Overexpression of the Caragana korshinskii com58276 Gene Enhances Tolerance to Drought in Cotton (Gossypium hirsutum L.)
Abstract
:1. Introduction
2. Results
2.1. Gene Cloning and Subcellular Localization
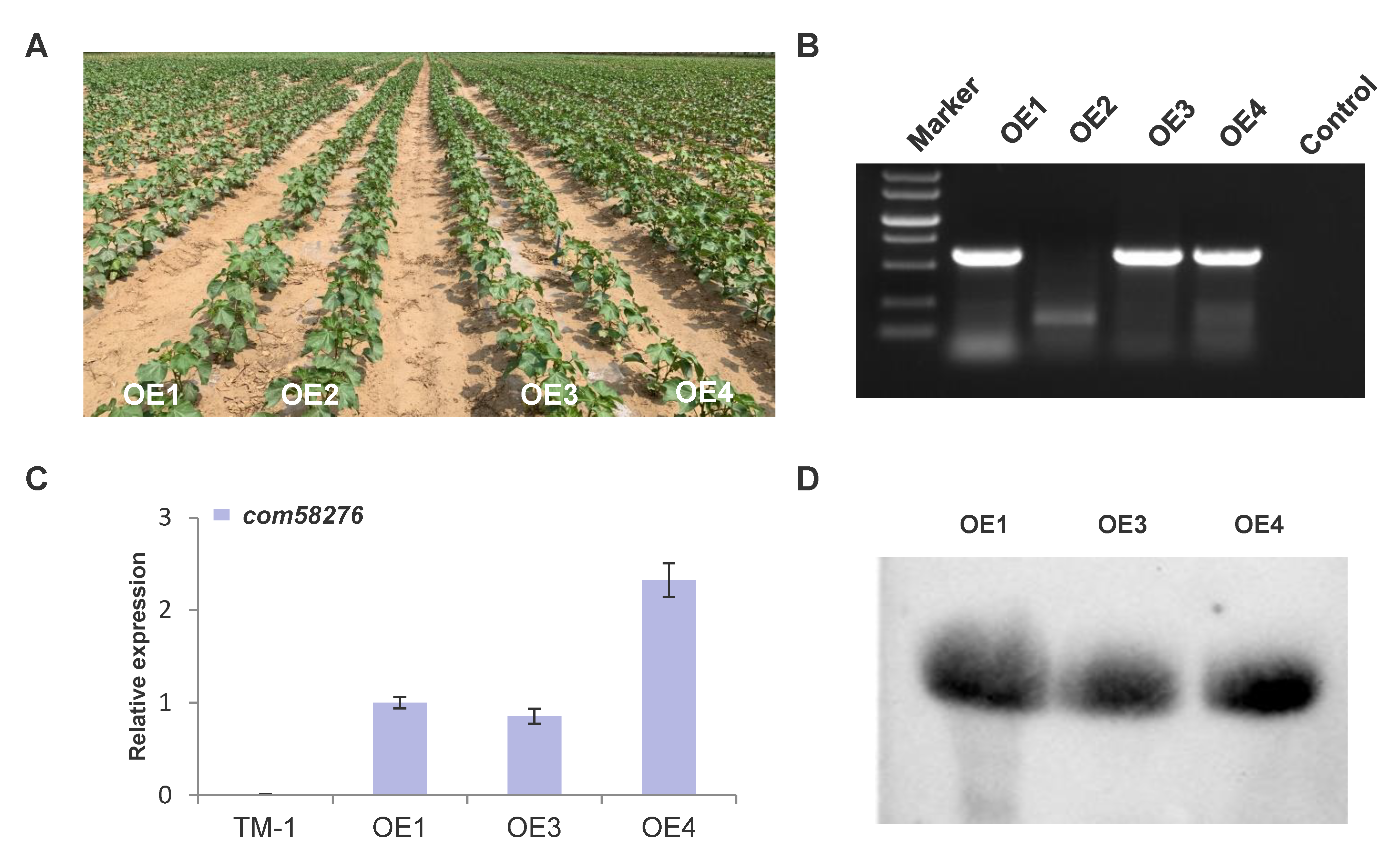
2.2. Genetic Transformation and Positive Identification
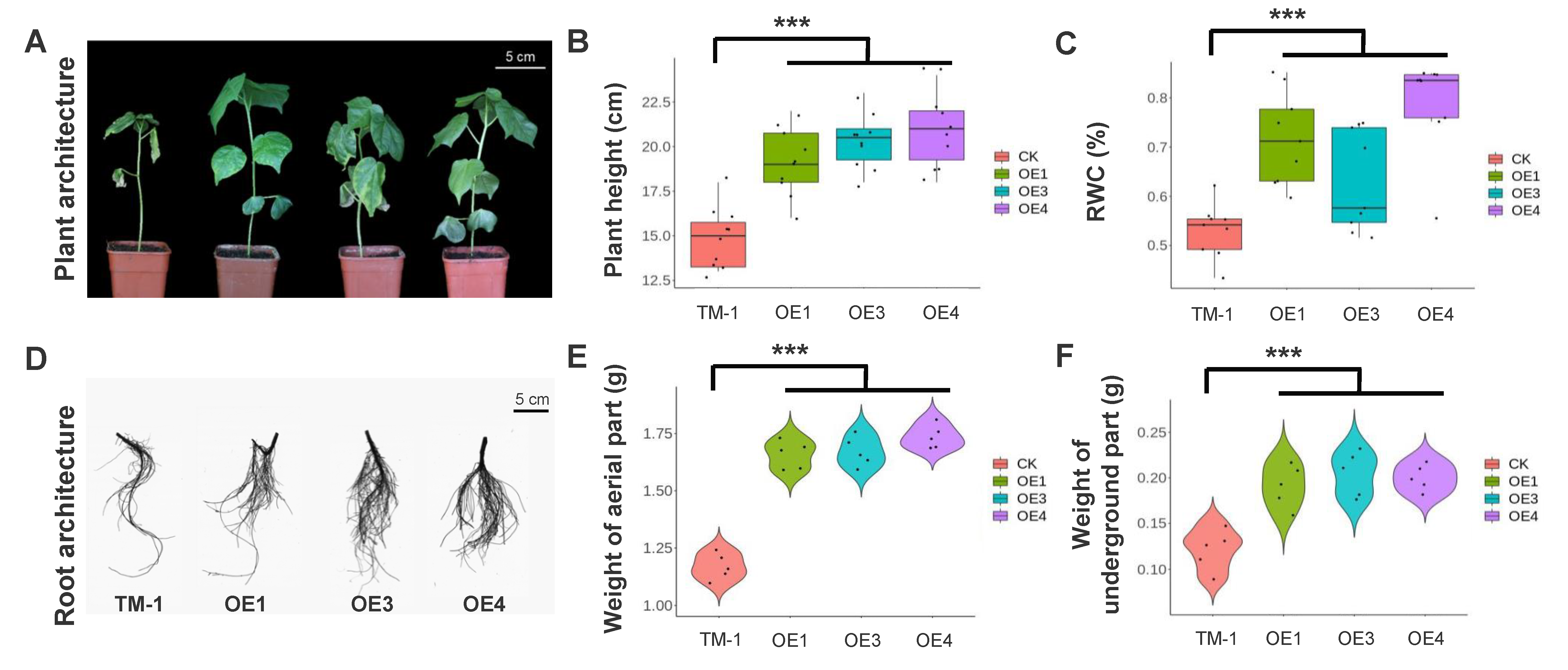
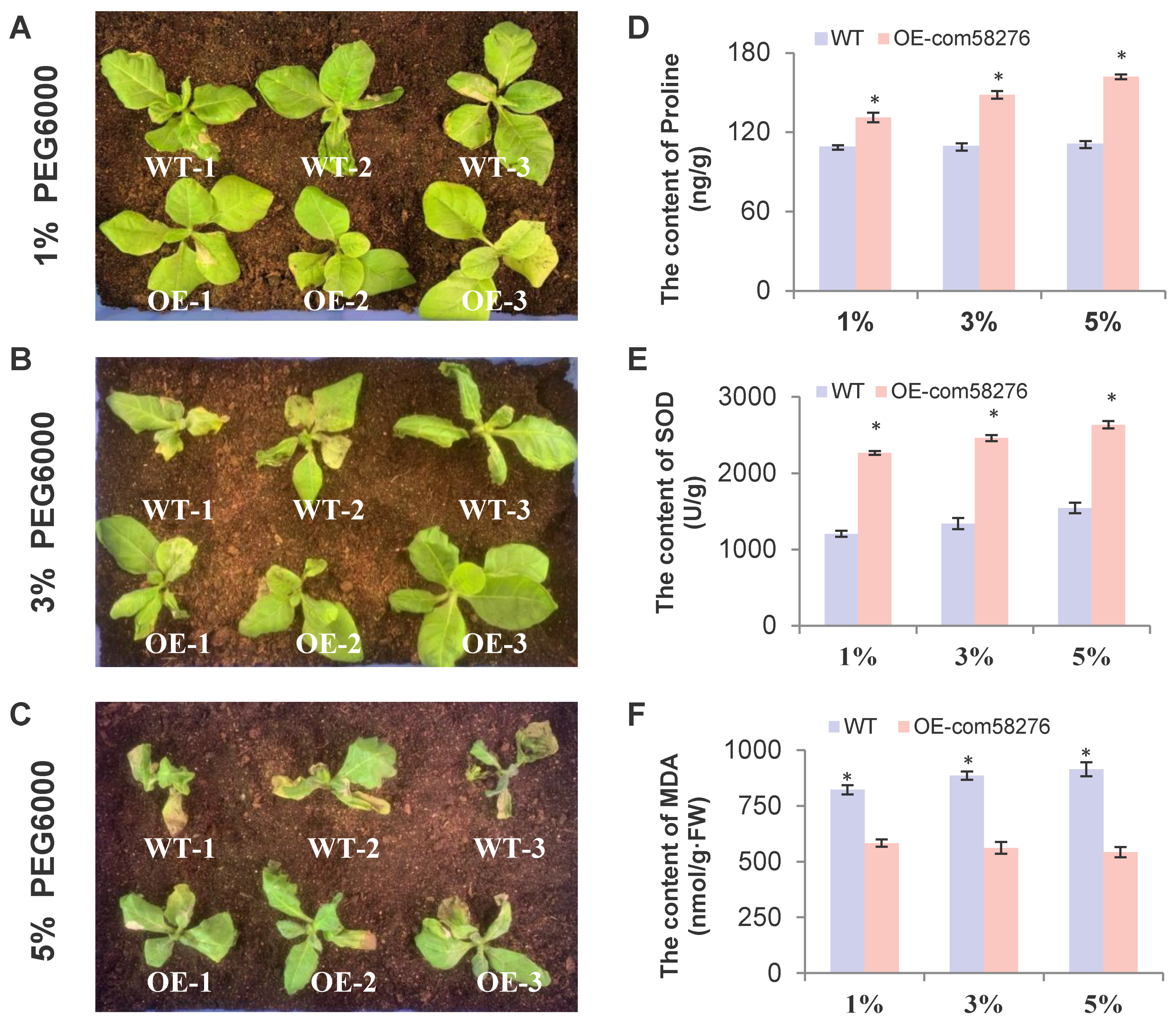
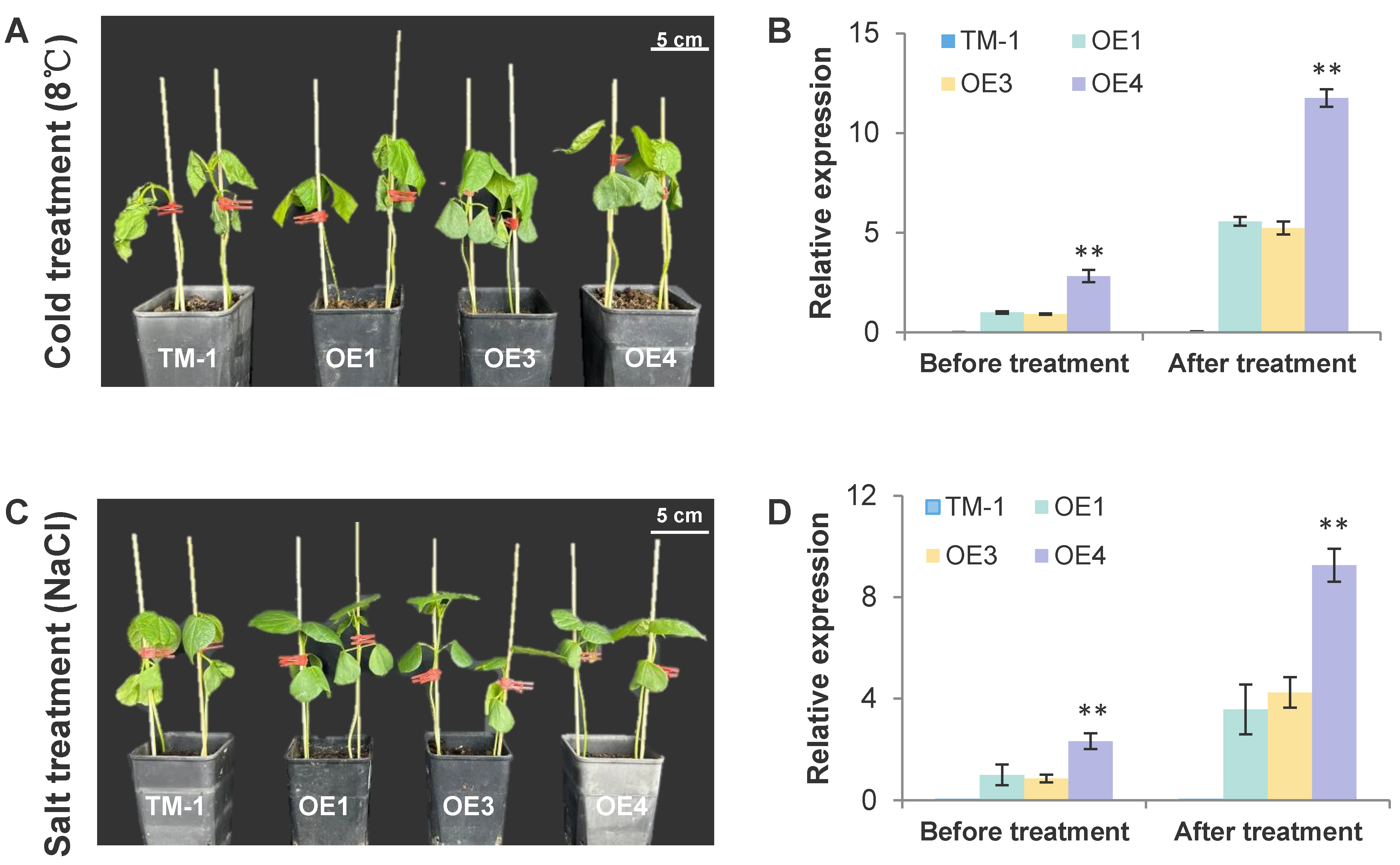
2.3. Overexpression of the com58276 Gene Enhances Drought Tolerance in Cotton
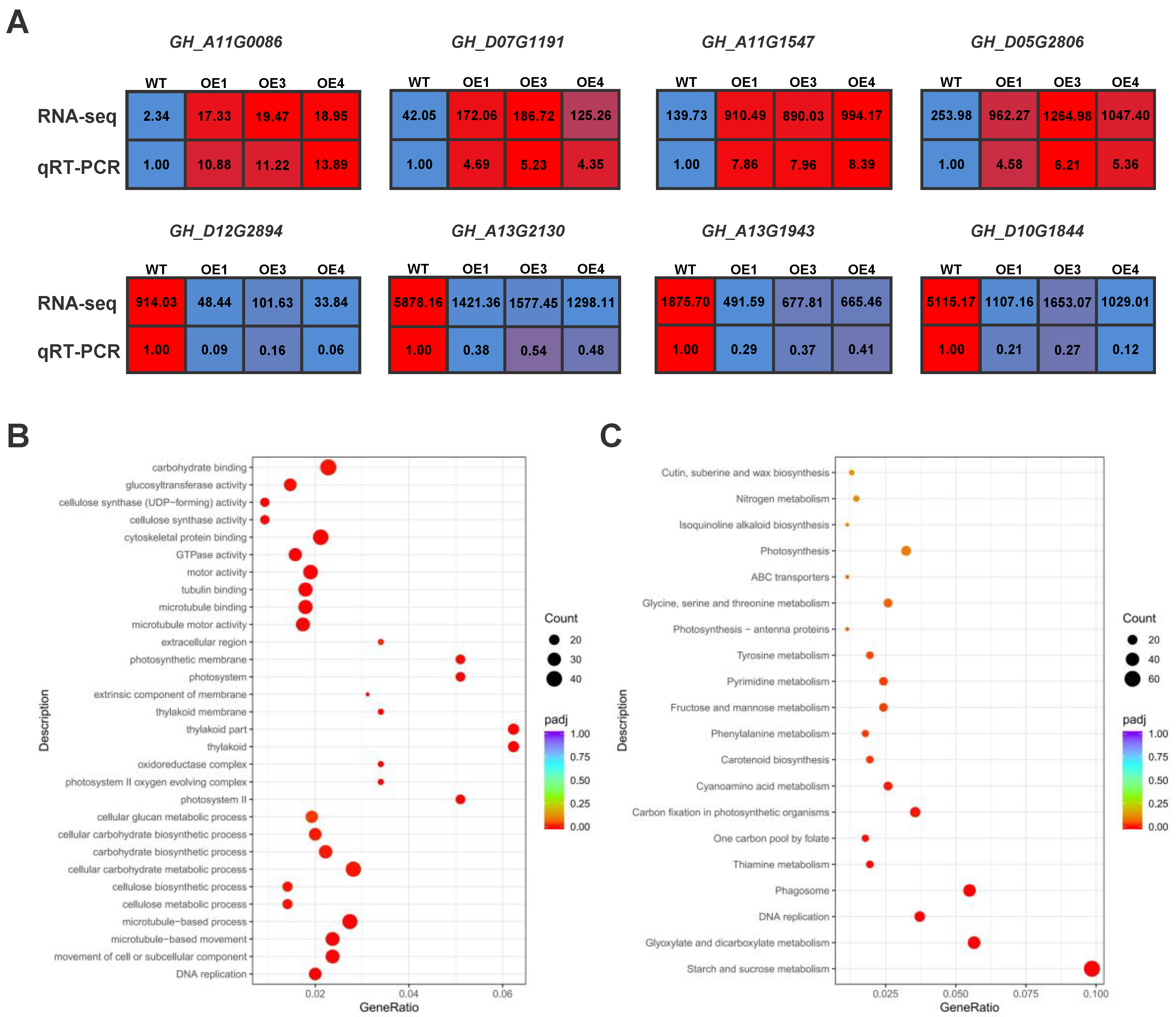
2.4. Transcriptome Analysis Reveals the Mechanisms of Drought Tolerance Induced by com58276
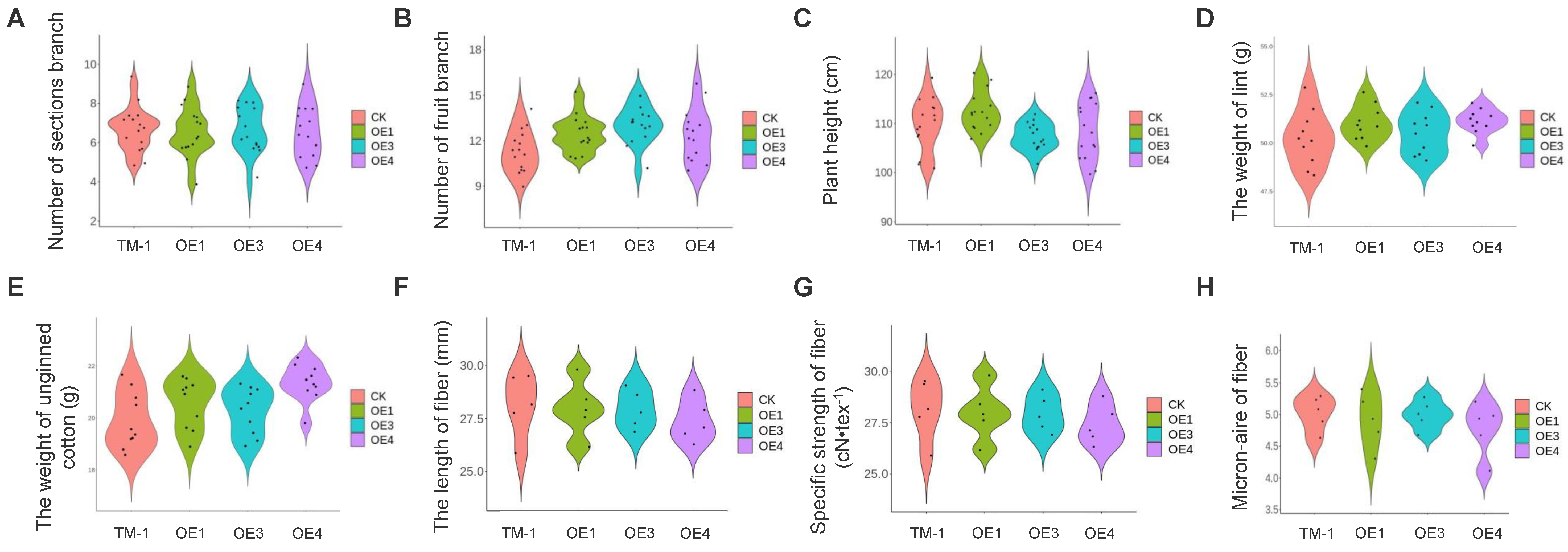
2.5. Overexpression of com58276 Has No Negative Effects on Cotton
3. Discussion
3.1. The Function of com58276 Is Conserved in Plants
3.2. com58276 Is Involved in a Variety of Adversity Stress Responses
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Stress Treatment
4.2. Determination of Proline, SOD, and CAT Activity
4.3. RNA Sequencing and Data Analysis
4.4. RNA Extraction, cDNA Preparation, and qRT-PCR Analyses
4.5. Subcellular Localization of COM58276 Proteins
4.6. Data Recording for Morphological Traits
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhu, Y.X. Breeding cotton with superior fiber quality: Identification and utilization of multiple elite loci and exotic genetic resources. Sci. China Life Sci. 2021, 64, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.; Liu, S.; Ma, H.; Chen, J.; Shen, Q.; Ge, C.; Zhang, X.; Pang, C.; Zhao, X. The compensation effects of physiology and yield in cotton after drought stress. J. Plant Physiol. 2018, 224, 30–48. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, H.; Yang, X.; Zhang, X. Drought coping strategies in cotton: Increased crop per drop. Plant Biotechnol. J. 2017, 15, 271–284. [Google Scholar] [CrossRef]
- Patterson, D.T.; Highsmith, M.T. Competition of spurred anoda (Anoda cristata) and velvetleaf (Abutilon theophrasti) with cotton (Gossypium hirsutum) during simulated drought and recovery. Weed Sci. 1989, 37, 658–664. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Ghany, A.G.A.A.; Zaki, E.A. Sequence heterogeneity of the envelope-like domain in the Egyptian cotton Gossypium barbadense. Afr. J. Biotechnol. 2003, 2, 341–344. [Google Scholar]
- Rehman, T.; Tabassum, B.; Yousaf, S.; Sarwar, G.; Qaisar, U. Consequences of Drought Stress Encountered During Seedling Stage on Physiology and Yield of Cultivated Cotton. Front. Plant Sci. 2022, 13, 906444. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, G.; Sun, Z.; Tang, Y.; Wu, Y. Cotton proteomics for deciphering the mechanism of environment stress response and fiber development. J. Proteom. 2014, 105, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, H.W. Progress on genetic diversity of genus caragana germplasm resources. J. Plant Genet. Resour. 2008, 9, 397–400. [Google Scholar]
- Li, Y.; Han, G. Hydrological physiological characteristics of Caragana korshinskii under water stress. J. Northwest For. Univ. 2008, 23, 1–4. [Google Scholar]
- Hui, Y.; Hu, X.; Li, F. Leaf photosynthesis, chlorophyll fluorescence, ion content and free amino acids in Caragana korshinskii Kom exposed to NaCl stress. Acta Physiol. Plantarum. 2012, 34, 2285–2295. [Google Scholar]
- Yang, Q.; Zhang, T.; Wang, Y.; Li, G.; Yin, J.; Han, X.; Qi, L.W.; Li, G.J.; Wang, R. Construction of a suppression subtractive hybridization library of Caragana korshinskii under drought stress and cloning of CkWRKY1 gene. Sci. Silvae Sin. 2013, 49, 62–68. [Google Scholar]
- Yang, Q.; Yin, J.J.; Wang, Y.; Wang, R.G.; Li, G.J. Cloning and expression analysis of CkLEA1 gene in Caragana korshinskii Kom. China Biotechnol. 2013, 33, 93–99. [Google Scholar]
- Ren, A.; Yi, J.; Gao, H.; Li, J.; Wang, X. Cloning and expression analysis of the promoter of Caragana korshinskii gene. Acta Pratac. Sin. 2013, 22, 165–170. [Google Scholar]
- Long, Y.; Liang, F.; Zhang, J.; Xue, M.; Zhang, T.; Pei, X. Identification of drought response genes by digital gene expression (DGE) analysis in Caragana korshinskii Kom. Gene 2020, 725, 144170. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Liang, C.Z.; Meng, Z.G.; Sun, G.Q.; Meng, Z.H.; Guo, S.D.; Zhang, R. CottonFGD: An integrated functional genomics database for cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pu, Y.; Abid, M.A.; Kang, L.; Ye, Y.; Zhang, M.; Liang, C.; Wei, Y.; Zhang, R.; Meng, Z. A Rapid and Efficient Method for Isolation and Transformation of Cotton Callus Protoplast. Int. J. Mol. Sci. 2022, 23, 8368. [Google Scholar] [CrossRef]
- Gwathmey, C.O.; Bange, M.P.; Brodrick, R. Cotton crop maturity: A compendium of measures and predictors. Field Crop Res. 2016, 191, 41–53. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Y.; Wang, P.; Xu, J.; Yang, Y.; Zhou, T.; Zheng, K.; Pei, X.; Chen, Q.; Sun, G. Overexpression of the Caragana korshinskii com58276 Gene Enhances Tolerance to Drought in Cotton (Gossypium hirsutum L.). Plants 2023, 12, 1069. https://doi.org/10.3390/plants12051069
Pu Y, Wang P, Xu J, Yang Y, Zhou T, Zheng K, Pei X, Chen Q, Sun G. Overexpression of the Caragana korshinskii com58276 Gene Enhances Tolerance to Drought in Cotton (Gossypium hirsutum L.). Plants. 2023; 12(5):1069. https://doi.org/10.3390/plants12051069
Chicago/Turabian StylePu, Yuanchun, Peilin Wang, Jiangling Xu, Yejun Yang, Ting Zhou, Kai Zheng, Xinwu Pei, Quanjia Chen, and Guoqing Sun. 2023. "Overexpression of the Caragana korshinskii com58276 Gene Enhances Tolerance to Drought in Cotton (Gossypium hirsutum L.)" Plants 12, no. 5: 1069. https://doi.org/10.3390/plants12051069
APA StylePu, Y., Wang, P., Xu, J., Yang, Y., Zhou, T., Zheng, K., Pei, X., Chen, Q., & Sun, G. (2023). Overexpression of the Caragana korshinskii com58276 Gene Enhances Tolerance to Drought in Cotton (Gossypium hirsutum L.). Plants, 12(5), 1069. https://doi.org/10.3390/plants12051069





