Isolation and Screening of Antagonistic Endophytes against Phytophthora infestans and Preliminary Exploration on Anti-oomycete Mechanism of Bacillus velezensis 6-5
Abstract
1. Introduction
2. Results
2.1. Isolation of Endophytes from Potato Roots and Screening of Endophytes with Anti-oomycete Activity against P. infestans
2.2. Stress Tolerance Analysis of Candidate Isolates
2.3. The Potential Application of Candidate Isolates against Potato Late Blight on Potato Tubers
2.4. Identification of Endophytes 6-5
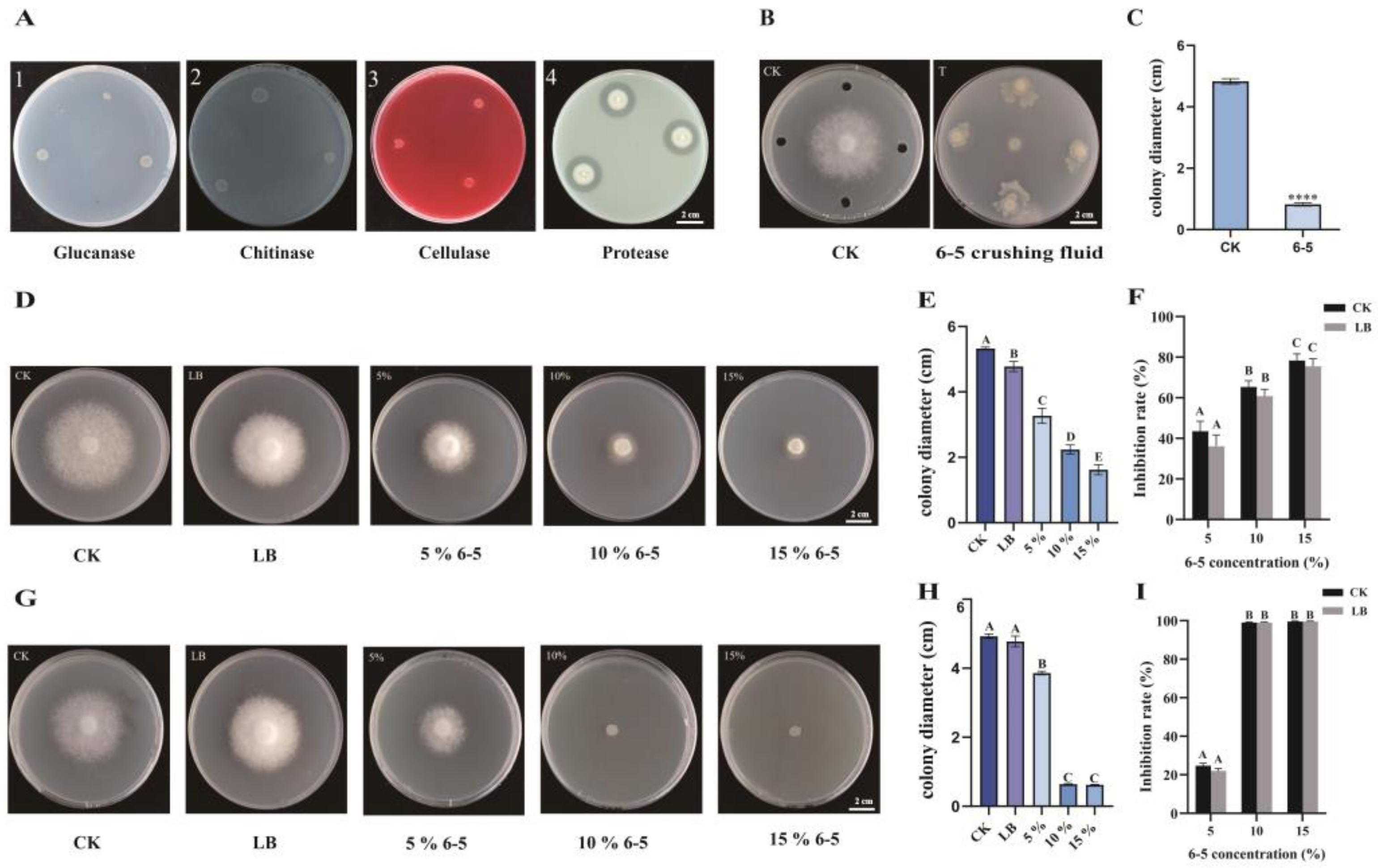
2.5. Effect of 6-5 on Mycelial Growth, Hyphal Phenotype, and Spore Germination of P. infestans
2.6. Detection of Extracellular Enzymes of 6-5 and Localization of Antagonistic Substances of 6-5 against P. infestans
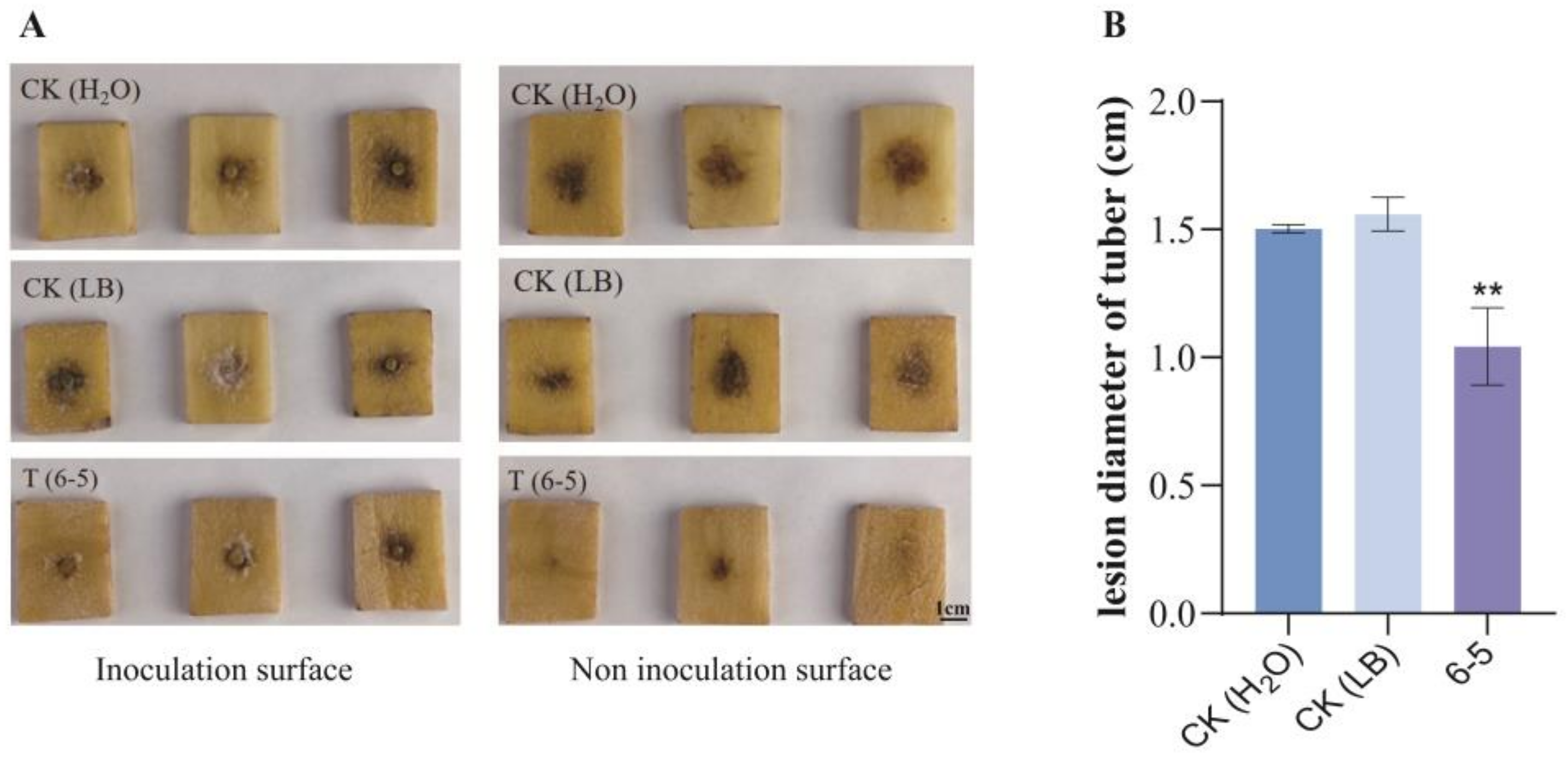
2.7. Effect of 6-5 on Inducing the Resistance of Potato Tubers to Late Blight
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation of Endophytes from Roots of Potato
4.3. Screening of Endophytes with Anti-oomycete Activity against P. infestans
4.4. Stress Tolerance Analysis of Candidate Isolates
4.5. Potato Tubers Test of Candidate Isolates
4.6. Identification of Endophytic Isolate 6-5
4.7. Effect of 6-5 on Mycelial Growth, Hyphal Phenotype, and Spore Germination of P. infestans
4.8. Detection of Extracellular Enzymes of 6-5
4.9. Localization of Antagonistic Substances of 6-5 against P. infestans
4.10. Effect of 6-5 on Inducing the Resistance of Potato Tubers to Late Blight
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.H.; Ma, Q.; Sun, Z.N.; Cui, H.C.; Liu, H.R. Biocontrol mechanism of Myxococcus fulvus B25-I-3 against Phytophthora infestans and its control efficiency on potato late blight. Folia Microbiol. 2021, 66, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Kondhare, K.R.; Natarajan, B.; Banerjee, A.K. Molecular signals that govern tuber development in potato. Int. J. Dev. Biol. 2020, 64, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Paluchowska, P.; Sliwka, J.; Yin, Z. Late blight resistance genes in potato breeding. Planta 2022, 255, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pruitt, R.N.; Nurnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Sorokan, A.; Benkovskaya, G.; Burkhanova, G.; Blagova, D.; Maksimov, I. Endophytic strain Bacillus subtilis 26DCryChS producing cry1Ia toxin from Bacillus thuringiensis promotes multifaceted potato defense against Phytophthora infestans (Mont.) de Bary and Pest Leptinotarsa decemlineata Say. Plants 2020, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Fry, W.E.; Birch, P.R.; Judelson, H.S.; Grunwald, N.J.; Danies, G.; Everts, K.L.; Gevens, A.J.; Gugino, B.K.; Johnson, D.A.; Johnson, S.B.; et al. Five reasons to consider Phytophthora infestans a reemerging pathogen. Phytopathology 2015, 105, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.A.; Ukladov, E.O.; Golubeva, T.S. Phytophthora infestans: An overview of methods and attempts to combat late blight. J. Fungi 2021, 7, 1071. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Effective control of two genotypes of Phytophthora infestans in the field by three oxathiapiprolin fungicidal mixtures. PLoS ONE 2021, 16, e0258280. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Coffey, A.; Guo, J.; Waters, D.M.; Arendt, E.K. Ecofriendly control of potato late blight causative agent and the potential role of lactic acid bacteria: A review. Appl. Microbiol. Biot. 2012, 96, 37–48. [Google Scholar] [CrossRef]
- Deahl, K.; Cooke, L.; Black, L.; Wang, T.; Perez, F.; Moravec, B.; Quinn, M.; Jones, R. Population changes in Phytophthora infestans in Taiwan associated with the appearance of resistance to metalaxyl. Pest Manag. Sci. 2002, 58, 951–958. [Google Scholar] [CrossRef]
- Hashemi, M.; Tabet, D.; Sandroni, M.; Benavent-Celma, C.; Seematti, J.; Andersen, C.B.; Grenville-Briggs, L.J. The hunt for sustainable biocontrol of oomycete plant pathogens, a case study of Phytophthora infestans. Fungal Biol. Rev. 2022, 40, 53–69. [Google Scholar] [CrossRef]
- Calvente, V.; Benuzzi, D.; de Tosetti, M.I.S. Antagonistic action of siderophores from Rhodotorula glutinis upon the postharvest pathogen Penicillium expansum. Int. Biodeter. Biodegr. 1999, 43, 167–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Zhang, W.; Lang, D.; Zhang, X.; Cui, G.; Zhang, X. Interactions between endophytes and plants: Beneficial effect of endophytes to ameliorate biotic and abiotic stresses in plants. J. Plant Biol. 2019, 62, 1–13. [Google Scholar] [CrossRef]
- Li, X.J.; Tang, H.Y.; Duan, J.L.; Gao, J.M.; Xue, Q.H. Bioactive alkaloids produced by Pseudomonas brassicacearum subsp. Neoaurantiaca, an endophytic bacterium from Salvia miltiorrhiza. Nat. Prod. Res. 2018, 27, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus Spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef]
- Brooks, S.; Klomchit, A.; Chimthai, S.; Jaidee, W.; Bastian, A.C. Xylaria feejeensis, SRNE2BP a fungal endophyte with biocontrol properties to control early blight and fusarium wilt disease in tomato and plant growth promotion activity. Curr. Microbiol. 2022, 79, 108. [Google Scholar] [CrossRef]
- Thomloudi, E.E.; Tsalgatidou, P.C.; Baira, E.; Papadimitriou, K.; Venieraki, A.; Katinakis, P. Genomic and metabolomic insights into secondary metabolites of the novel Bacillus halotolerans Hil4, an endophyte with promising antagonistic activity against gray mold and plant growth promoting potential. Microorganisms 2021, 9, 2508. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J.; Li, P.; Feng, S.; Dong, P.; Ren, M. Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J. Sci. Food Agric. 2021, 101, 1744–1757. [Google Scholar] [CrossRef]
- Asad, S.; He, P.; He, P.; Li, Y.; Wu, Y.; Ahmed, A.; Wang, Y.; Munir, S.; He, Y. Interactions between indigenous endophyte Bacillus subtilis L1-21 and nutrients inside citrus in reducing Huanglongbing pathogen Candidatus Liberibacter Asiaticus. Pathogens 2021, 10, 1304. [Google Scholar] [CrossRef]
- Munir, S.; Li, Y.; He, P.; He, P.; He, P.; Cui, W.; Wu, Y.; Li, X.; Li, Q.; Zhang, S.; et al. Defeating Huanglongbing pathogen Candidatus Liberibacter asiaticus with indigenous citrus endophyte Bacillus subtilis L1-21. Front. Plant Sci. 2021, 12, 789065. [Google Scholar] [CrossRef]
- Nanjani, S.; Soni, R.; Paul, D.; Keharia, H. Genome analysis uncovers the prolific antagonistic and plant growth-promoting potential of endophyte Bacillus velezensis K1. Gene 2022, 836, 146671. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhang, Q.; Zhang, A.L.; Gao, J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.-J.; Hussain, A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Anyasi, R.O.; Atagana, H.I. Endophyte: Understanding the microbes and its applications. Pak. J. Biol. Sci. 2019, 22, 154–167. [Google Scholar] [CrossRef]
- Anyasi, R.O.; Atagana, H.I.; Sutherland, R. Comparative study of the colonization of Chromolaena and tobacco plants by Bacteria safensis CS4 using different methods of inoculation. Pak. J. Biol. Sci. 2019, 22, 309–317. [Google Scholar] [CrossRef]
- Zhang, J.; Islam, M.S.; Wang, J.; Zhao, Y.; Dong, W. Isolation of potato endophytes and screening of Chaetomium globosum antimicrobial genes. Int. J. Mol. Sci. 2022, 23, 4611. [Google Scholar] [CrossRef]
- Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.J.; Hasterok, R. Defining the genetic basis of plant-endophytic bacteria interactions. Int. J. Mol. Sci. 2019, 20, 1947. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martínez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant-Microbe Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef]
- El-Hasan, A.; Ngatia, G.; Link, T.I.; Voegele, R.T. Isolation, identification, and biocontrol potential of root fungal endophytes associated with Solanaceous plants against potato late blight (Phytophthora infestans). Plants 2022, 11, 1605. [Google Scholar] [CrossRef]
- Singh, R.; Pandey, K.D.; Singh, M.; Singh, S.K.; Hashem, A.; Al-Arjani, A.F.; Abd Allah, E.F.; Singh, P.K.; Kumar, A. Isolation and characterization of endophytes bacterial strains of Momordica charantia L. and their possible approach in stress management. Microorganisms 2022, 10, 290. [Google Scholar] [CrossRef]
- La Fua, J.; Sabaruddin, L.; Santiaji Bande, O.; Leomo, S.; Kade Sutariati, G.A.; Khaeruni, A.; Safuan, O.; Hs, G.; Corona Rakian, T.; Muhidin; et al. Isolation of drought-tolerant endophyte bacteria from local tomato plants. Pak. J. Biol. Sci. 2021, 24, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Gul Jan, F.; Hamayun, M.; Hussain, A.; Jan, G.; Iqbal, A.; Khan, A.; Lee, I.J. An endophytic isolate of the fungus Yarrowia lipolytica produces metabolites that ameliorate the negative impact of salt stress on the physiology of maize. BMC Microbiol. 2019, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Stress response signaling and virulence: Insights from entomopathogenic fungi. Curr. Genet. 2015, 61, 239–249. [Google Scholar] [CrossRef]
- Mizubuti, E.S.; Aylor, D.E.; Fry, W.E. Survival of Phytophthora infestans sporangia exposed to solar radiation. Phytopathology 2000, 90, 78–84. [Google Scholar] [CrossRef]
- Huang, X.; You, Z.; Luo, Y.; Yang, C.; Ren, J.; Liu, Y.; Wei, G.; Dong, P.; Ren, M. Antifungal activity of chitosan against Phytophthora infestans, the pathogen of potato late blight. Int. J. Biol. Macromol. 2021, 166, 1365–1376. [Google Scholar] [CrossRef]
- Ren, J.; Tong, J.; Li, P.; Huang, X.; Dong, P.; Ren, M. Chitosan is an effective inhibitor against potato dry rot caused by Fusarium oxysporum. Physiol. Mol. Plant Pathol. 2021, 113, 101601. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Feng, S.; Lin, X.; Ren, M.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Technol. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Park, J.H. Control efficacy of Bacillus velezensis AFB2-2 against potato late blight caused by Phytophthora infestans in organic potato cultivation. Plant Pathol. J. 2021, 37, 580–595. [Google Scholar] [CrossRef]
- Yan, H.; Qiu, Y.; Yang, S.; Wang, Y.; Wang, K.; Jiang, L.; Wang, H. Antagonistic activity of Bacillus velezensis SDTB038 against Phytophthora infestans in Potato. Plant Dis. 2021, 105, 1738–1747. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a probiotic in animal feed: A review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Cheffi Azabou, M.; Gharbi, Y.; Medhioub, I.; Ennouri, K.; Barham, H.; Tounsi, S.; Triki, M.A. The endophytic strain Bacillus velezensis OEE1: An efficient biocontrol agent against Verticillium wilt of olive and a potential plant growth promoting bacteria. Biol. Control 2020, 142, 104168. [Google Scholar] [CrossRef]
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and characterization of endophyte Bacillus velezensis KOF112 from grapevine shoot xylem as biological control agent for fungal diseases. Plants 2021, 10, 1815. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, D.; Xiong, Q.; Bao, B.; Zhang, W.; Dai, T.; Zhao, Y.; Borriss, R.; Fan, B. The Plant-Beneficial Rhizobacterium Bacillus velezensis FZB42 Controls the Soybean Pathogen Phytophthora sojae Due to Bacilysin Production. Appl. Environ. Microbiol. 2021, 87, e0160121. [Google Scholar] [CrossRef]
- Nigris, S.; Baldan, E.; Tondello, A.; Zanella, F.; Vitulo, N.; Favaro, G.; Guidolin, V.; Bordin, N.; Telatin, A.; Barizza, E.; et al. Biocontrol traits of Bacillus licheniformis GL174, a culturable endophyte of Vitis vinifera cv. Glera. BMC Microbiol. 2018, 18, 133. [Google Scholar] [CrossRef]
- Alfiky, A.; L’Haridon, F.; Abou-Mansour, E.; Weisskopf, L. Disease inhibiting effect of strain Bacillus subtilis EG21 and its metabolites against potato pathogens Phytophthora infestans and Rhizoctonia solani. Phytopathology 2022, 112, 2099–2109. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zhang, M.Q.; Wang, L.S.; Mei, Y.Z.; Dai, C.C. Overexpression of chitinase in the endophyte Phomopsis liquidambaris enhances wheat resistance to Fusarium graminearum. Fungal Genet. Biol. 2022, 158, 103650. [Google Scholar] [CrossRef]
- Martins, J.; Verissimo, P.; Canhoto, J. Isolation and identification of Arbutus unedo L. fungi endophytes and biological control of Phytophthora cinnamomi in vitro. Protoplasma 2022, 259, 659–677. [Google Scholar] [CrossRef]
- Szekeres, A.; Kredics, L.; Antal, Z.; Kevei, F.; Manczinger, L. Isolation and characterization of protease overproducing mutants of Trichoderma harzianum. FEMS Microbiol. Lett. 2004, 233, 215–222. [Google Scholar] [CrossRef]
- Flores, A.; Chet, I.; Herrera-Estrella, A. Improved biocontrol activity of Trichoderma harzianum by over-expression of the proteinase-encoding gene prb1. Curr. Genet. 1997, 31, 30–37. [Google Scholar] [CrossRef]
- Chinchilla, D.; Bruisson, S.; Meyer, S.; Zuhlke, D.; Hirschfeld, C.; Joller, C.; L’Haridon, F.; Mene-Saffrane, L.; Riedel, K.; Weisskopf, L. A sulfur-containing volatile emitted by potato-associated bacteria confers protection against late blight through direct anti-oomycete activity. Sci. Rep. 2019, 9, 18778. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Stoll, A.; Salvatierra-Martinez, R.; Gonzalez, M.; Araya, M. The role of surfactin production by Bacillus velezensis on colonization, biofilm formation on tomato root and leaf surfaces and subsequent protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef]
- Feng, S.; Jin, L.; Tang, S.; Jian, Y.; Li, Z. Combination of rhizosphere bacteria isolated from resistant potato plants for biocontrol of potato late blight. Pest Manag. Sci. 2022, 78, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.; Kasnak, C.; Palamutoglu, R. Effects of endophytic Bacillus Subtilis and salicylic acid on postharvest diseases (Phytophthora infestans, Fusarium oxysporum) development in stored potato tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.E.; Gibbons, N.E. Berger Bacterial Identification Manual; Science Press: Beijing, China, 1984. [Google Scholar]
- Dong, X.; Cai, M. Common Bacterial System Identification Manual; Science Press: Beijing, China, 2001. [Google Scholar]
- Zhai, Y.; Zhu, J.X.; Tan, T.M.; Xu, J.P.; Shen, A.R.; Yang, X.B.; Li, J.L.; Zeng, L.B.; Wei, L. Isolation and characterization of antagonistic Paenibacillus polymyxa HX-140 and its biocontrol potential against Fusarium wilt of cucumber seedlings. BMC Microbiol. 2021, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Salem, E.; Naceur, D.; Olfa, T.; Adel, H.; Bacem, M.; Ridha, M.; Mohamed, S.; Ferid, L. Evaluation of antifungal activity from Bacillus strains against Rhizoctonia solani. Afr. J. Biotechnol. 2012, 11, 4196–4201. [Google Scholar] [CrossRef]
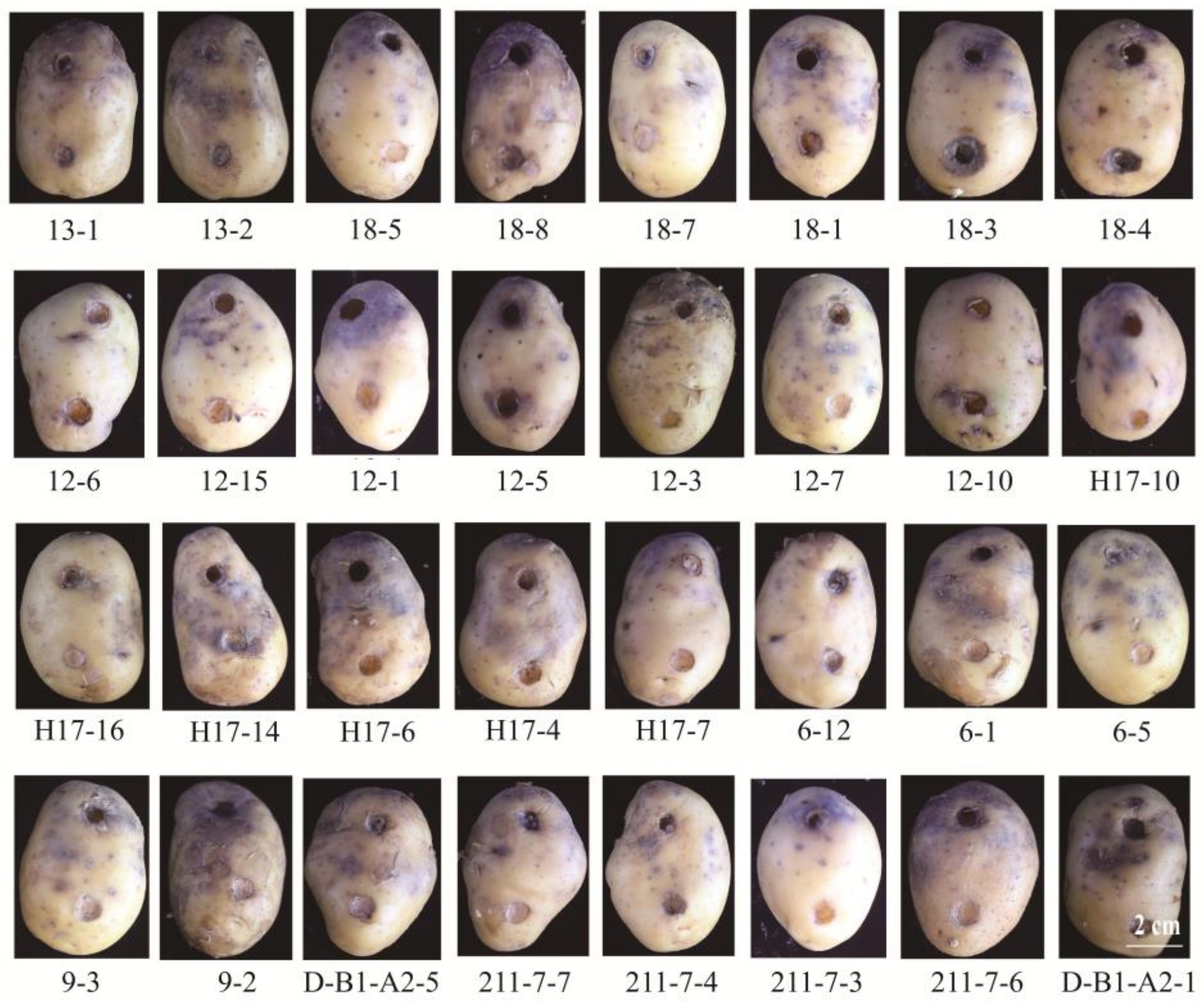

| Candidate Isolates | pH 5 | pH 9 | 57 °C | 17 °C | UV 40 min | 10% Cr3+ | 20% Cr3+ | 40% Cr3+ | 30% NaCl |
|---|---|---|---|---|---|---|---|---|---|
| 13-1 | ++ | ++ | ++ | + | ++ | ++ | ++ | − | ++ |
| 13-2 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 18-5 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 18-8 | ++ | ++ | ++ | ++ | ++ | ++ | − | − | ++ |
| 18-7 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 18-1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 18-3 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 18-4 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 12-6 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 12-15 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 12-1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 12-5 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 12-3 | ++ | ++ | ++ | ++ | ++ | ++ | − | − | ++ |
| 12-7 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 12-10 | ++ | − | ++ | ++ | ++ | ++ | ++ | − | ++ |
| H17-10 | ++ | + | ++ | ++ | ++ | ++ | ++ | − | ++ |
| H17-16 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| H17-14 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| H17-6 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| H17-4 | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| H17-7 | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 6-12 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 6-1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 6-5 | ++ | ++ | + | + | ++ | ++ | ++ | − | ++ |
| 9-3 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 9-2 | ++ | ++ | ++ | ++ | ++ | ++ | − | − | ++ |
| D-B1-A2-5 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 211-7-7 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 211-7-4 | − | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 211-7-3 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
| 211-7-6 | − | ++ | ++ | ++ | ++ | ++ | − | − | ++ |
| D-B1-A2-1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Huang, X.; Hou, Y.; Xia, X.; Zhu, Z.; Huang, A.; Feng, S.; Li, P.; Shi, L.; Dong, P. Isolation and Screening of Antagonistic Endophytes against Phytophthora infestans and Preliminary Exploration on Anti-oomycete Mechanism of Bacillus velezensis 6-5. Plants 2023, 12, 909. https://doi.org/10.3390/plants12040909
Zhang J, Huang X, Hou Y, Xia X, Zhu Z, Huang A, Feng S, Li P, Shi L, Dong P. Isolation and Screening of Antagonistic Endophytes against Phytophthora infestans and Preliminary Exploration on Anti-oomycete Mechanism of Bacillus velezensis 6-5. Plants. 2023; 12(4):909. https://doi.org/10.3390/plants12040909
Chicago/Turabian StyleZhang, Jiaomei, Xiaoqing Huang, Yuqin Hou, Xiangning Xia, Zhiming Zhu, Airong Huang, Shun Feng, Peihua Li, Lei Shi, and Pan Dong. 2023. "Isolation and Screening of Antagonistic Endophytes against Phytophthora infestans and Preliminary Exploration on Anti-oomycete Mechanism of Bacillus velezensis 6-5" Plants 12, no. 4: 909. https://doi.org/10.3390/plants12040909
APA StyleZhang, J., Huang, X., Hou, Y., Xia, X., Zhu, Z., Huang, A., Feng, S., Li, P., Shi, L., & Dong, P. (2023). Isolation and Screening of Antagonistic Endophytes against Phytophthora infestans and Preliminary Exploration on Anti-oomycete Mechanism of Bacillus velezensis 6-5. Plants, 12(4), 909. https://doi.org/10.3390/plants12040909







