Response of Rowan Berry (Sorbus redliana) Shoot Culture to Slow Growth Storage Conditions
Abstract
1. Introduction
2. Results
2.1. Morphological Traits of In Vitro Shoot Cultures during Storage
2.1.1. Shoot Length
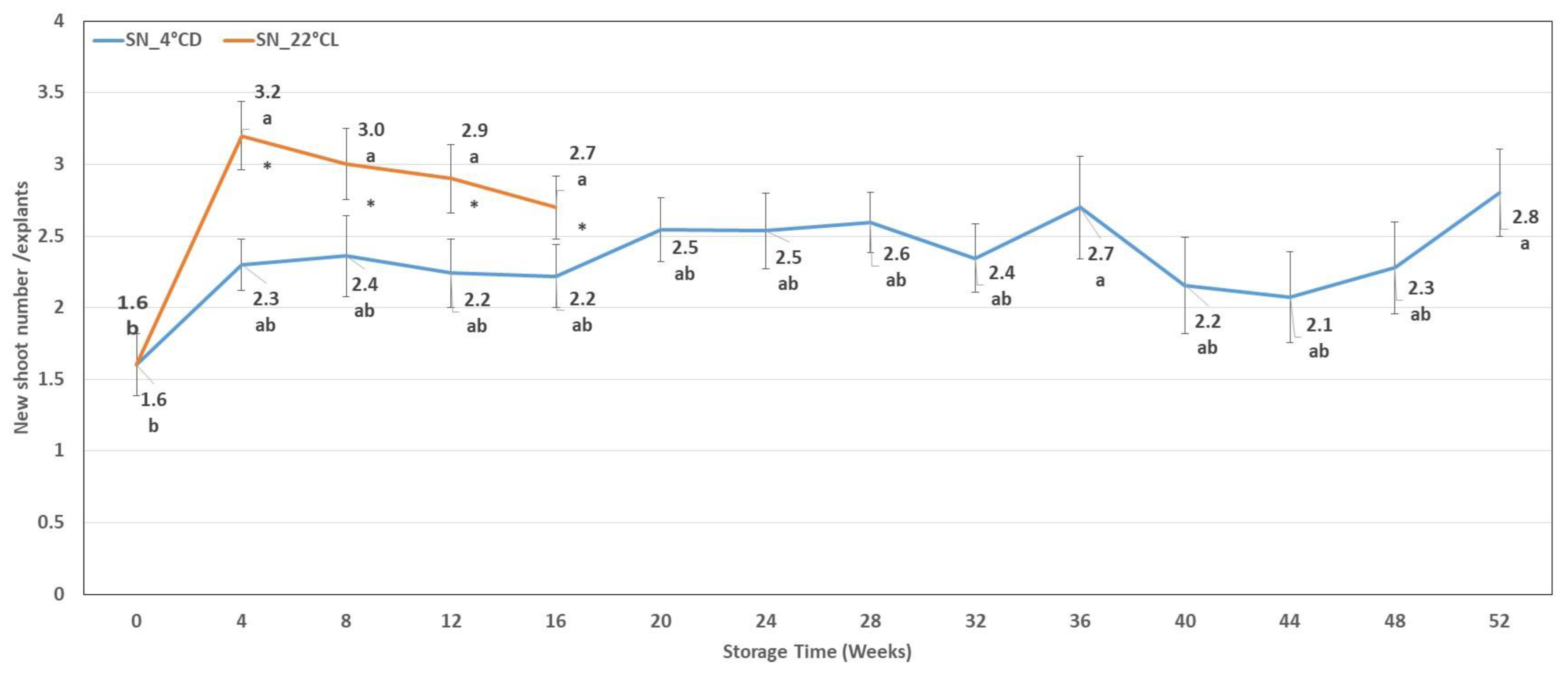
2.1.2. Shoot Number
2.2. Physiological Traits of In Vitro Shoot Cultures during Storage
2.2.1. Chlorophyll Content of Leaves
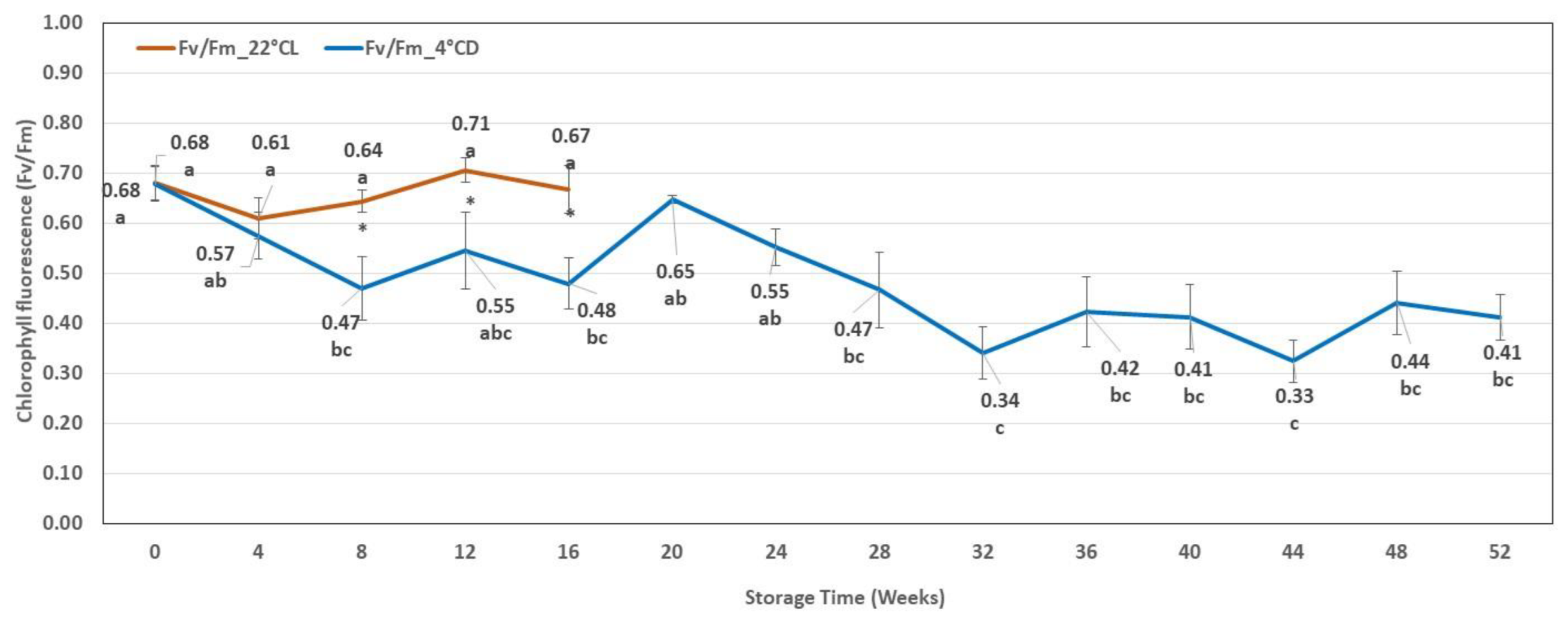
2.2.2. Chlorophyll Fluorescence Results
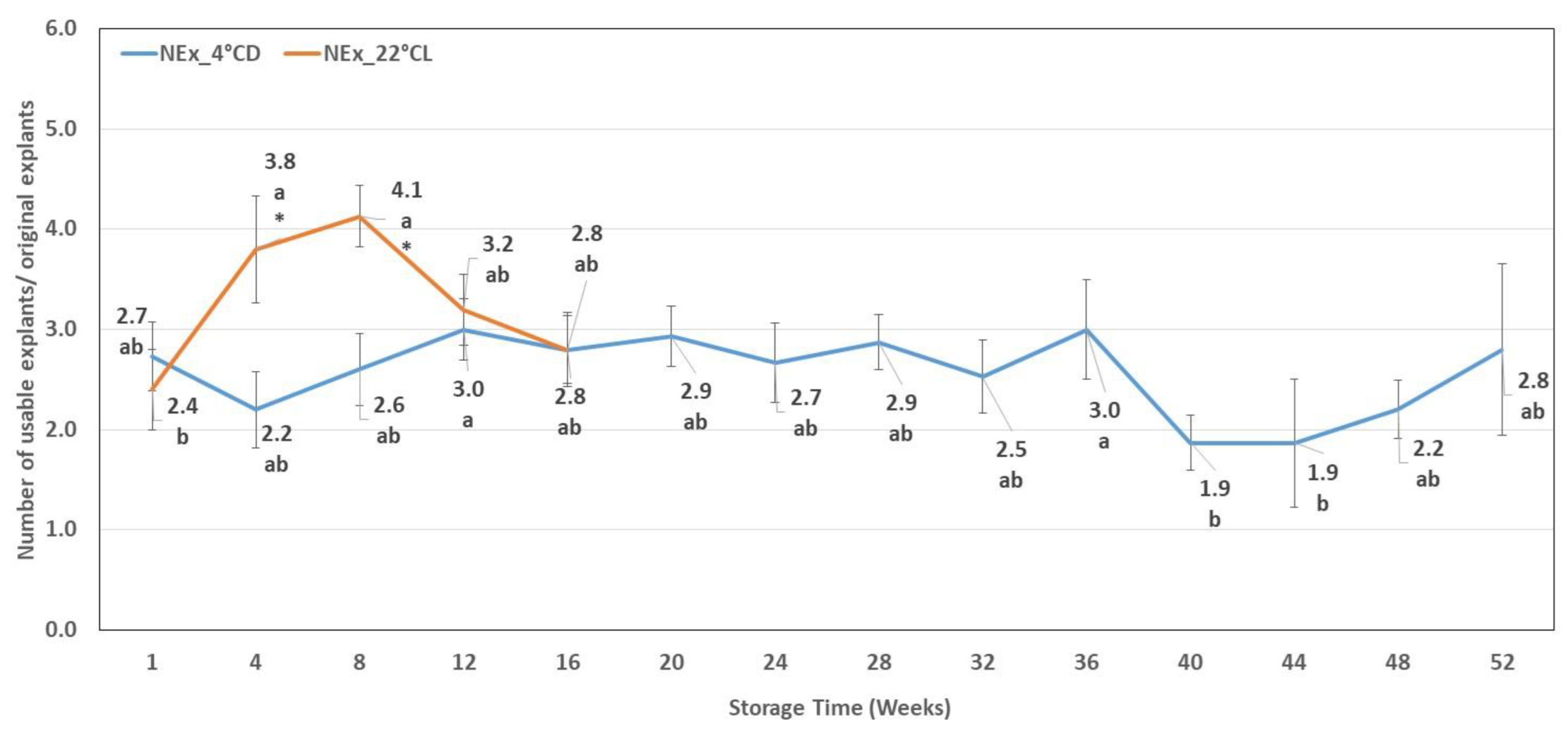
2.3. Number of Usable Explants
2.4. Shoot Proliferation Parameters of Explants from Different Storage Conditions
2.4.1. Length of Shoots at the End of Subculture Period
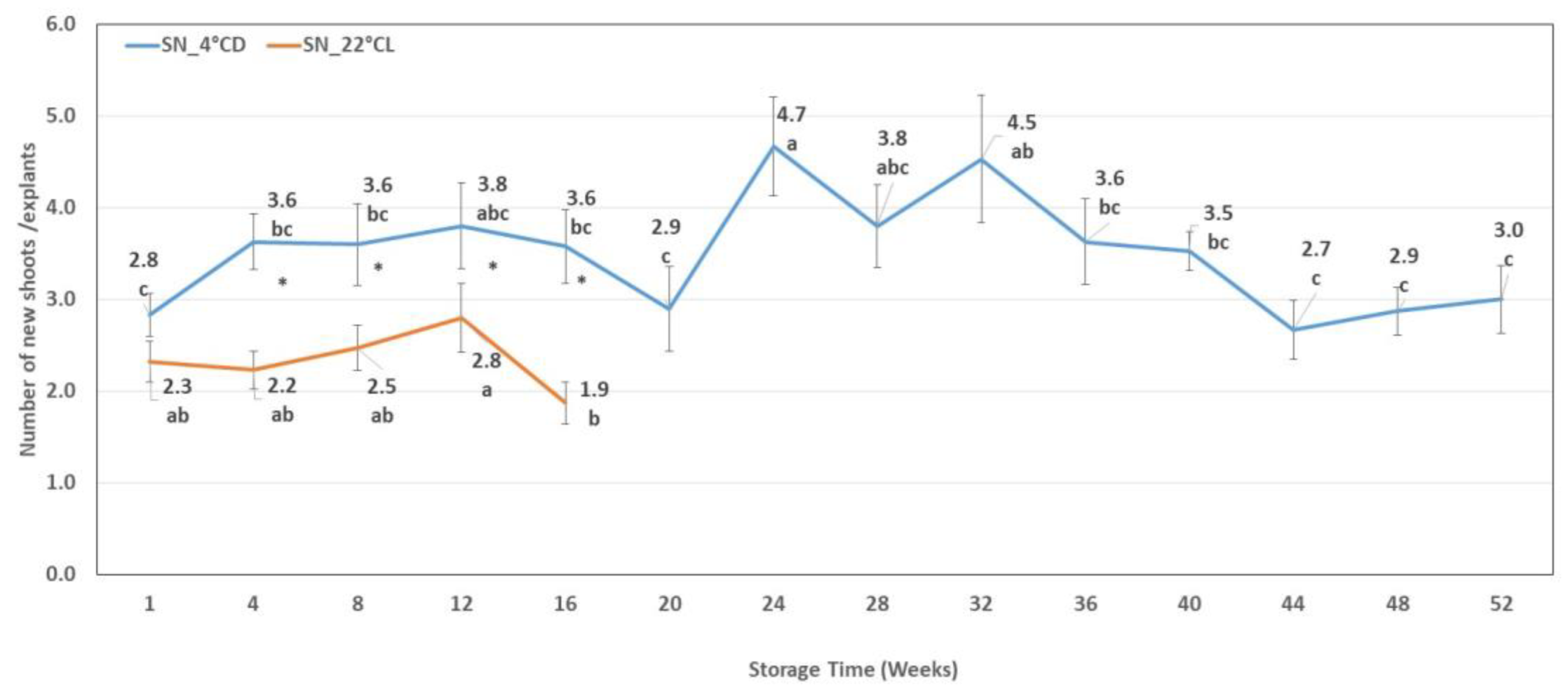
2.4.2. The number of New Shoots at the End of the Subculture Period
3. Discussion
4. Materials and Methods
4.1. Establishment and Maintainance of S. redliana In Vitro Cultures and Experimental Conditions
4.2. Morphological Observations on Stored Shoot Cultures
4.3. Measurements to Detect Physiological Changes in Stored Cultures
4.3.1. Measurement of the Chlorophyll Content in Leaves
4.3.2. Measurement of Chlorophyll Fluorescence
4.3.3. Number of Usable Explants after Storage
4.4. Morphological Observations on Subcultured Shoot Cultures
4.5. Data Collection and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phipps, J.B.; Robertson, K.R.; Smith, P.G.; Rohrer, J.R. A checklist of the subfamily Maloideae (Rosaceae). Can. J. Bot. 1990, 68, 2209–2269. [Google Scholar] [CrossRef]
- Campbell, C.S.; Donoghue, M.J.; Baldwin, B.G.; Martin, F.; Wojciechowski, M.F. Phylogenetic relationships in Maloideae (Rosaceae): Evidence from sequences of the internal transcribed spacers of nuclear ribosomal DNA and its congruence with morphology. Am. J. Bot. 1995, 82, 903–918. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Hebda, A.; Kempf, M.; Wachowiak, W.; Pluciński, B.; Kauzal, P.; Tomasz Zwijacz-Kozica, T. Hybridization and introgression of native and foreign Sorbus tree species in unique environments of protected mountainous areas. AoB Plants 2021, 13, plaa070. [Google Scholar] [CrossRef]
- Robertson, K.R.; Phipps, J.B.; Rohrer, J.R.; Smith, P.G. A Synopsis of Genera in Maloideae (Rosaceae). Syst. Bot. 1991, 16, 376–394. [Google Scholar] [CrossRef]
- Wolf, H.; Arenhövel, W.; Behm, A.; Franke, A.; Kleinschmit, J.; Rogge, M.; Schneck, D.; Schneck, V.; Schulzke, R.; Tabel, U. Conservation and breeding of wild fruit tree species in forestry. Acta Hortic. 2000, 538, 57–62. [Google Scholar] [CrossRef]
- Ördögh, M.; Jámbor-Benczúr, E.; Tilly Mándy, A.; Lelik, L. The effects of growth regulators in proliferation of Sorbus redliana ‘Burokvölgy’. Int. J. Hortic. Sci. 2006, 12, 77–83. [Google Scholar] [CrossRef]
- Enescu, C.M.; de Rigo, D.; Houston Durrant, T.; Caudullo, G. Sorbus domestica in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; European Commission: Luxembourg, 2016; p. e019db5. [Google Scholar]
- Rivers, M.C.; Beech, E.; Bazos, I.; Bogunić, F.; Buira, A.; Caković, D.; Carapeto, A.; Carta, A.; Cornier, B.; Fenu, G.; et al. European Red List of Trees; IUCN: Cambridge, UK; Brussels, Belgium, 2019; p. 60. [Google Scholar] [CrossRef]
- Hamston, T.J.; de Vere, N.; King, R.A.; Pellicer, J.; Fay, M.F.; Cresswell, J.E.; Stevens, J.R. Apomixis and Hybridization Drives Reticulate Evolution and Phyletic Differentiation in Sorbus L.: Implications for Conservation. Front. Plant Sci. 2018, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Aravanopoulos, F.A. Conservation and Monitoring of Tree Genetic Resources in Temperate Forests. Curr. For. Rep. 2016, 2, 119–129. [Google Scholar] [CrossRef]
- Lambardi, M.; Ozudogru, A. Advances in the safe storage of micropropagated woody plants at low temperature. Acta Hortic. 2013, 988, 29–42. [Google Scholar] [CrossRef]
- Postman, J.D. The USDA Quince and Pear Genebank in Oregon, a World Source of Fire Blight Resistance. Acta Hort. 2008, 793, 357–362. [Google Scholar] [CrossRef]
- Keller, E.R.J.; Senula, A.; Leunufna, S.; Grübe, M. Slow growth storage and cryopreservation—Tools to facilitate germplasm maintenance of vegetatively propagated crops in living plant collections. Int. J. Refrig. 2006, 29, 411–417. [Google Scholar] [CrossRef]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Engelmann, F. Biotechnology and Conservation of Plant Biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Agrawal, A.; Singh, S.; Malhotra, E.V.; Meena, D.P.S.; Tyagi, R.K. In Vitro Conservation and Cryopreservation of Clonally Propagated Horticultural Species. In Conservation and Utilization of Horticultural Genetic Resources; Rajasekharan, P., Rao, V., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Li, J.; He, M.; Xu, X.; Huang, T.; Tian, H.; Zhang, W. In Vitro Techniques for Shipping of Micropropagated Plant Materials. Horticulturae 2022, 8, 609. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, V.; Quaraishi, A. In vitro conservation through slow-growth storage. In Synthetic Seeds; Faisal, M., Alatar, A.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 397–415. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Kostas, S.; Joachim, M.; Economou, A. Regeneration of Viburnum dentatum L. from Alginate-Encapsulated Shoot Explants after Short-Term Cold Storage and Assessment of Genetic Stability Using ISSR Analysis. Agronomy 2020, 10, 1660. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Moat, J.F.; Ferraz, J.B.S.; Marks, T.R.; Camargo, J.L.C.; Nadarajan, J.; Ferraz, I.D.K. Innovative approaches to the preservation of forest trees. Forest Ecol. Manag. 2014, 333, 88–98. [Google Scholar] [CrossRef]
- Rotach, P. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Service Tree (Sorbus domestica); International Plant Genetic Resources Institute: Rome, Italy, 2003; 6p. [Google Scholar]
- Rich, T.C.G.; Houston, L.; Goodwin, A.; Morgan, V.; Bird, S.; Jones, R.; May, R.; Shiel, D.; Stockdale, R. Conservation status of Sorbus cuneifolia (Rosaceae), Llangollen whitebeam. Br. Ir. Bot. 2019, 1, 231–242. [Google Scholar] [CrossRef]
- Prknová, H. Long-term storage of service tree (Sorbus domestica L.) seeds and induction of their germination. J. For. Sci. 2015, 61, 417–421. [Google Scholar] [CrossRef]
- Reed, B.M. The in vitro genebank of temperate fruit and nut crops at the National Clonal Germplasm Repository-Corvallis. In Management of Field and In Vitro Germplasm Collections; International Plant Genetic Resources Institute: Rome, Italy, 1999; pp. 132–135. [Google Scholar]
- Arrillaga, I.; Marzo, T.; Segura, J. Micropropagation of juvenile and adult Sorbus domestica L. Plant Cell Tiss. Org. 1991, 27, 341–348. [Google Scholar] [CrossRef]
- Máchová, P.; Malá, J.; Cvrčková, H.; Dostál, J.; Buriánek, V. In vitro reproduction of rare and endemic species of rowan tree. J. For. Sci. 2013, 59, 386–390. [Google Scholar] [CrossRef]
- Kļaviņa, D.; Ievinsh, G. Growth of tissue culture and changes in oxidative enzyme activity of Sorbus and tayberry cultivars during cold storage. Acta Univ. Latv. 2008, 745, 179–186. [Google Scholar]
- Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In Vitro Technology in Plant Conservation: Relevance to Biocultural Diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef]
- Schenk, R.V.; Hildebrandt, A.C. Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phys. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Chalupa, V. In vitro propagation of mature trees of Sorbus aucuparia L. and field performance of micropropagated trees. J. For. Sci. 2002, 48, 529–535. [Google Scholar] [CrossRef]
- Dobránszki, J.; Magyar-Tábori, K.; Jevcsák, M.; Ördögh, M.; Benczúr, E.J. Improving the in vitro rooting of micro-shoots of Sorbus rotundifolia ‘Bükk szépe’ by the sequential application of Humus® FW and Wuxal® Super organic and chemical fertilisers. J. Hortic. Sci. Biotech. 2012, 87, 509–513. [Google Scholar] [CrossRef]
- Jámbor-Benczúr, E.; Ónadi, K.; Kissimon, J.; Horváth, G. The effect of carbon source on in vitro multiplication, photosynthesis and anatomical structure of Sorbus rotundifolia L. Acta Hortic. 1997, 447, 157–159. [Google Scholar]
- Šedivá, J.; Velebil, J.; Zahradník, D. Micropropagation as a Tool for the Conservation of Autochthonous Sorbus Species of Czechia. Plants 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Lambardi, M.; De Carlo, A. Application of Tissue Culture to the Germplasm Conservation of Temperate Broad-Leaf Trees. In Micropropagation of Woody Trees and Fruits; Forestry Sciences; Jain, S.M., Ishii, K., Eds.; Springer: Dordrecht, Germany, 2003; p. 75. [Google Scholar] [CrossRef]
- Benelli, C.; Tarraf, W.; Izgu, T.; De Carlo, A. In Vitro Conservation through Slow Growth Storage Technique of Fruit Species: An Overview of the Last 10 Years. Plants 2022, 11, 3188. [Google Scholar] [CrossRef]
- González-Arnao, M.T.; Dolce, N.; González-Benito, M.E.; Martínez, C.R.C.; Cruz-Cruz, C.A. Approaches for In Vitro Conservation of Woody Plants Germplasm Chapter 13. In Biodiversity and Conservation of Woody Plants; Sustainable Development and, Biodiversity; Ahuja, M.R., Jain, S.M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 17, pp. 355–419. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, S.; Wang, H.; Dai, J. Seasonal variations of cold hardiness and dormancy depth in five temperate woody plants in China. Front. For. Glob. Change 2022, 5, 1061191. [Google Scholar] [CrossRef]
- Pruski, K.; Kozai, T.; Lewis, T.; Astatkie, T.; Nowak, J. Sucrose and light effects on in vitro cultures of potato, chokocherry and saskatoon berry during low temperature storage. Plant Cell Tiss. Org. 2000, 63, 215–221. [Google Scholar] [CrossRef]
- Negri, V.; Tosti, N.; Standardi, A. Slow-growth storage of single node shoots of apple genotypes. Plant Cell Tiss. Org. 2000, 62, 159–162. [Google Scholar] [CrossRef]
- Orlikowska, T. Effect of in vitro storage at 4 °C on survival and proliferation of two apple rootstocks. Plant Cell Tiss. Org. Cult. 1992, 31, 1–7. [Google Scholar] [CrossRef]
- Janeiro, L.; Vieitez, A.; Ballester, A. Cold storage of in vitro cultures of wild cherry, chestnut and oak. Ann. Sci. For. 1995, 52, 287–293. [Google Scholar] [CrossRef]
- Kamińska, M.; Skrzypek, E.; Wilmowicz, E.; Tretyn, A.; Trejgell, A. Effect of light conditions and ABA on cold storage and post-storage propagation of Taraxacum pieninicum. Plant Cell Tiss. Org. 2016, 127, 25–34. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.C.; Zhang, J.H.; Yang, H.; Xu, L.; Wang, Q.F.; Sack, L.; Wu, X.Q.; Hou, J.H.; He, N.P. Variation in leaf chlorophyll concentration from tropical to cold-temperate forests: Association with gross primary productivity. Ecol. Indic. 2018, 85, 383–389. [Google Scholar] [CrossRef]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2005, 56, 435–447. [Google Scholar] [CrossRef]
- Dale, M.P.; Causton, D.R. Use of the chlorophyll a/b ratio as a bioassay for the light environment of a plant. Funct. Ecol. 1992, 6, 190–196. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of Low Temperature on Chlorophyll Biosynthesis and Chloroplast Biogenesis of Rice Seedlings during Greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef] [PubMed]
- Ptushenko, V.V.; Ptushenko, O.S.; Tikhonov, A.N. Chlorophyll Fluorescence Induction, Chlorophyll Content, and Chromaticity Characteristics of Leaves as Indicators of Photosynthetic Apparatus Senescence in Arboreous Plants. Biochemistry 2014, 79, 260–272. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Aazami, M.A.; Asghari-Aruq, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Baron, M.; Sochor, J. Low Temperature Stress Mediates the Antioxidants Pool and Chlorophyll Fluorescence in Vitis vinifera L. Cultivars. Plants 2021, 10, 1877. [Google Scholar] [CrossRef]
- Desjardins, Y.; Dubuc, J.-F.; Badr, A. In vitro culture of plants: A stressful activity! Acta Hortic. 2009, 812, 29–50. [Google Scholar] [CrossRef]
- Vosnjak, M.; Sircelj, H.; Hudina, M.; Usenik, V. Response of chloroplast pigments, sugars and phenolics of sweet cherry leaves to chilling. Sci. Rep. 2021, 11, 7210. [Google Scholar] [CrossRef] [PubMed]
- Riva-Roveda, L.; Escale, B.; Giauffret, C.; Périlleux, C. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biol. 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, D.; Orlikowska, T.; Maciorowski, R.; Kunka, M.; Niewiadomska-Wnuk, A. Storage of proliferating gooseberry cultures under slow growth conditions. Hort. Sci. 2021, 48, 134–140. [Google Scholar] [CrossRef]
- Jámbor-Benczúr, E.; Márta-Riffer, A. In vitro propagation of Philodendron tuxtlanum bunting with benzylaminopurine. Acta Agron. Hung. 1990, 39, 341–348. [Google Scholar]
- Heller, R. Recherces sur la Nutrition Minérale des Tissus Cultivés in Vitro. Ann. Sci. Nat. Bot. Veg. 1953, 2, 1–225. [Google Scholar]
- Jámbor-Benczúr, E.; Géczi, J.; Kissné, I.; Szafián Zs Nagy, T. Results in micropropagation of Sorbus redliana Karp. I. Culture initiation and multiplication. Publ. Untv. Hortic. Ind. Aliment. 1998, LVII, 77–82. [Google Scholar]
- Felföldy, L. A biológiai vízminősítés. Budapest: Vízgazdálkodási Intézet. Vízügyi Hidrobiol. 1987, 16, 141–148. [Google Scholar]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta. 1975, 376, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]





| Storage Weeks | Hyperhydration (%) | Discoloured Leaves (%) | Necrotic Tissues (%) | Etiolated Shoots (%) |
|---|---|---|---|---|
| 4 °CD | ||||
| 0 | 6.2 | 0.0 | 0.0 | 0.0 |
| 4 | 15.9 | 0.0 | 0.0 | 0.0 |
| 8 | 11.2 | 0.0 | 0.0 | 0.0 |
| 12 | 24.5 | 0.0 | 0.0 | 0.0 |
| 16 | 16.5 | 0.0 | 0.0 | 0.0 |
| 20 | 21.4 | 0.0 | 0.0 | 0.0 |
| 24 | 25.6 | 0.0 | 0.0 | 0.0 |
| 28 | 34.2 | 4.6 | 4.6 | 29.1 |
| 32 | 37.8 | 45.9 | 0.0 | 51.6 |
| 36 | 38.5 | 45.8 | 0.0 | 71.5 |
| 40 | 29.2 | 58.2 | 0.0 | 90.0 |
| 44 | 51.1 | 64.5 | 5.2 | 100.0 |
| 48 | 60.7 | 73.8 | 28.7 | 100.0 |
| 52 | 46.6 | 87.1 | 31.4 | 87.4 |
| 22 °CL | ||||
| 0 | 3.5 | 0.0 | 0.0 | 0.0 |
| 4 | 12.7 | 0.0 | 0.0 | 0.0 |
| 8 | 14.0 | 14.4 | 0.0 | 0.0 |
| 12 | 25.0 | 79.3 | 16.5 | 0.0 |
| 16 | 27.2 | 100.0 | 85.0 | 0.0 |
| Weeks in Storage | Chl a | Chl b | Chl a/b | |||
|---|---|---|---|---|---|---|
| mg g−1 Fresh Weight | ||||||
| 4 °CD | 22 °CL | 4 °CD | 22 °CL | 4 °CD | 22 °CL | |
| 0 | 0.62 ± 0.06 a | 0.62 ± 0.06 a | 0.27 ± 0.02 a | 0.27 ± 0.03 a | 2.29 ± 0.03 ab | 2.29 ± 0.03 a |
| 4 | 0.56 ± 0.03 b | 0.47 ± 0.03 b | 0.24 ± 0.01 b | 0.22 ± 0.01 b | 2.38 ± 0.03 ab | 2.16 ± 0.02 a |
| 8 | 0.49 ± 0.02 bc * | 0.28 ± 0.02 bc | 0.20 ± 0.01 b * | 0.13 ± 0.01 c | 2.45 ± 0.03 ab * | 2.09 ± 0.03 a |
| 12 | 0.54 ± 0.02 b * | 0.31 ± 0.03 bc | 0.21 ± 0.01 b * | 0.14 ± 0.01 c | 2.54 ± 0.02 ab * | 2.29 ± 0.04 a |
| 16 | 0.53 ± 0.02 b * | 0.22 ± 0.02 c | 0.21 ± 0.01 b * | 0.10 ± 0.01 c | 2.50 ± 0.03 a * | 2.30 ± 0.03 a |
| 20 | 0.46 ± 0.02 bc | - | 0.17 ± 0.01 bc | - | 2.63 ± 0.02 ab | - |
| 24 | 0.33 ± 0.02 cd | - | 0.13 ± 0.01 cd | - | 2.59 ± 0.04 ab | - |
| 28 | 0.21 ± 0.02 de | - | 0.08 ± 0.01 de | - | 2.48 ± 0.04 ab | - |
| 32 | 0.27 ± 0.04 de | - | 0.11 ± 0.01 de | - | 2.54 ± 0.04 ab | - |
| 36 | 0.21 ± 0.02 de | - | 0.08 ± 0.01 de | - | 2.57 ± 0.04 ab | - |
| 40 | 0.18 ± 0.02 de | - | 0.07 ± 0.01 de | - | 2.56 ± 0.04 ab | - |
| 44 | 0.09 ± 0.01 e | - | 0.04 ± 0.004 e | - | 2.27 ± 0.03 b | - |
| 48 | 0.15 ± 0.02 e | - | 0.06 ± 0.01 de | - | 2.47 ± 0.05 ab | - |
| 52 | 0.14 ± 0.02 e | - | 0.06 ± 0.01 de | - | 2.50 ± 0.05 ab | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendler-Drienyovszki, N.; Magyar-Tábori, K. Response of Rowan Berry (Sorbus redliana) Shoot Culture to Slow Growth Storage Conditions. Plants 2023, 12, 1287. https://doi.org/10.3390/plants12061287
Mendler-Drienyovszki N, Magyar-Tábori K. Response of Rowan Berry (Sorbus redliana) Shoot Culture to Slow Growth Storage Conditions. Plants. 2023; 12(6):1287. https://doi.org/10.3390/plants12061287
Chicago/Turabian StyleMendler-Drienyovszki, Nóra, and Katalin Magyar-Tábori. 2023. "Response of Rowan Berry (Sorbus redliana) Shoot Culture to Slow Growth Storage Conditions" Plants 12, no. 6: 1287. https://doi.org/10.3390/plants12061287
APA StyleMendler-Drienyovszki, N., & Magyar-Tábori, K. (2023). Response of Rowan Berry (Sorbus redliana) Shoot Culture to Slow Growth Storage Conditions. Plants, 12(6), 1287. https://doi.org/10.3390/plants12061287







