Antagonistic Strain Bacillus halotolerans Jk-25 Mediates the Biocontrol of Wheat Common Root Rot Caused by Bipolaris sorokiniana
Abstract
:1. Introduction
2. Results
2.1. Screening of Antagonistic Bacteria
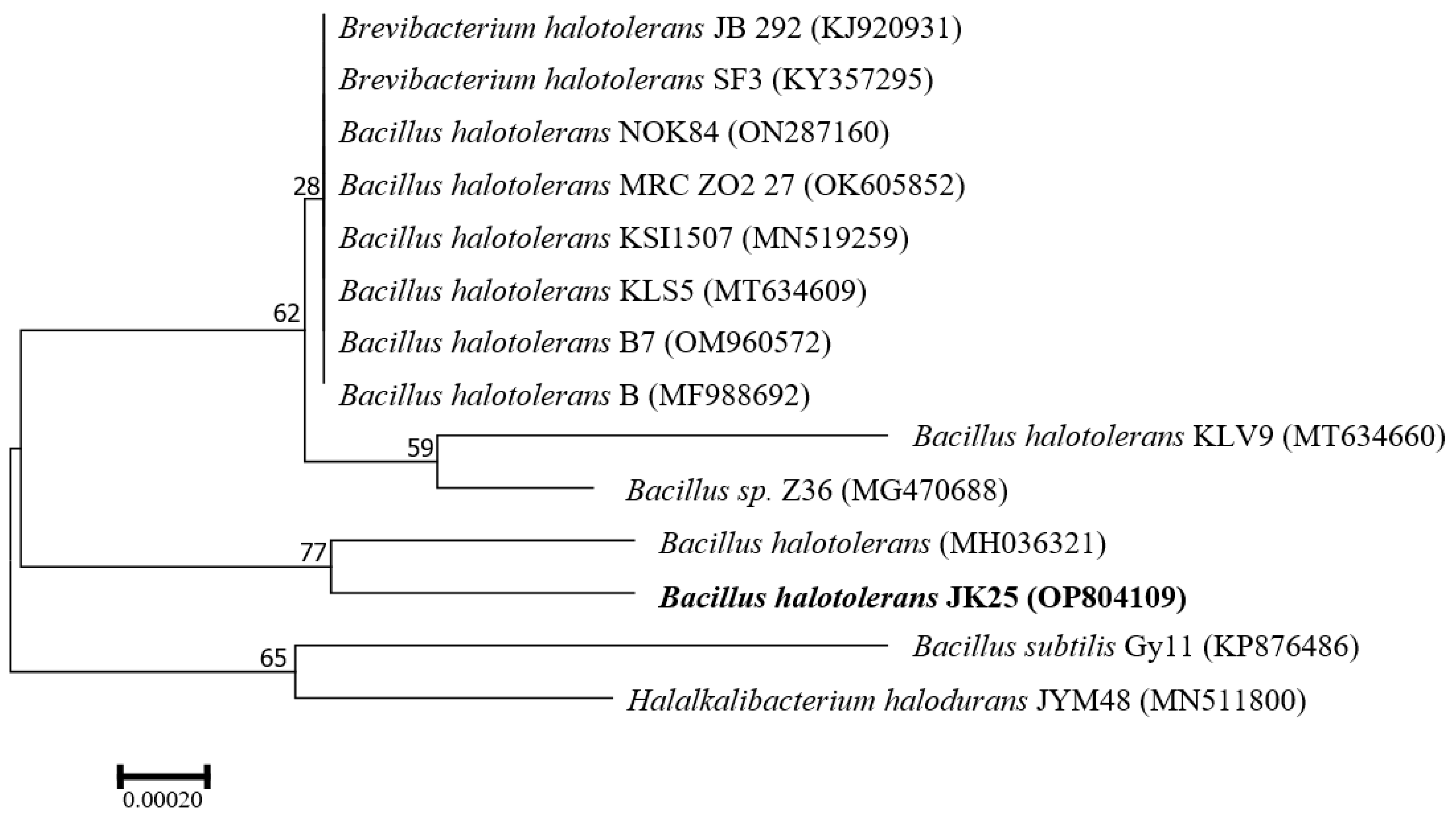
2.2. Identification of Antagonistic Strain JK-25
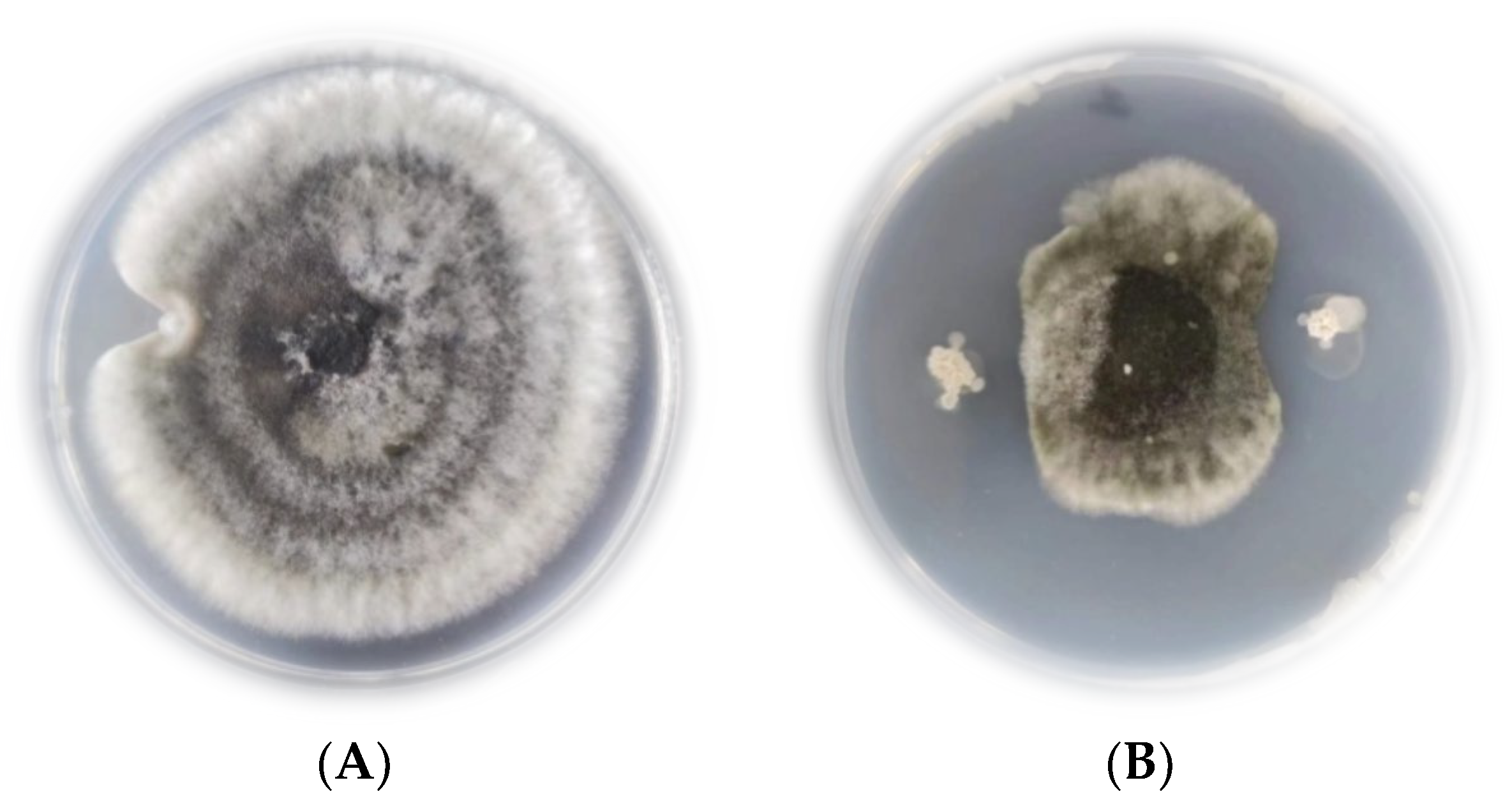
2.3. Inhibitory Effect of the Antagonistic Strain JK-25 in Cultured fermentation broth (CF) on B. sorokiniana
2.4. Detection and Identification of Antifungal Metabolites
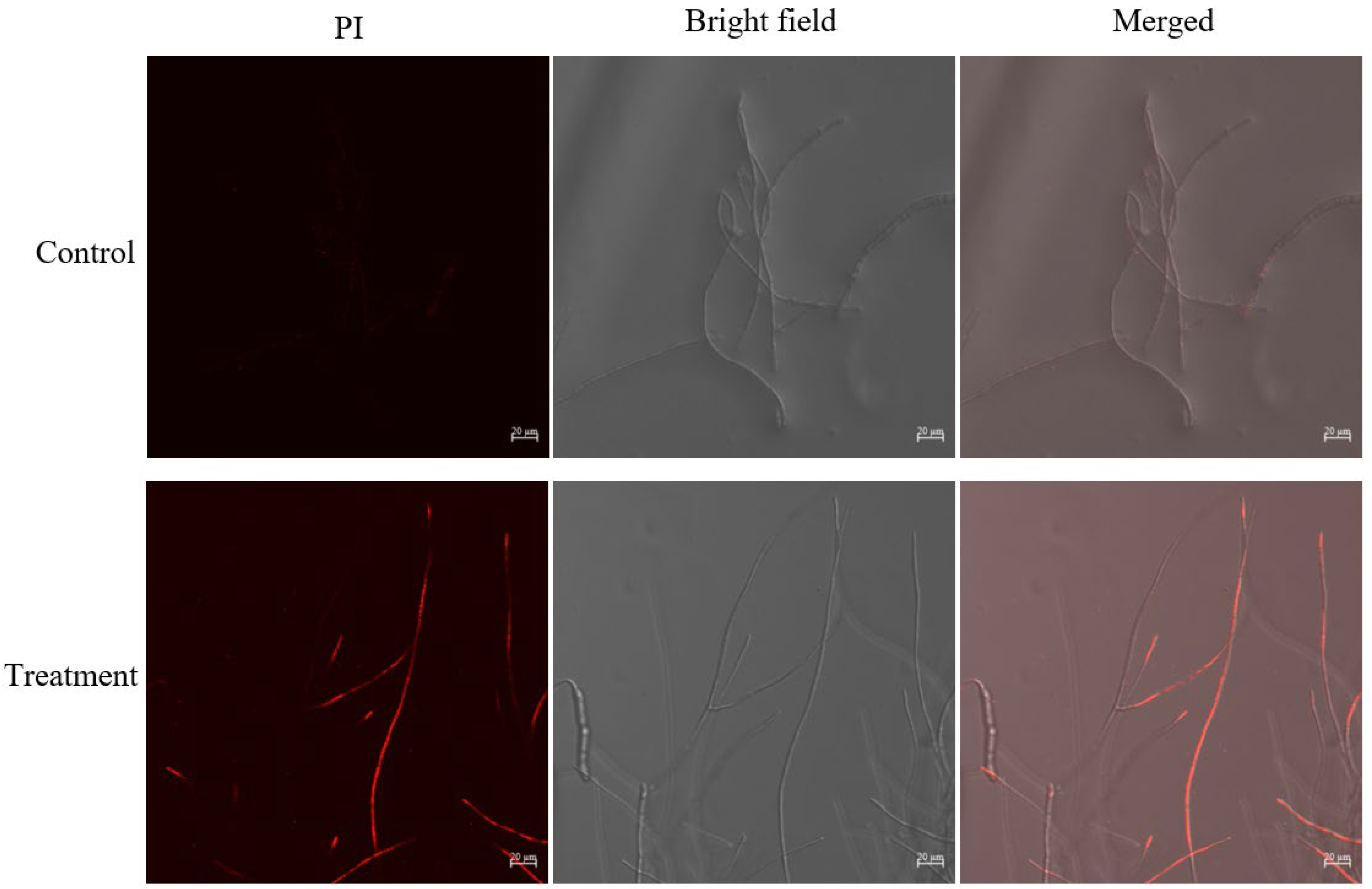
2.5. Effect of Crude Extract on Cell Membrane of B. sorokiniana
2.6. Detection of the Effect of Crude Extract on the Antioxidant Activity of B. sorokiniana
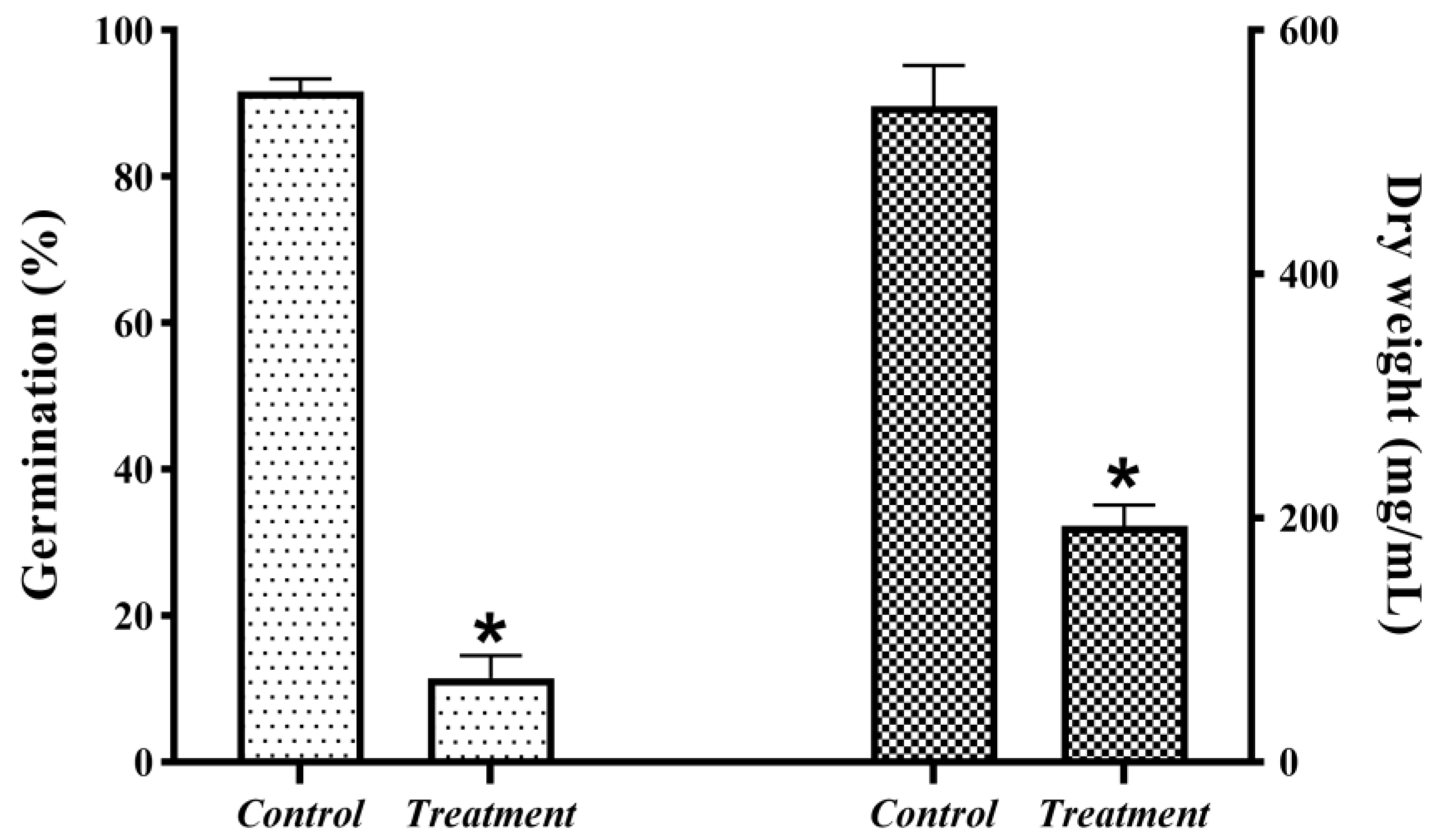
2.7. Biocontrol of Wheat Rot under Greenhouse Conditions
2.8. Production of Extracellular Enzymes and Growth Promotion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation and Screening of Antagonistic Bacteria
4.3. Identification of Strain JK-25
4.4. Inhibitory Effect of the Antagonistic Strain JK-25 Cultured Fermentation Broth (CF) on B. sorokiniana
4.5. Detection and Identification of Antagonistic Metabolites
4.6. Effect of Crude Extract on Cell Membrane of B. sorokiniana
4.7. Detection of the Effect of Crude Extract on the Antioxidant Activity of B. sorokiniana
4.8. Pot Experiments
4.9. Production of Extracellular Enzymes and Growth Promotion
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alomari, D.Z.; Eggert, K.; von Wiren, N.; Alqudah, A.M.; Polley, A.; Plieske, J.; Ganal, M.W.; Pillen, K.; Roder, M.S. Identifying Candidate Genes for Enhancing Grain Zn Concentration in Wheat. Front. Plant Sci. 2018, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Dhanarajan, G.; Rangarajan, V.; Sridhar, P.R.; Sen, R. Development and Scale-up of an Efficient and Green Process for HPLC Purification of Antimicrobial Homologues of Commercially Important Microbial Lipopeptides. ACS Sustain. Chem. Eng. 2016, 4, 6638–6646. [Google Scholar] [CrossRef]
- Su, J.; Zhao, J.; Zhao, S.; Li, M.; Pang, S.; Kang, Z.; Zhen, W.; Chen, S.; Chen, F.; Wang, X. Genetics of Resistance to Common Root Rot (Spot Blotch), Fusarium Crown Rot, and Sharp Eyespot in Wheat. Front. Genet. 2021, 12, 699342. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, A.M. Bipolaris sorokiniana-Induced Black Point, Common Root Rot, and Spot Blotch Diseases of Wheat: A Review. Front. Cell. Infect. Microbiol. 2021, 11, 584899. [Google Scholar] [CrossRef] [PubMed]
- Poole, G.J.; Harries, M.; Huberli, D.; Miyan, S.; MacLeod, W.J.; Lawes, R.; McKay, A. Predicting Cereal Root Disease in Western Australia Using Soil DNA and Environmental Parameters. Phytopathology 2015, 105, 1069–1079. [Google Scholar] [CrossRef]
- Xu, F.; Yang, G.; Wang, J.; Song, Y.; Liu, L.; Zhao, K.; Li, Y.; Han, Z. Spatial Distribution of Root and Crown Rot Fungi Associated with Winter Wheat in the North China Plain and Its Relationship with Climate Variables. Front. Microbiol. 2018, 9, 1054. [Google Scholar] [CrossRef]
- Allali, K.; Goudjal, Y.; Zamoum, M.; Bouznada, K.; Sabaou, N.; Zitouni, A. Nocardiopsis dassonvillei strain MB22 from the Algerian Sahara promotes wheat seedlings growth and potentially controls the common root rot pathogen Bipolaris sorokiniana. J. Plant Pathol. 2019, 101, 1115–1125. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.-H.; Sarthou, J.-P.; Cellier, V.; Messéan, A.; Aubertot, J.-N. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017, 37, 1–25. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Roper, M.M.; Myers, C.A.; Thatcher, L.F. Developing Actinobacterial Endophytes as Biocontrol Products for Fusarium pseudograminearum in Wheat. Front. Bioeng. Biotechnol. 2021, 9, 691770. [Google Scholar] [CrossRef]
- Campanella, V.; Mandalà, C.; Angileri, V.; Miceli, C. Management of common root rot and Fusarium foot rot of wheat using Brassica carinata break crop green manure. Crop. Prot. 2019, 130, 105073. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Ulrich, D.; Brandt, S.A.; Zentner, R.P.; Wang, H.; Thomas, A.G.; Olfert, O. Crop Management Effects on Root and Crown Rot of Wheat in West—Central Saskatchewan, Canada. Agron. J. 2011, 103, 756–765. [Google Scholar] [CrossRef]
- Le, C.N.; Mendes, R.; Kruijt, M.; Raaijmakers, J.M. Genetic and Phenotypic Diversity of Sclerotium rolfsii in Groundnut Fields in Central Vietnam. Plant Dis. 2012, 96, 389–397. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, S.; Gupta, A.; Singh, U.B.; Brahmaprakash, G.P.; Saxena, A.K. Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen Sclerotium rolfsii in tomato. Biol. Control 2019, 137, 104014. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, U.; Chakraborty, B.N. Induced defense response in wheat plants against Bipolaris sorokiniana following application of Bacillus safensis and Ochrobactrum pseudogrignonense. Indian Phytopathol. 2018, 71, 49–58. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Singh, S.; Kumar, M.; Sahu, P.K.; Singh, H.V.; Kumar, S.; Roy, M.; Imran, M.; Rai, J.P.; et al. Trichoderma harzianum- and Methyl Jasmonate-Induced Resistance to Bipolaris sorokiniana Through Enhanced Phenylpropanoid Activities in Bread Wheat (Triticum aestivum L.). Front. Microbiol. 2019, 10, 1697. [Google Scholar] [CrossRef]
- Ullah, H.; Yasmin, H.; Mumtaz, S.; Jabeen, Z.; Naz, R.; Nosheen, A.; Hassan, M.N. Multitrait Pseudomonas spp. Isolated from Monocropped Wheat (Triticum aestivum) Suppress Fusarium Root and Crown Rot. Phytopathology 2020, 110, 582–592. [Google Scholar] [CrossRef]
- Villa-Rodríguez, E.; Parra-Cota, F.; Castro-Longoria, E.; López-Cervantes, J.; de los Santos-Villalobos, S. Bacillus subtilis TE3: A promising biological control agent against Bipolaris sorokiniana, the causal agent of spot blotch in wheat (Triticum turgidum L. subsp. durum). Biol. Control 2019, 132, 135–143. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X.H.; Shan, C.; Deng, Z.; Ji, Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz. J. Microbiol. 2015, 46, 977–989. [Google Scholar] [CrossRef]
- Miljakovic, D.; Marinkovic, J.; Balesevic-Tubic, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Villarreal-Delgado, M.F.; Villa-Rodríguez, E.D.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Parra-Cota, F.I.; De los Santos-Villalobos, S. El género Bacillus como agente de control biológico y sus implicaciones en la bioseguridad agrícola. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2018, 36, 95–130. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Yi, Y.; Shan, Y.; Liu, S.; Yang, Y.; Liu, Y.; Yin, Y.; Hou, Z.; Luan, P.; Li, R. Antagonistic Strain Bacillus amyloliquefaciens XZ34-1 for Controlling Bipolaris sorokiniana and Promoting Growth in Wheat. Pathogens 2021, 10, 1526. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.D.; Chong, X.Y.; Xin, Q.H.; Wang, D.X.; Bian, K. Seed-borne endophytic Bacillus velezensis LHSB1 mediate the biocontrol of peanut stem rot caused by Sclerotium rolfsii. J. Appl. Microbiol. 2020, 128, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aswini, K.; Sai Prasad, J.; Kumar, N.; Pathak, D.; Gond, S.; Venkadasamy, G.; Suman, A. Characterization of actinobacteria from wheat seeds for plant growth promoting traits and protection against fungal pathogens. J. Basic Microbiol. 2022, 62, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Backman, P.A.; Sikora, R.A. Endophytes: An emerging tool for biological control. Biol. Control 2008, 46, 1–3. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Cura, J.A. Insights into the Interactions among Roots, Rhizosphere, and Rhizobacteria for Improving Plant Growth and Tolerance to Abiotic Stresses: A Review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Zhang, C.; Wang, L.; Gao, W.; Jiang, J. Bacillus megaterium WL-3 Lipopeptides Collaborate Against Phytophthora infestans to Control Potato Late Blight and Promote Potato Plant Growth. Front. Microbiol. 2020, 11, 1602. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liang, J.; Wu, L.; Gao, W.; Jiang, J. Iturin A Extracted From Bacillus subtilis WL-2 Affects Phytophthora infestans via Cell Structure Disruption, Oxidative Stress, and Energy Supply Dysfunction. Front. Microbiol. 2020, 11, 536083. [Google Scholar] [CrossRef]
- Rajer, F.U.; Samma, M.K.; Ali, Q.; Rajar, W.A.; Wu, H.; Raza, W.; Xie, Y.; Tahir, H.A.S.; Gao, X. Bacillus spp.-Mediated Growth Promotion of Rice Seedlings and Suppression of Bacterial Blight Disease under Greenhouse Conditions. Pathogens 2022, 11, 1251. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, Q.; Yin, L.; Zhang, Y.; Yang, Q.; Liu, K.; Wang, Y.; Han, S.; Zhao, H.; Zhao, H. Isolation and characterization of a mycosubtilin homologue antagonizing Verticillium dahliae produced by Bacillus subtilis strain Z15. PLoS ONE 2022, 17, e0269861. [Google Scholar] [CrossRef]
- Villa-Rodriguez, E.; Moreno-Ulloa, A.; Castro-Longoria, E.; Parra-Cota, F.I.; de Los Santos-Villalobos, S. Integrated omics approaches for deciphering antifungal metabolites produced by a novel Bacillus species, B. cabrialesii TE3(T), against the spot blotch disease of wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2021, 251, 126826. [Google Scholar] [CrossRef]
- Kaur, P.K.; Joshi, N.; Singh, I.P.; Saini, H.S. Identification of cyclic lipopeptides produced by Bacillus vallismortis R2 and their antifungal activity against Alternaria Altern. J. Appl. Microbiol. 2017, 122, 139–152. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, H.; Zou, J.; Wang, X.; Zhang, R.; Xiang, Y.; Chen, Z. Bacillomycin L and surfactin contribute synergistically to the phenotypic features of Bacillus subtilis 916 and the biocontrol of rice sheath blight induced by Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2015, 99, 1897–1910. [Google Scholar] [CrossRef]
- Rodríguez, J.; Tonelli, M.L.; Figueredo, M.S.; Ibáñez, F.; Fabra, A. The lipopeptide surfactin triggers induced systemic resistance and priming state responses in Arachis hypogaea L. Eur. J. Plant Pathol. 2018, 152, 845–851. [Google Scholar] [CrossRef]
- Hazarika, D.J.; Goswami, G.; Gautom, T.; Parveen, A.; Das, P.; Barooah, M.; Boro, R.C. Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol. 2019, 19, 71. [Google Scholar] [CrossRef]
- Jamali, H.; Sharma, A.; Srivastava, A.K. Biocontrol potential of Bacillus subtilis RH5 against sheath blight of rice caused by Rhizoctonia solani. J. Basic Microbiol. 2020, 60, 268–280. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Q.; Yang, H.; Huang, J.; Li, Y. Characterization of a new Bacillus velezensis as a powerful biocontrol agent against tomato gray mold. Pestic. Biochem. Physiol. 2022, 187, 105199. [Google Scholar] [CrossRef]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef]
- Wang, D.; Gong, N.; Liu, C.; Li, S.; Guo, Z.; Wang, G.; Shang, Q.; Wang, D.; Ji, X.; Xin, Y. MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis). Int. J. Mol. Sci. 2022, 23, 3372. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Mageshwaran, V.; Gupta, R.; Singh, S.; Sahu, P.K.; Singh, U.B.; Chakdar, H.; Bagul, S.Y.; Paul, S.; Singh, H.V. Endophytic Bacillus subtilis antagonize soil-borne fungal pathogens and suppress wilt complex disease in chickpea plants (Cicer arietinum L.). Front. Microbiol. 2022, 13, 994847. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ji, X.; Ge, Y.; Li, J.; Qi, W.; Qiao, K. Characterization of Antagonistic Bacillus methylotrophicus Isolated From Rhizosphere and Its Biocontrol Effects on Maize Stalk Rot. Phytopathology 2019, 109, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Zhao, M.; Chen, D.; Cheng, J.; Li, J.; Feng, Z.; Ma, Z.; An, D. Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop. Prot. 2013, 44, 29–37. [Google Scholar] [CrossRef]
- Bergey, D.H. Bergey’s Manual of Determinative Bacteriology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Alfiky, A.; L’Haridon, F.; Abou-Mansour, E.; Weisskopf, L. Disease Inhibiting Effect of Strain Bacillus subtilis EG21 and Its Metabolites Against Potato Pathogens Phytophthora infestans and Rhizoctonia solani. Phytopathology 2022, 112, 2099–2109. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.; Fu, L.; Xu, G.; Lin, X.; Qiao, J.; Xia, Y. Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8. Pathogens 2022, 11, 595. [Google Scholar] [CrossRef]
- Chen, L.; Heng, J.; Qin, S.; Bian, K. A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE 2018, 13, e0198560. [Google Scholar] [CrossRef]
- Yu, C.; Liu, X.; Zhang, X.; Zhang, M.; Gu, Y.; Ali, Q.; Mohamed, M.S.R.; Xu, J.; Shi, J.; Gao, X.; et al. Mycosubtilin Produced by Bacillus subtilis ATCC6633 Inhibits Growth and Mycotoxin Biosynthesis of Fusarium graminearum and Fusarium verticillioides. Toxins 2021, 13, 791. [Google Scholar] [CrossRef]
- Gu, Q.; Qiao, J.; Wang, R.; Lu, J.; Wang, Z.; Li, P.; Zhang, L.; Ali, Q.; Khan, A.R.; Gao, X.; et al. The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize. Int. J. Mol. Sci. 2022, 23, 6431. [Google Scholar] [CrossRef]
- Leenders, F.; Stein, T.H.; Kablitz, B.; Franke, P.; Vater, J. Rapid typing of Bacillus subtilis strains by their secondary metabolites using matrix-assisted laser desorption/ionization mass spectrometry of intact cells. Rapid Commun. Mass Spectrom. 1999, 13, 943–949. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, R.; Ji, S.; Fan, H.; Wang, J.; Wang, Y.; Liu, Z. Isolation of Trichoderma from forestry model base and the antifungal properties of isolate TpsT17 toward Fusarium oxysporum. Microbiol. Res. 2020, 231, 126371. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Wei, X.; Xue, Q.; Lai, H. Biocontrol effects of Penicillium griseofulvum against monkshood (Aconitum carmichaelii Debx.) root diseases caused by Sclerotium rolfsiii and Fusarium spp. J. Appl. Microbiol. 2019, 127, 1532–1545. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Mejdoub-Trabelsi, B.; Tounsi, S. Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol. Control 2021, 152, 104444. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]



| Strain | Inhibition Rate (%) | |||
|---|---|---|---|---|
| Bipolaris sorokiniana | Fusarium oxysporum | Fusarium graminearum | Rhizoctonia zeae | |
| JK-5 | 78.38 ± 2.63 ab | 76.97 ± 1.22 a | 69.63 ± 1.46 b | 25.73 ± 7.9 c |
| JK-13 | 75.4 ± 2.79 b | 71 ± 2.08 b | 65.2 ± 2.75 bc | 45.7 ± 2.21 b |
| JK-25 | 82.63 ± 0.67 a | 80.47 ± 1.4 a | 75.33 ± 1.27 a | 59.73 ± 2.45 a |
| JK-44 | 66.06 ± 2.07 c | 57.2 ± 1.97 c | 62.47 ± 3.2 c | 30 ± 8.94 c |
| JK-58 | 67.2 ± 3.44 c | 55.3 ± 3.22 c | 64.87 ± 3.33 bc | 32.47 ± 2.54 c |
| Biochemical Tests | Reaction | Colony Morphology | Description |
|---|---|---|---|
| Gram stain | + | Endospores | Present |
| Methyl red test | − | Morphology | Rounded |
| Voges-Proskauer test | + | Pigment | Creamy white |
| Indole test | − | Surface | Neat and smooth |
| Nitrate reduction | + | Margin | rough |
| Catalase | + | Opacity | Opaque |
| 10% salt tolerance test | + | ||
| Glucose fermentation | + | ||
| Starch hydrolysis | + | ||
| Citrate test | + | ||
| Gelatin liquefaction | + |
| Treatments | Disease Incidence Rate (%) | Disease Index | Control Efficacy (%) |
|---|---|---|---|
| Sterile water control | 98.75 ± 3.81 a | 71.59 ± 7.24 a | - |
| Carbendazim control | 42.37 ± 8.96 c | 15.85 ± 2.41 b | 77.86 ± 4.32 |
| CF treatment | 49.65 ± 9.54 b | 20.20 ± 3.58 b | 72.06 ± 6.94 |
| Bioboosters | Reaction |
|---|---|
| Protease | + |
| Pectinase | + |
| Cellulase | + |
| Chitinase | − |
| Siderophores | + |
| Indoleacetic acid (IAA) | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.; Niu, Z.; Zhang, W.; Wei, S.; Lv, Y.; Hu, Y. Antagonistic Strain Bacillus halotolerans Jk-25 Mediates the Biocontrol of Wheat Common Root Rot Caused by Bipolaris sorokiniana. Plants 2023, 12, 828. https://doi.org/10.3390/plants12040828
Kang K, Niu Z, Zhang W, Wei S, Lv Y, Hu Y. Antagonistic Strain Bacillus halotolerans Jk-25 Mediates the Biocontrol of Wheat Common Root Rot Caused by Bipolaris sorokiniana. Plants. 2023; 12(4):828. https://doi.org/10.3390/plants12040828
Chicago/Turabian StyleKang, Kun, Zhipeng Niu, Wei Zhang, Shan Wei, Yangyong Lv, and Yuansen Hu. 2023. "Antagonistic Strain Bacillus halotolerans Jk-25 Mediates the Biocontrol of Wheat Common Root Rot Caused by Bipolaris sorokiniana" Plants 12, no. 4: 828. https://doi.org/10.3390/plants12040828
APA StyleKang, K., Niu, Z., Zhang, W., Wei, S., Lv, Y., & Hu, Y. (2023). Antagonistic Strain Bacillus halotolerans Jk-25 Mediates the Biocontrol of Wheat Common Root Rot Caused by Bipolaris sorokiniana. Plants, 12(4), 828. https://doi.org/10.3390/plants12040828






