Salicylic Acid Modulates the Osmotic System and Photosynthesis Rate to Enhance the Drought Tolerance of Toona ciliata
Abstract
:1. Introduction
2. Results
2.1. Growth and Morphology
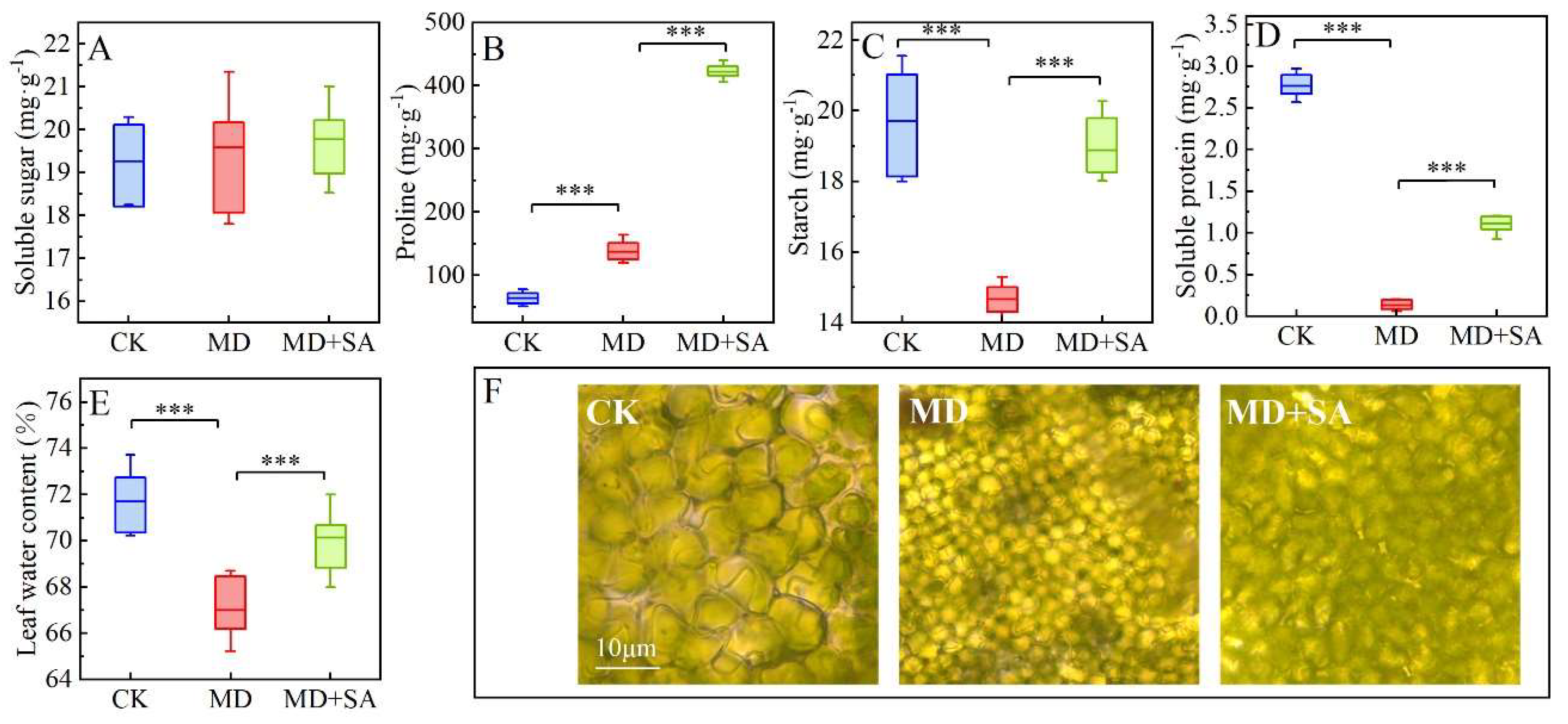
2.2. Osmotic Substances
2.3. Photosynthetic Index
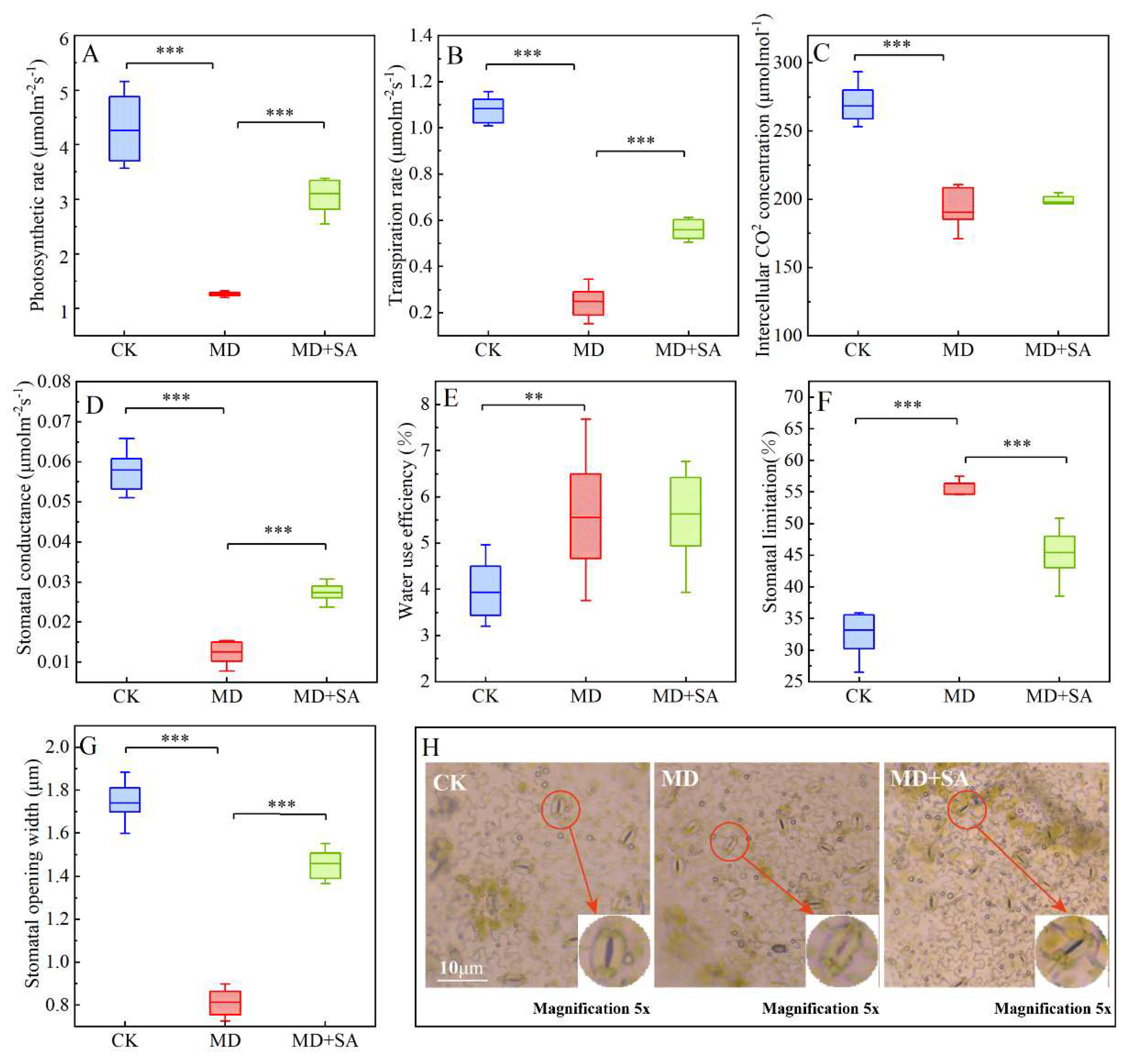
2.3.1. Gas Exchange Parameters
2.3.2. Chlorophyll Fluorescence Parameters
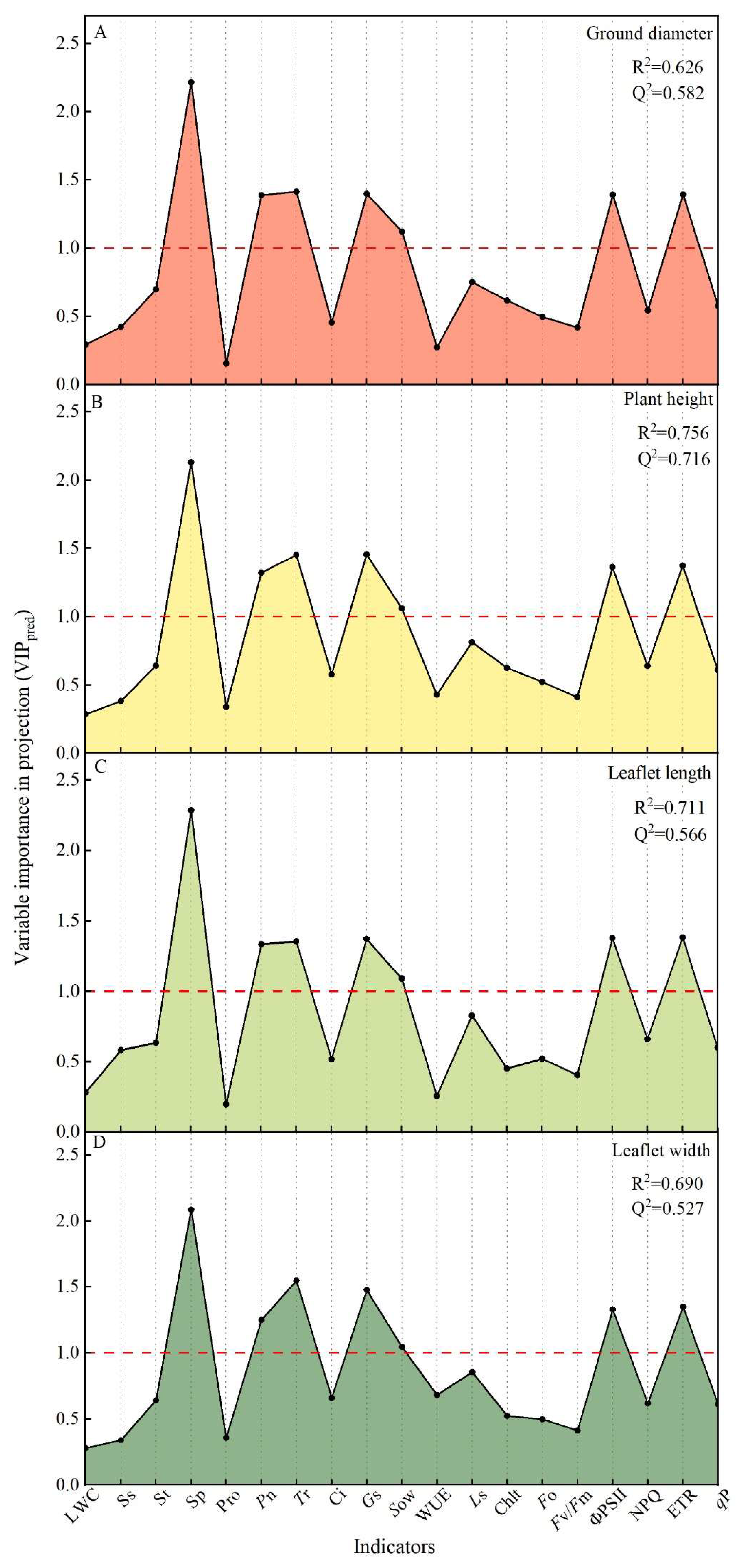
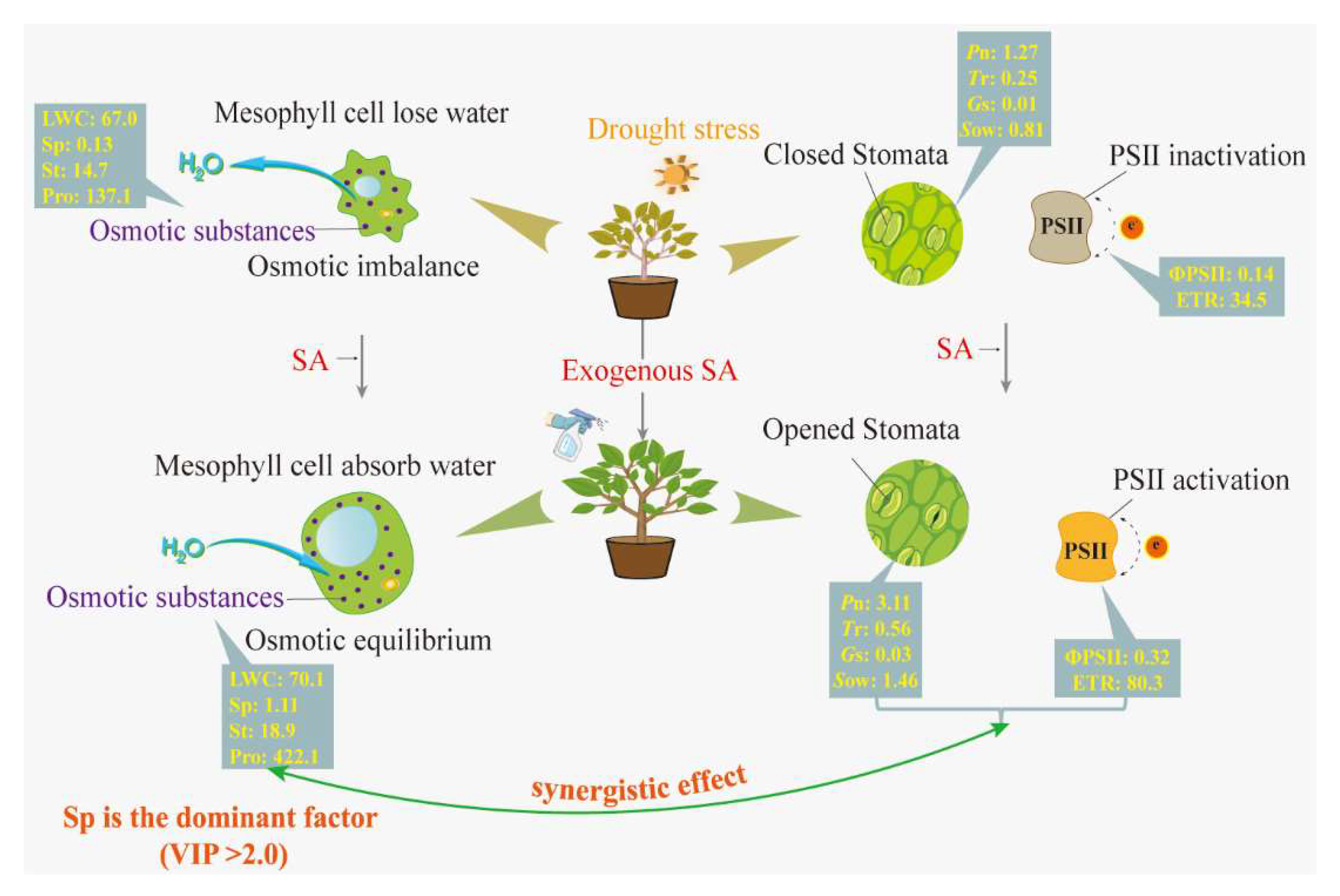
2.4. Major Regulatory Mechanisms of Exogenous SA Affecting Drought Injury in T. ciliata
2.5. Synergistic Regulation among the Major Factors
3. Discussion
3.1. Exogenous SA Regulates the Apparent Morphology of T. ciliata under Drought Stress
3.2. Exogenous SA Modulates the Osmotic System to Enhance the Drought Tolerance of T. ciliata
3.3. Exogenous SA Modulates Photosynthesis to Enhance Drought Tolerance in T. ciliata
3.4. Synergistic Physiological Processes of Exogenous SA which Enhance Drought Tolerance in T. ciliata
4. Materials and Methods
4.1. Source of Plants
4.2. Treatment and Training Conditions of Test Materials
4.3. Growth and Morphology
4.4. Osmotic Substances
4.5. Photosynthetic Index
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fadiji, A.; Yadav, A.N.; Santoyo, G.; Babalola, O. Understanding the plant-microbe interactions in environments exposed to abiotic stresses: An overview. Microbiol. Res. 2023, 271, 127368. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 25–260. [Google Scholar] [CrossRef] [PubMed]
- Pritzkow, C.; Szota, C.; Williamson, V.; Arndt, S.K. Previous drought exposure leads to greater drought resistance in eucalypts through changes in morphology rather than physiology. Tree Physiol. 2021, 41, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Ayegboyin, K.; Akinrinde, E. Effect of Water Deficit Imposed during the Early Developmental Phase on Photosynthesis of Cocoa (Theobroma cacao L.). Agric. Sci. 2016, 7, 11–19. [Google Scholar]
- Li, Y.P.; Li, H.B.; Li, Y.Y.; Zhang, H.Q. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Hu, Y.; Xiang, W.; Schäfer, K.V.R.; Lei, P.; Deng, X.; Forrester, D.I.; Fang, X.; Zeng, Y.; Ouyang, S.; Chen, L.; et al. Photosynthetic and hydraulic traits influence forest resistance and resilience to drought stress across different biomes. Sci. Total Environ. 2022, 828, 154517. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lenka, S.K.; Gupta, S.; Rawal, R.K. Agonist, antagonist and signaling modulators of ABA receptor for agronomic and post-harvest management. Plant Physiol. Biochem. 2020, 148, 10–25. [Google Scholar] [CrossRef]
- Chapman, K.M.; Marchi-Werle, L.; Hunt, T.E.; Heng-Moss, T.M.; Louiset, J. Abscisic and Jasmonic Acids Contribute to Soybean Tolerance to the Soybean Aphid (Aphis glycines Matsumura). Sci. Rep. 2018, 8, 1514. [Google Scholar] [CrossRef]
- Zhu, M.; Benfey, P.N. Plant physiology: The to-and-fro of hormone signals to respond to drought. Curr. Biol. 2023, 33, 114–117. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, A.; Ramakrishnan, M.; Ha, C.V.; Zheng, B.; Bhardwaj, M.; Tran, L.P.S. Roles of abscisic acid and auxin in plants during drought: A molecular point of view. Plant Physiol. Biochem. 2023, 204, 108129. [Google Scholar] [CrossRef] [PubMed]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Muhammad, A.R.; Mohammad, S.; Khan, T.; Farhat, F.; Yasmeen, S.; Raja, D.; Rasool, F.; Sial, M.; Yan, Z. Recent Insights into Signaling Responses to Cope Drought Stress in Rice. Rice Sci. 2022, 29, 105–117. [Google Scholar]
- Liu, B.H.; Liang, J.; Tang, G.M.; Wang, X.F.; Liu, F.C.; Zhao, D.C. Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019, 250, 230–235. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Muhammad, A.S. Impact of Salicylic Acid and PGPR on the Drought Tolerance and Phytoremediation Potential of Helianthus annus. Front. Microbiol. 2018, 9, 2507. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Yu, W.; Sheng, S.; Zhong, M.; Cheng, Z.; Wu, J.Q.; Sun, J.; Guo, S.R. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar]
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2021, 3, 100228. [Google Scholar] [CrossRef]
- Song, X.H.; Guo, H.H.; Liu, Y. Effects of salicylic acid and sucrose on pigment content in Pistacia chinensis leaves. Sci. Hortic. 2020, 259, 108783. [Google Scholar] [CrossRef]
- Masoumeh, K.; Adel, S.; Ebrahim, R.; Sara, K. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar]
- Tuğba, K. Seed treatments with salicylic and succinic acid to mitigate drought stress in flowering kale cv. ‘Red Pigeon F1′. Sci. Hortic. 2023, 313, 111939. [Google Scholar]
- Huang, C.; Liao, J.; Huang, W.; Qin, N. Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System. Int. J. Mol. Sci. 2022, 23, 14819. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.W.; Liu, Y.M.; Liu, Y.M. Effects of Three Exogenous Substances on the Physiological Characteristics of Toona ciliata Roem. under Drought Stress. J. Southwest Univ. (Nat. Sci.) 2022, 44, 48–56. [Google Scholar]
- Editorial Board of Dendrography of China. Afforestation Techniques of Main Tree Species in China, 2nd ed.; China Forestry Publishing House Beijing: Beijing, China, 2021; p. 613. [Google Scholar]
- Mizanur, R.; Rofiqul, I.; Mahmuda, I. Long-term growth decline in Toona ciliata in a moist tropical forest in Bangladesh: Impact of global warming. Acta Oecol. 2017, 80, 8–17. [Google Scholar]
- Huang, Y.; Deng, X.; Zhao, Z.; Xiang, W.; Lei, P. Monthly radial growth model of chinese fir (Cunninghamia lanceolata (lamb.) hook.), and the relationships between radial increment and climate factors. Forests 2019, 10, 757. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.S.M.S.; Hussain, H.A.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Role of exogenous-applied salicylic acid, zinc and glycine betaine to improve drought-tolerance in wheat during reproductive growth stages. BMC Plant Biol. 2021, 21, 574. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.L.; Zhu, Y.L.; Qiu, M.; Ren, Q.; Wu, M. Effects of drought stress and rehydration on growth and physiological characteristics of flax stem in Hongbei Mountain, Karst area. J. Northwest AF Univ. (Nat. Sci. Ed.) 2023, 51, 1–10. [Google Scholar]
- Muthulakshmi, S.; Lingakumar, K. Role of salicylic acid (SA) in plants: A review. Int. J. Appl. Res. 2017, 3, 33–37. [Google Scholar]
- Iqbal, M.; Khan, R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Zahra, S.; Amin, B.; Ali, V.S.M.; Ali, Y.; Mehdi, Y. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl). J. Biophys. Struct. Biol. 2010, 2, 35–41. [Google Scholar]
- Barcia, R.A.; Pena, L.B.; Zawoznik, M.S.; María, P.B.; Susana, M.G. Osmotic adjustment and maintenance of the redox balance in root tissue may be key points to overcome a mild water deficit during the early growth of wheat. Plant Growth Regul. 2014, 74, 107–117. [Google Scholar] [CrossRef]
- Bengu, T.U.; Oguzhan, M.; Ethem, A. Effects of exogenous salicylic acid on antioxidant activity and proline accumulation in apple (Malus domestica L.). Hortic. Environ. Biotechnol. 2015, 56, 606–611. [Google Scholar]
- Wellpott, K.; Jozefowicz, A.M.; Meise, P.; Schum, A.; Seddig, S.; Mock, H.P.; Winkelmann, T.; Bündig, C. Combined nitrogen and drought stress leads to overlapping and unique proteomic responses in potato. Planta 2023, 257, 58. [Google Scholar] [CrossRef] [PubMed]
- Rudić, J.; Pantelić, D.; Oljača, J.; Momčilović, I. Effects of Elevated Temperature and Salicylic Acid on Heat Shock Response and Growth of Potato Microplants. Horticulturae 2022, 8, 372. [Google Scholar] [CrossRef]
- Clarke, S.M.; Mur, L.A.J.; Wood, J.E.; Scott, I.M. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J. 2004, 38, 432–447. [Google Scholar] [CrossRef] [PubMed]
- Xolani, I.; Siboza, I.B.; Alfred, O. Enzymatic antioxidants in response to methyl jasmonate and salicylic acid and their effect on chilling tolerance in lemon fruit [Citrus limon (L.) Burm. F.]. Sci. Hortic. 2017, 225, 659–667. [Google Scholar]
- He, Q.; Zhao, S.; Ma, Q.; Zhang, Y.; Huang, L.; Li, G.; Hao, L. Endogenous salicylic acid levels and signaling positively regulate Arabidopsis response to polyethylene glycol-simulated drought stress. Plant Growth Regul. 2014, 33, 871–880. [Google Scholar] [CrossRef]
- Li, Z.; Tan, X.F.; Lu, K.; Zhang, L. Influence of drought stress on the growth, leaf gas exchange, and chlorophyll fluorescence in two varieties of tung tree seedlings. Acta Ecol. Sin. 2017, 37, 1515–1524. [Google Scholar]
- Seong, H.L.; Janusz, J.Z. Regulation of water transport in Arabidopsis by methyl jasmonate. Plant Physiol. Biochem. 2019, 139, 540–547. [Google Scholar]
- Dong, C.J.; Wang, X.L.; Shang, Q.M. Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci. Hortic. 2011, 129, 629–636. [Google Scholar] [CrossRef]
- Hubbard, K.; Webb, A. Circadian Rhythms in Stomata: Physiological and Molecular Aspects. In Rhythms in Plants: Dynamic Responses in a Dynamic Environment; Springer: Cham, Switzerland, 2015; Volume 6, pp. 231–255. [Google Scholar]
- Susanne, V.C.; Neil, B. The Biology of Transpiration. From Guard Cells to Globe. Plant Physiol. 2007, 143, 3. [Google Scholar]
- Kibler, C.L.; Trugman, A.T.; Roberts, D.A.; Still, C.J.; Scott, R.L.; Caylor, K.K.; Stella, J.C.; Singer, M.B. Evapotranspiration regulates leaf temperature and respiration in dryland vegetation. Agric. For. Meteorol. 2023, 339, 109560. [Google Scholar] [CrossRef]
- Eisenhut, M.; Roell, M.S.; Weber, A.P.M. Mechanistic understanding of photorespiration paves the way to a new green revolution. New Phytol. 2019, 223, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Wang, L.J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.J.; Cheng, J.S.; Luo, H.B.; Li, S.H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Deger, A.G.; Scherzer, S.; Nuhkat, M.; Kedzierska, J.; Kollist, H.; Brosché, M. Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 2015, 208, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, G.; Zhao, S.; Yang, Y. Soluble Protein Content, Bioactive Compounds and the Antioxidant Activity in Seeds of Ten Rheum tanguticum Lines from Qinghai-Tibet Plateau. Chem Biodivers. 2023, 20, e202200901. [Google Scholar] [CrossRef]
- Lee, K.; Bae, D.W.; Kim, S.H.; Han, H.J.; Liu, X.; Park, H.C. Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. Plant Physiol. 2010, 167, 161–168. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef] [PubMed]
- Estefanía, U.; Michael, H.; Óscar, L.; Wolfgang, P.S.; Mónica, B. An Arabidopsis soluble chloroplast proteomic analysis reveals the participation of the Executer pathway in response to increased light conditions. J. Exp. Bot. 2015, 66, 2067–2077. [Google Scholar]
- Klára, K.; Pavel, V.; Ilja, T.P.; Jenny, R. Plant proteome changes under abiotic stress—Contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar]
- Maleeva, Y.V.; Neverov, K.V.; Obukhov, Y.N. Water Soluble Chlorophyll-Binding Proteins of Plants: Structure, Properties and Functions. Mol. Biol. 2019, 53, 876–888. [Google Scholar] [CrossRef]
- GB/T 20481-2017; Grades of meteorological drought. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China; Standardization Administration: Beijing, China, 2017.
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, L.; Lv, X. Capability of leaf water content and its threshold values in reflection of soil–plant water status in maize during prolonged drought. Ecol. Indic. 2021, 124, 107395. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determina-tion of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Beatriz, G.P.; Lennart, E.; Johan, T. Variable influence on projection (VIP) for OPLS models and its applicability in multivariate time series analysis. Chemom. Intell. Lab. Syst. 2015, 146, 297–304. [Google Scholar]
- Galindo, P.B.; Eriksson, L.; Trygg, J. Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). J. Chemom. 2014, 28, 623–632. [Google Scholar] [CrossRef]





| Treatment | Ground Diameter Growth Rate (%) | Height Growth Rate (%) | Leaflet Length Growth Rate (%) | Leaflet Width Growth Rate (%) |
|---|---|---|---|---|
| CK | 5.67 ± 0.44 a | 4.59 ± 0.51 a | 2.70 ± 0.39 a | 10.02 ± 2.18 a |
| MD | 4.80 ± 1.50 b | 2.78 ± 0.56 b | 1.88 ± 0.24 b | 6.05 ± 2.21 b |
| MD + SA | 5.64 ± 0.59 a | 4.42 ± 0.53 a | 2.63 ± 0.47 a | 9.32 ± 2.44 a |
| Treatment | Leaf Color | Degree of Leaf Wilting | Morphological Description |
|---|---|---|---|
| CK | A | C | The trees are straight and dark green, spotless and wilting. The tree is straight; its leaves are the lightest and they have white spots. |
| MD | C | A | The tips of the leaflets are dry, the veins are red, and the petioles turn yellow and fall off from below, leaving only four rounds of compound leaves at the top. |
| MD + SA | B | B | The tree body is straight, the leaves are thicker and larger, without spots, the degree of wilting is lighter, and the bottom leaves become yellow and fall off. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Liu, Y.; Liu, Y.; Dai, C.; Zhang, Y.; Zhou, F.; Zhu, Y. Salicylic Acid Modulates the Osmotic System and Photosynthesis Rate to Enhance the Drought Tolerance of Toona ciliata. Plants 2023, 12, 4187. https://doi.org/10.3390/plants12244187
Gao Q, Liu Y, Liu Y, Dai C, Zhang Y, Zhou F, Zhu Y. Salicylic Acid Modulates the Osmotic System and Photosynthesis Rate to Enhance the Drought Tolerance of Toona ciliata. Plants. 2023; 12(24):4187. https://doi.org/10.3390/plants12244187
Chicago/Turabian StyleGao, Qi, Yamin Liu, Yumin Liu, Chongwen Dai, Yulin Zhang, Fanbo Zhou, and Yating Zhu. 2023. "Salicylic Acid Modulates the Osmotic System and Photosynthesis Rate to Enhance the Drought Tolerance of Toona ciliata" Plants 12, no. 24: 4187. https://doi.org/10.3390/plants12244187
APA StyleGao, Q., Liu, Y., Liu, Y., Dai, C., Zhang, Y., Zhou, F., & Zhu, Y. (2023). Salicylic Acid Modulates the Osmotic System and Photosynthesis Rate to Enhance the Drought Tolerance of Toona ciliata. Plants, 12(24), 4187. https://doi.org/10.3390/plants12244187






