Drought stress by Regulating Nitrogen Metabolism and Biological Nitrogen Fixation in Trigonella foenum-graecum. Plant Stress 2022, 6, 100112. [Google Scholar] [CrossRef]
- Bisht, S.; Sharma, V.; Kumari, N. Biosynthesized magnetite nanoparticles from Polyalthia longifolia leaves improve photosynthetic performance and yield of Trigonella foenum-graecum under drought stress. Plant Stress 2022, 5, 100090. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; ur Rehman, M.Z.; Hussain, A.; Hussain, K.; Chatha, S.A.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef]
- Ahmed, F.; Javed, B.; Razzaq, A.; Mashwani, Z.U. Applications of copper and silver nanoparticles on wheat plants to induce drought tolerance and increase yield. IET Nanobiotechnol. 2021, 15, 68–78. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Shoarian, N.; Jamei, R.; Pasban Eslam, B.; Salehi Lisar, S.Y. Titanium dioxide nanoparticles increase resistance of Lallemantia iberica to drought stress due to increased accumulation of protective antioxidants. Iran. J. Plant Physiol. 2020, 10, 3343–3354. [Google Scholar]
- Foroutan, L.; Solouki, M.; Abdossi, V.; Fakheri, B.A. The effects of zinc oxide nanoparticles on enzymatic and osmoprotectant alternations in different Moringa peregrina populations under drought stress. Int. J. Basic Sci. Med. 2018, 3, 178–187. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Rao, Y.S.; Balaji, T.; Prathima, B.; Jyothi, N.V. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater. Lett. 2013, 100, 241–244. [Google Scholar] [CrossRef]
- Herrera-Becerra, R.; Rius, J.; Zorrilla, C. Tannin biosynthesis of iron oxide nanoparticles. Appl. Phys. A 2010, 100, 453–459. [Google Scholar] [CrossRef]
- Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria× ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 511–523. [Google Scholar]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Tsegaye, M.M.; Chouhan, G.; Fentie, M.; Tyagi, P.; Nand, P. Therapeutic Potential of Green Synthesized Metallic Nanoparticles Against Staphylococcus aureus. Curr. Drug Res. Rev. Former. Curr. Drug Abus. Rev. 2021, 13, 172–183. [Google Scholar] [CrossRef]
- Prabakaran, S.; Rajan, M. Biosynthesis of nanoparticles and their roles in numerous areas. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–47. [Google Scholar]
- Bolade, O.P.; Williams, A.B.; Benson, N.U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100279. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Al-Zahrani, F.A.; Salem, S.S.; Al-Ghamdi, H.A.; Nhari, L.M.; Lin, L.; El-Shishtawy, R.M. Green synthesis and antibacterial activity of Ag/Fe2O3 nanocomposite using Buddleja lindleyana extract. Bioengineering 2022, 9, 452. [Google Scholar] [CrossRef]
- Freire, A.; Chung, E.; Mendoza, I.; Jaén, J.A. Green synthesis of iron oxide nanoparticles using Caesalpinia coriaria (Jacq.) Willd. fruits extract. Hyperfine Interact. 2023, 244, 6. [Google Scholar] [CrossRef]
- Wahid, A.; Perveen, M.; Gelani, S.; Basra, S.M. Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J. Plant Physiol. 2007, 164, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.M.; Ishtiaq, M.; Maqbool, M.; Akram, R.; Shahid, A.; Shokralla, S.; Al-Ghobari, H.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Seed priming with iron oxide nanoparticles raises biomass production and agronomic profile of water-stressed flax plants. Agronomy 2022, 12, 982. [Google Scholar] [CrossRef]
- Abbas, S.F.; Bukhari, M.A.; Raza, M.A.S.; Abbasi, G.H.; Ahmad, Z. Seed nanopriming with zinc oxide improves wheat growth and photosynthetic performance in wheat under drought stress. R. square 2023. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. S. Afr. J. Bot. 2022, 151, 889–899. [Google Scholar] [CrossRef]
- Moacăl, E.A.; Watz, C.G.; Păcurariu, C.; Tudoran, L.B.; Ianoș, R.; Socoliuc, V.; Drăghici, G.A.; Iftode, A.; Liga, S.; Dragoș, D.; et al. Biosynthesis of iron oxide nanoparticles: Physico-chemical characterization and their In vitro cytotoxicity on healthy and tumorigenic cell lines. Nanomaterials 2022, 12, 2012. [Google Scholar] [CrossRef] [PubMed]
- Bouafia, A.; Laouini, S.E. Green synthesis of iron oxide nanoparticles by aqueous leaves extract of Mentha pulegium L.: Effect of ferric chloride concentration on the type of product. Mater. Lett. 2020, 265, 127364. [Google Scholar] [CrossRef]
- Gottimukkala, K.; Harika, R.; Zamare, D. Green synthesis of iron nanoparticles using green tea leaves extract. J. Nanomed. Biother. Discov. 2017, 7, 151. [Google Scholar]
- Pattanayak, M.; Nayak, P. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Azadirachta indica (Neem). World J. Nano Sci. Technol. 2013, 2, 06–09. [Google Scholar]
- Madivoli, E.S.; Kareru, P.G.; Maina, E.G.; Nyabola, A.O.; Wanakai, S.I.; Nyang’au, J. Biosynthesis of iron nanoparticles using Ageratum conyzoides extracts, their antimicrobial and photocatalytic activity. SN Appl. Sci. 2019, 1, 500. [Google Scholar] [CrossRef]
- Razack, S.A.; Suresh, A.; Sriram, S.; Ramakrishnan, G.; Sadanandham, S.; Veerasamy, M.; Nagalamadaka, R.B.; Sahadevan, R. Green synthesis of iron oxide nanoparticles using Hibiscus rosa-sinensis for fortifying wheat biscuits. SN Appl. Sci. 2020, 2, 898. [Google Scholar] [CrossRef]
- Anchan, S.; Pai, S.; Sridevi, H.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Biogenic synthesis of ferric oxide nanoparticles using the leaf extract of Peltophorum pterocarpum and their catalytic dye degradation potential. Biocatal. Agric. Biotechnol. 2019, 20, 101251. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Sharifi, I. Biosynthesis of bimetallic and core–shell nanoparticles: Their biomedical applications—A review. IET Nanobiotechnol. 2018, 12, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, W.; Khan, M.A.; Nazir, M.; Siddiquah, A.; Mushtaq, S.; Hashmi, S.S.; Abbasi, B. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater. Sci. Eng. C 2019, 103, 109740. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total Environ. 2014, 466, 210–213. [Google Scholar] [CrossRef]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar] [CrossRef]
- Wani, S.H.; Sah, S. Biotechnology and abiotic stress tolerance in rice. J. Rice Res. 2014, 2, e105. [Google Scholar] [CrossRef]
- Rosenow, D.T.; Quisenberry, J.E.; Wendt, C.W.; Clark, L.E. Drought tolerant sorghum and cotton germplasm. Agric. Water Manag. 1983, 7, 207–222. [Google Scholar] [CrossRef]
- Punia, S. Barley starch modifications: Physical, chemical and enzymatic-A review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar Sprayed Green Zinc Oxide Nanoparticles Mitigate Drought-Induced Oxidative Stress in Tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Fang, R.; Ma, J.; Hannan, F.; Huang, Q.; Sun, Y.; Javed, M.; Ali, S.; Zhou, W.; Farooq, M.A. Calcium nanoparticles (Ca-NPs) improve drought stress tolerance in Brassica napus by modulating the photosystem II, nutrient acquisition and antioxidant performance. NanoImpact 2022, 28, 100423. [Google Scholar] [CrossRef] [PubMed]
- Asoufi, H.M.; Al-Antary, T.M.; Awwad, A.M. Green route for synthesis hematite (α-Fe2O3) nanoparticles: Toxicity effect on the green peach aphid, Myzus persicae (Sulzer). Environ. Nanotechnol. Monit. Manag. 2018, 9, 107–111. [Google Scholar]
- Rufus, A.; Sreeju, N.; Vilas, V.; Philip, D. Biosynthesis of hematite (α-Fe2O3) nanostructures: Size effects on applications in thermal conductivity, catalysis, and antibacterial activity. J. Mol. Liq. 2017, 242, 537–549. [Google Scholar] [CrossRef]
- Atkin, O.K.; Macherel, D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 2009, 103, 581–597. [Google Scholar] [CrossRef]
- Patel, M.; Fatnani, D.; Parida, A.K. Silicon-induced mitigation of drought stress in peanut genotypes (Arachis hypogaea L.) through ion homeostasis, modulations of antioxidative defense system, and metabolic regulations. Plant Physiol. Biochem. 2021, 166, 290–313. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Farroh, K.Y.; Amin, H.E.; Atta, A.M. Green synthesis of zinc oxide nanoparticles: Fortification for rice grain yield and nutrients uptake enhancement. Molecules 2021, 26, 584. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; AlShammari, T.M.; Bargouti, M.; Ozdemir, M.; Tombuloglu, G.; Akhtar, S.; Sabit, H.; Hakeem, K.R.; Almessiere, M.; et al. Uptake, translocation, and physiological effects of hematite (α-Fe2O3) nanoparticles in barley (Hordeum vulgare L.). Environ. Pollut. 2020, 266, 115391. [Google Scholar] [CrossRef]
- Van Nhan, L.; Ma, C.; Rui, Y.; Cao, W.; Deng, Y.; Liu, L.; Xing, B. The effects of Fe2O3 nanoparticles on physiology and insecticide activity in non-transgenic and Bt-transgenic cotton. Front. Plant Sci. 2016, 6, 1263. [Google Scholar] [CrossRef]
- Jovanić, B.R.; Radenković, B.; Despotović-Zrakić, M.; Bogdanović, Z.; Barać, D. Effect of UV-B radiation on chlorophyll fluorescence, photosynthetic activity and relative chlorophyll content of five different corn hybrids. J. Photochem. Photobiol. 2022, 10, 100115. [Google Scholar] [CrossRef]
- Umair, H.M.; Aamer, M.; Umer, C.M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Kalefetoğlu, T.; Ekmekci, Y. The effects of drought on plants and tolerance mechanisms. Gazi Univ. J. Sci. 2005, 18, 723–740. [Google Scholar]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Younis, A.; Riaz, A.; Mansoor, F.; Hameed, M.; Akram, N.A.; Abideen, Z. Morpho-anatomical adaptations of two Tagetes erecta L. cultivars with contrasting response to drought stress. Pak. J. Bot. 2020, 52, 801–810. [Google Scholar] [CrossRef]
- Pacheco-Silva, N.V.; Donato, A.M. Morpho-anatomy of the leaf of Myrciaria glomerata. Rev. Bras. De Farmacogn. 2016, 26, 275–280. [Google Scholar] [CrossRef]
- Hwang, B.G.; Ryu, J.; Lee, S.J. Vulnerability of protoxylem and metaxylem vessels to embolisms and radial refilling in a vascular bundle of maize leaves. Front. Plant Sci. 2016, 7, 941. [Google Scholar] [CrossRef]
- da Silva Folli-Pereira, M.; Ramos, A.C.; Canton, G.C.; da Conceição, J.M.; de Souza, S.B.; Cogo, A.J.; Figueira, F.F.; Eutrópio, F.J.; Rasool, N. Foliar application of trace elements in alleviating drought stress. Water Stress Crop Plants A Sustain. Approach 2016, 2, 669–681. [Google Scholar]
- Reguera, M.; Bassil, E.; Blumwald, E. Intracellular NHX-type cation/H+ antiporters in plants. Mol. Plant 2014, 7, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J. Structures and stabilities of endohedral fullerenes: X@ C24H24 (X = H, Li, Li+, Na, Na+, K, K+, Be, Mg, Mg2+, Ca, Ca2+, B, Al, C, Si, N and P). J. Mol. Struct. THEOCHEM 2009, 896, 25–29. [Google Scholar] [CrossRef]
- Pardo, J.M.; Rubio, F. Na+ and K+ Transporters in Plant Signaling. In Transporters and Pumps in Plant Signaling; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–98. [Google Scholar]
- Tang, R.J.; Zhao, F.G.; Garcia, V.J.; Kleist, T.J.; Yang, L.; Zhang, H.X.; Luan, S. Tonoplast CBL–CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3134–3139. [Google Scholar] [CrossRef]
- Raya-Díaz, S.; Sánchez-Rodríguez, A.R.; Segura-Fernández, J.M.; Del Campillo, M.D.; Quesada-Moraga, E. Entomopathogenic fungi-based mechanisms for improved Fe nutrition in sorghum plants grown on calcareous substrates. PLoS ONE 2017, 12, e0185903. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. Significance and role of Si in crop production. Adv. Agron. 2017, 146, 83–166. [Google Scholar]
- Yamauchi, Y.; Furutera, A.; Seki, K.; Toyoda, Y.; Tanaka, K.; Sugimoto, Y. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem. 2008, 46, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, S.; Liu, Z.; Yang, F.; Yin, G. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Youssef, O.A.; Tammam, A.A.; El-Bakatoushi, R.F.; Alframawy, A.M.; Emara, M.M.; El-Sadek, L.M. Hematite nanoparticles influence ultrastructure, antioxidant defenses, gene expression, and alleviate cadmium toxicity in Zea mays. J. Plant Interact. 2020, 15, 54–74. [Google Scholar] [CrossRef]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.; Chau, N.H.; Van, N.T. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Pacifici, R.E.; Davies, K.J. Protein, lipid and DNA repair systems in oxidative stress: The free-radical theory of aging revisited. Gerontology 1991, 37, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B 2005, 827, 65–75. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Muthusamy, M.; Uma, S.; Backiyarani, S.; Saraswathi, M.S.; Chandrasekar, A. Transcriptomic changes of drought-tolerant and sensitive banana cultivars exposed to drought stress. Front. Plant Sci. 2016, 7, 1609. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, K.; Raghunath, A.; Panneerselvam, L.; Perumal, E. Iron oxide nanoparticles modulate heat shock proteins and organ specific markers expression in mice male accessory organs. Toxicol. Appl. Pharmacol. 2017, 317, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sillu, D.; Kumar, A.; Agnihotri, S. Dual nanozyme characteristics of iron oxide nanoparticles alleviate salinity stress and promote the growth of an agroforestry tree, Eucalyptus tereticornis Sm. Environ. Sci. Nano 2021, 8, 1308–1325. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Zuo, S.; Zuo, Y.; Gu, W.; Wei, S.; Li, J. Exogenous Proline Optimizes Osmotic Adjustment Substances and Active Oxygen Metabolism of Maize Embryo under Low-Temperature Stress and Metabolomic Analysis. Processes 2022, 10, 1388. [Google Scholar] [CrossRef]
- Mushtaq, N.; Iqbal, S.; Hayat, F.; Raziq, A.; Ayaz, A.; Zaman, W. Melatonin in Micro-Tom Tomato: Improved Drought Tolerance via the Regulation of the Photosynthetic Apparatus, Membrane Stability, Osmoprotectants, and Root System. Life 2022, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Giglou, M.T.; Giglou, R.H.; Esmaeilpour, B.; Azarmi, R.; Padash, A.; Falakian, M.; Śliwka, J.; Gohari, G.; Lajayer, H.M. A new method in mitigation of drought stress by chitosan-coated iron oxide nanoparticles and growth stimulant in peppermint. Ind. Crops Prod. 2022, 187, 115286. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef] [PubMed]
- Noor, R.; Yasmin, H.; Ilyas, N.; Nosheen, A.; Hassan, M.N.; Mumtaz, S.; Khan, N.; Ahmad, A.; Ahmad, P. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere 2022, 292, 133201. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Sadiq, H.M.; Shah, N.S.; Khan, A.U.; Muhammad, N.; Hassan, S.U.; Tahir, K.; Khan, F.U.; Imran, M.; Ahmad, N.; et al. Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J. Photochem. Photobiol. B Biol. 2019, 192, 147–157. [Google Scholar] [CrossRef]
- Badmapriya, D.; Asharani, I. Dye degradation studies catalysed by green synthesized iron oxide nanoparticles. Int. J. ChemTech Res. 2016, 9, 409–416. [Google Scholar]
- Saad, R.; Gamal, A.; Zayed, M.; Ahmed, A.M.; Shaban, M.; BinSabt, M.; Rabia, M.; Hamdy, H. Fabrication of ZnO/CNTs for application in CO2 sensor at room temperature. Nanomaterials 2021, 11, 3087. [Google Scholar] [CrossRef]
- Mulaudzi, T.; Hendricks, K.; Mabiya, T.; Muthevhuli, M.; Ajayi, R.F.; Mayedwa, N.; Gehring, C.; Iwuoha, E. Calcium Improves germination and growth of Sorghum bicolor seedlings under salt stress. Plants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, M.A.; Ahmad, Z.; Ashraf, M.Y.; Afzal, M.; Nawaz, F.; Nafees, M.; Jatoi, W.N.; Malghani, N.A.; Shah, A.N.; Manan, A. Silicon mitigates drought stress in wheat (Triticum aestivum L.) through improving photosynthetic pigments, biochemical and yield characters. Silicon 2021, 13, 4757–4772. [Google Scholar] [CrossRef]
- Gaafar, Y.Z.A.; Ziebell, H. Comparative study on three viral enrichment approaches based on RNA extraction for plant virus/viroid detection using high-throughput sequencing. PLoS ONE 2020, 15, e0237951. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]

| Growth Conditions | FW (g) | DW (g) | Chla (mg/g FW) | Chlb (mg/g FW) | Chlt (mg/g FW) |
|---|---|---|---|---|---|
| WW (control) | 0.650 ± 0.046 ab | 0.074 ± 0.002 a | 9.222 ± 0.892 ab | 3.752 ± 0.248 a | 11.499 ± 0.55 ab |
| WD (stressed) | 0.481 ± 0.009 c | 0.054 ± 0.003 c | 3.930 ± 0.101 c | 0.195 ± 0.032 c | 6.356 ± 0.856 c |
| 5 mg/L αFe2O3 NPs + WD | 0.611 ± 0.024 b | 0.068 ± 0.002 ab | 7.936 ± 0.958 b | 2.458 ± 0.405 b | 10.694 ± 0.957 b |
| 10 mg/L αFe2O3 NPs + WD | 0.744 ± 0.045 a | 0.073 ± 0.003 a | 10.243 ± 0.270 a | 3.963 ± 0.388 a | 12.982 ± 0.895 a |
| 15 mg/L αFe2O3 NPs + WD | 0.578 ± 0.040 bc | 0.061 ± 0.003 bc | 7.933 ± 0.066 b | 3.218 ± 0.217 ab | 9.901 ± 0.121 b |
| Growth Condition | |||||
|---|---|---|---|---|---|
| Elements | WW Wt% | WD Wt% | 5WD (Wt%) | 10WD (Wt%) | 15WD (Wt%) |
| Mg | 0.43 ± 0.08 a | 0.21 ± 0.008 c | 0.263 ± 0.142 b | 0.46 ± 0.09 a | 0.22 ± 0.05 c |
| Si | 0.28 ± 0.07 c | 0 ± 0 d | 2.8 ± 0.973 a | 0.52 ± 0.02 b | 0.22 ± 0.08 c |
| P | 1.37 ± 0.01 a | 0.16 ± 0.069 c | 0.53 ± 0.055 b | 0.62 ± 0.02 b | 0.18 ± 0.078 c |
| K | 15.45 ± 0.11 a | 4.135 ± 2.240 c | 6.295 ± 3.204b c | 12.7 ± 0.65 ab | 4.9 ± 2.63 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndou, N.; Rakgotho, T.; Nkuna, M.; Doumbia, I.Z.; Mulaudzi, T.; Ajayi, R.F. Green Synthesis of Iron Oxide (Hematite) Nanoparticles and Their Influence on Sorghum bicolor Growth under Drought Stress. Plants 2023, 12, 1425. https://doi.org/10.3390/plants12071425
Ndou N, Rakgotho T, Nkuna M, Doumbia IZ, Mulaudzi T, Ajayi RF. Green Synthesis of Iron Oxide (Hematite) Nanoparticles and Their Influence on Sorghum bicolor Growth under Drought Stress. Plants. 2023; 12(7):1425. https://doi.org/10.3390/plants12071425
Chicago/Turabian StyleNdou, Nzumbululo, Tessia Rakgotho, Mulisa Nkuna, Ibrahima Zan Doumbia, Takalani Mulaudzi, and Rachel Fanelwa Ajayi. 2023. "Green Synthesis of Iron Oxide (Hematite) Nanoparticles and Their Influence on Sorghum bicolor Growth under Drought Stress" Plants 12, no. 7: 1425. https://doi.org/10.3390/plants12071425
APA StyleNdou, N., Rakgotho, T., Nkuna, M., Doumbia, I. Z., Mulaudzi, T., & Ajayi, R. F. (2023). Green Synthesis of Iron Oxide (Hematite) Nanoparticles and Their Influence on Sorghum bicolor Growth under Drought Stress. Plants, 12(7), 1425. https://doi.org/10.3390/plants12071425










