Carotenoid Composition of Telekia speciosa
Abstract
:1. Introduction
2. Results
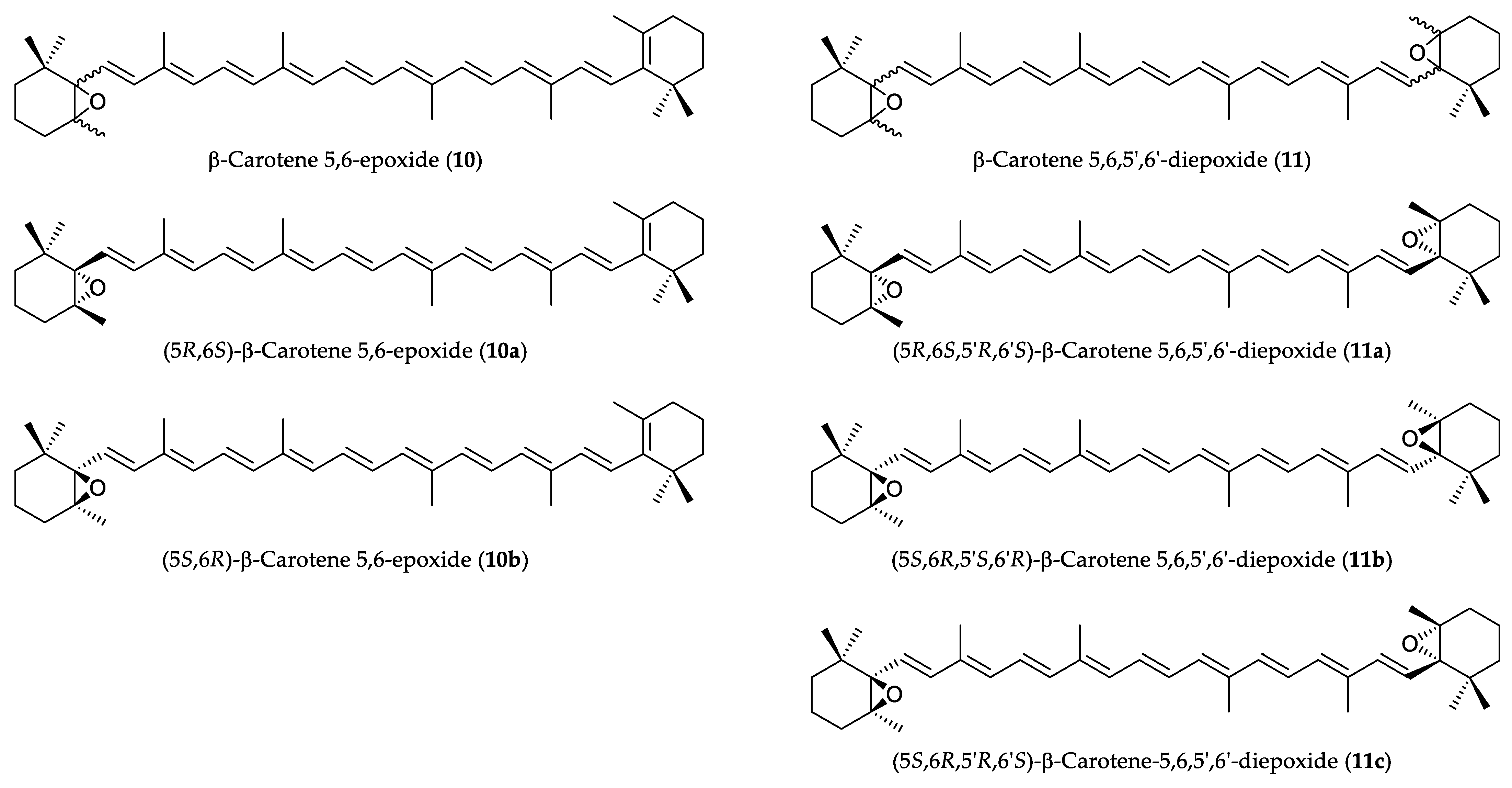
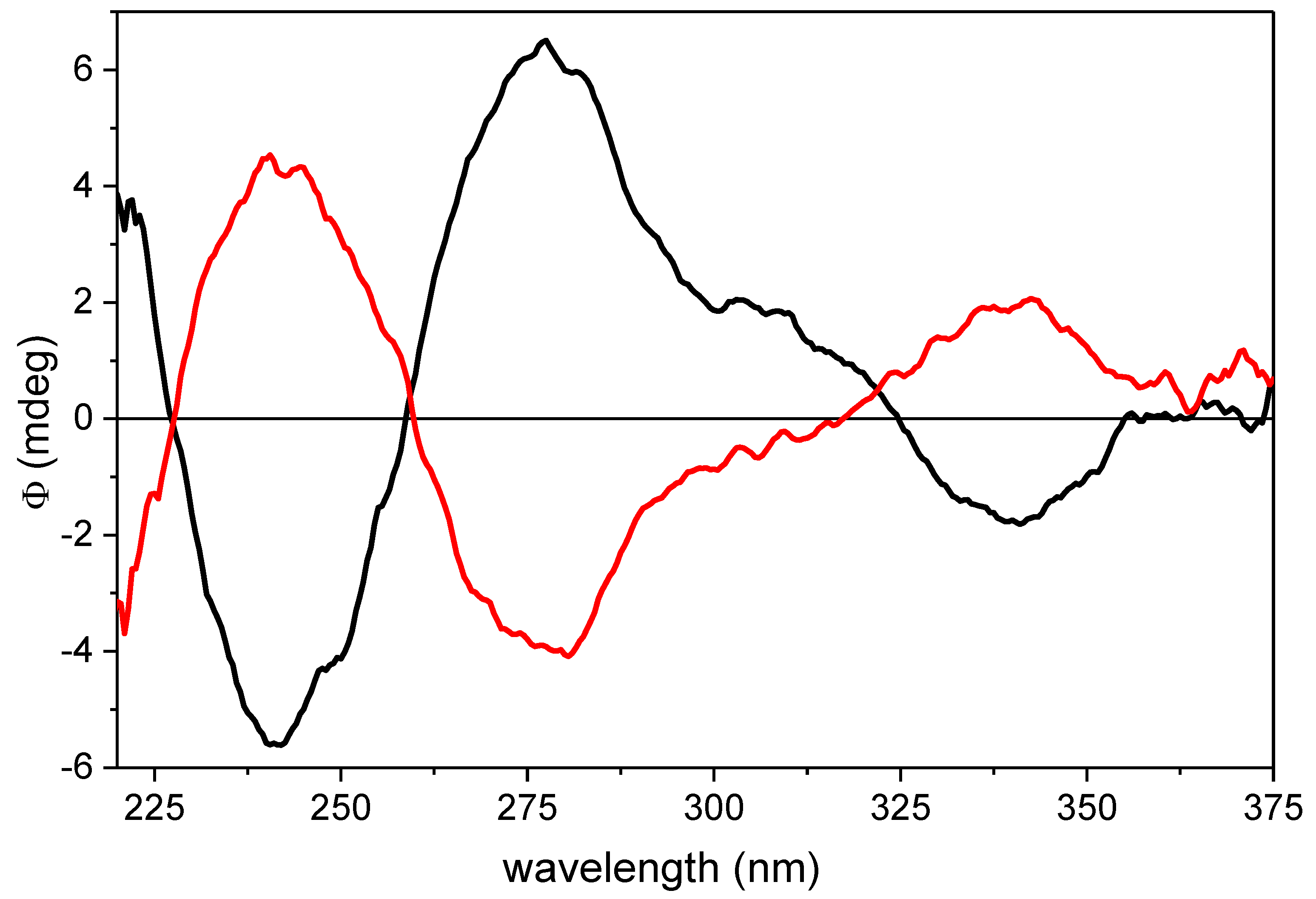
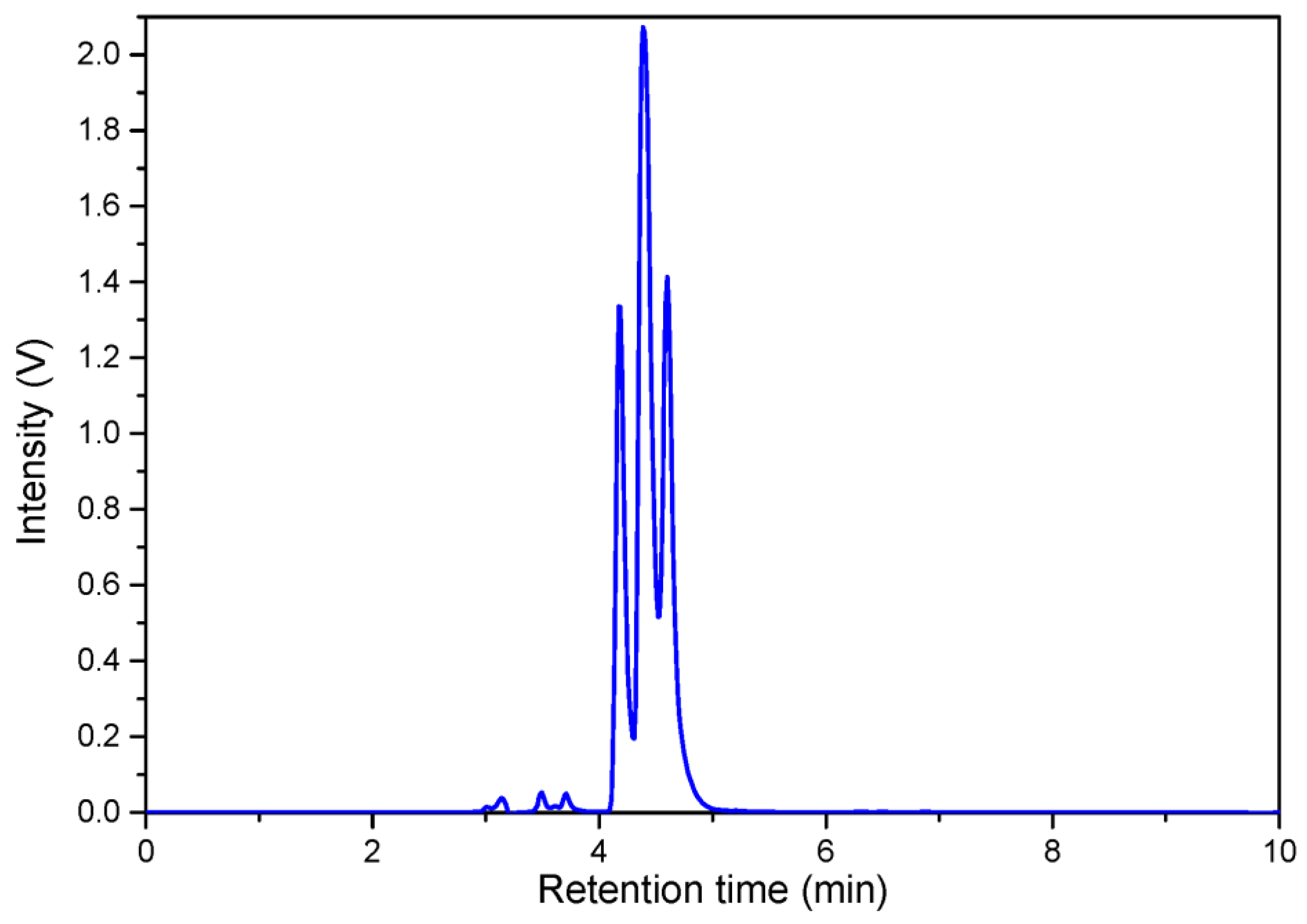
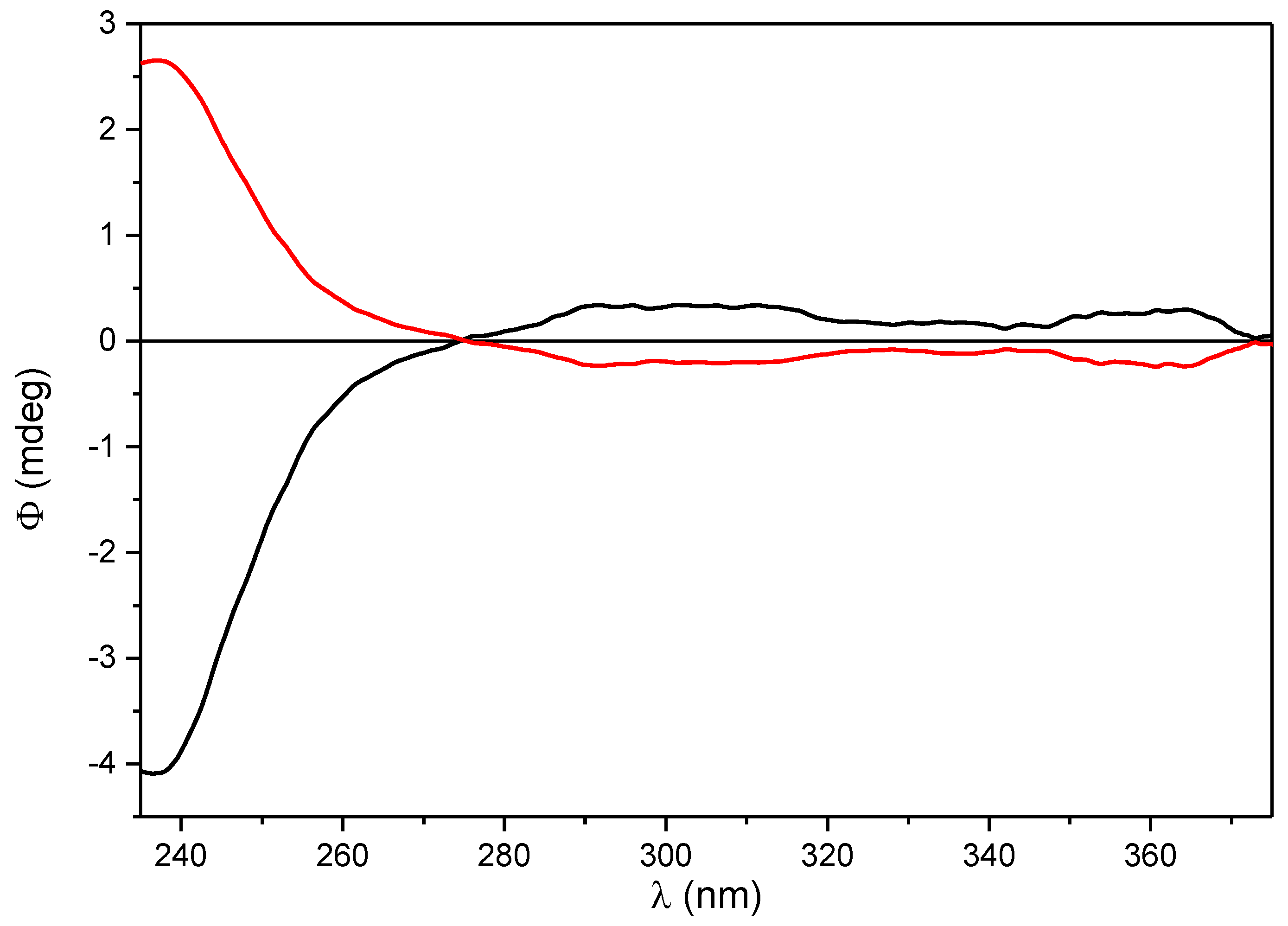
2.1. Semisynthesis and Identification of β-Carotene-5,6-epoxide (10) and 5,6,5′,6′-Diepoxide (11)
2.2. Analysis of Telekia speciosa
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Pigment Extraction, Determination of Carotenoid Content
4.2. General Experimental Procedures
4.2.1. Equipment for HPLC-DAD Separations on a C30 Stationary Phase
4.2.2. Equipment for HPLC-MS Separations at a C30 Stationary Phase
4.2.3. Equipments for Chiral HPLC and HPLC-ECD Analysis
4.2.4. Identification of the Peaks
4.3. Preparation of β-Carotene 5,6-Epoxide (10) and β-Carotene 5,6,5′,6′-Diepoxide (11)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Englund, M.; Pornpongrungrueng, P.; Gustafsson, M.H.G.; Anderberg, A.A. Phylogenetic relationships and generic delimitation in Inuleae subtribe Inulinae (Asteraceae) based on ITS and cpDNA sequence data. Cladistics 2009, 25, 319–352. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Mahanta, P.K. Naturally occurring terpene derivatives. 192. Two new pseudoguaianolides from Telekia speciosa. Phytochemistry 1979, 18, 887–888. [Google Scholar] [CrossRef]
- Bohlmann, F.; Jakupovic, J.; Schuster, A. Naturally occurring terpene derivatives. Part 348. Further eudesmanolides and xanthanolides from Telekia speciosa. Phytochemistry 1981, 20, 1891–1983. [Google Scholar] [CrossRef]
- Heilmann, J.; Muller, E.; Merfort, I. Flavonoid glucosides and dicaffeoylquinic acids from flowerheads of Buphthalmum salicifolium. Phytochemistry 1999, 51, 713–718. [Google Scholar] [CrossRef]
- Rácz, G.; Rácz-Kotilla, E.; Szabó, L.G. Gyógynövények Ismerete; Galenus Kiadó: Budapest, Hungary, 2012. [Google Scholar]
- Chalchat, J.C.; Maksimović, Z.; Petrović, S. Isoalantolactone, the principal constituent of the essential oil of Telekia speciosa (Schreb.) Baumg., Asteraceae underground parts. Arhiv. Farm. 2004, 54, 15–23. [Google Scholar]
- Wajs-Bonikowska, A.; Stojakowska, A.; Kalimba, D. Chemical composition of essential oils from a multiple shoot culture of Telekia speciosa and different plant organs. Nat. Prod. Commun. 2012, 7, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Malarz, J.; Zylewski, M.; Kisiel, W. Acylated hydroxycinnamic acid glucosides from flowers of Telekia speciosa. Phytochem. Lett. 2015, 12, 257–261. [Google Scholar] [CrossRef]
- Gentili, A.; Caretti, F.; Ventura, S.; Pérez-Fernández, V.; Venditti, A.; Curini, R. Screening of carotenoids in tomato fruits by using liquid chromatography with diode array–linear ion trap mass spectrometry detection. J. Agric. Food. Chem. 2015, 63, 7428–7439. [Google Scholar] [CrossRef]
- Ohmiya, A. Diversity of carotenoid composition in flower petals. Jpn. Agric. Res. Q. (JARQ) 2011, 5, 163–171. [Google Scholar] [CrossRef]
- Gagezl, A.; Thiéryl, V.; Pasquetl, V.; Cadore, J.P.; Picotl, L. Epoxycarotenoids and cancer. Review. Curr. Bioact. Comp. 2012, 8, 109–141. [Google Scholar] [CrossRef]
- Berger, R.; Liaaen-Jensen, S.; McAlister, V.; Guillard, R.R.L. Carotenoids of Prymnesiophyceae (Haptophyceae). Biochem. Syst. Ecol. 1977, 5, 71–75. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Bobbio, P.A.; Bobbio, F.A. Carotenoid composition and vitamin A value of the brasilian fruit Cyphomandra betacea. Food Chem. 1983, 12, 61–65. [Google Scholar] [CrossRef]
- Ignasiak, T.; Lesins, K. Carotenoids in petals of perennial Medicago species. Biochem. Syst. Ecol. 1975, 2, 177–180. [Google Scholar] [CrossRef]
- Murillo, E.; Turcsi, E.; Szabó, I.; Mosquera, Y.; Agócs, A.; Nagy, V.; Gulyás-Fekete, G.; Deli, J. Carotenoid compositon of the fruit of red mamey (Pouteria sapota). J. Agric. Food Chem. 2016, 64, 7148–7155. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.B.; Penteado, M.V.C.; Britton, G.; Eebelhart, P.; Acemoglu, M.; Eugster, C.H. Isolation and absolute configuration of β-carotene 5,6-diepoxide. Helv. Chim. Acta 1988, 71, 31–32. [Google Scholar] [CrossRef]
- Eschenmoser, W.; Eugster, C.H. Synthese und Chiralitat von (5S,6R)-5,6-Epoxy-5,6-dihydro-β,β-carotin und (5R,6R)-5,6-Dihydro-β,β-carotin-5,6-diol, einem Carotinoid mit ungewohnlichen Eigenschaften. Helv. Chim. Acta 1978, 61, 822–831. [Google Scholar] [CrossRef]
- Acemoglu, M.; Eugster, C.H. (5R,6S,5′R,6′S)-5,6,5′,6‘-Diepoxy-β,β-carotin: Synthese, spektroskopische, chiroptische und chromatographische Eigenschaften. Helv. Chim. Acta 1984, 67, 184–190. [Google Scholar] [CrossRef]
- Schiedt, K.; Liaaen-Jensen, S. Isolation and analysis. In Carotenoids; Britton, G., Liaaen Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1995; Volume 1A, pp. 81–108. [Google Scholar]
- Molnár, P.; Deli, J.; Matus, Z.; Tóth, G.; Steck, A.; Pfander, H. Partial synthesis and characterization of the mono- and diepoxides of β-cryptoxanthin. Helv. Chim. Acta 1997, 80, 221–229. [Google Scholar] [CrossRef]
- Turcsi, E.; Nagy, V.; Deli, J. Study on the elution order of carotenoids on endcapped C18 and C30 reverse silica stationary phase. A rewiev of the database. J. Food Comp. Anal. 2016, 47, 101–112. [Google Scholar] [CrossRef]
- Gulyás-Fekete, G.; Murillo, E.; Kurtán, T.; Papp, T.; Illyés, T.Z.; Drahos, L.; Visy, J.; Agócs, A.; Turcsi, E.; Deli, J. Cryptocapsinepoxide-type carotenoids from red mamey, Pouteria sapota. J. Nat. Prod. 2013, 76, 607–614. [Google Scholar] [CrossRef]
- Turcsi, E.; Murillo, E.; Kurtán, T.; Szappanos, Á.; Illyés, T.Z.; Gulyás-Fekete, G.; Agócs, A.; Avar, P.; Deli, J. Isolation of β-cryptoxanthin-epoxides, precursors of cryptocapsin and 3′-deoxycapsanthin, from red mamey (Pouteria sapota). J. Agric. Food Chem. 2015, 63, 6059–6065. [Google Scholar] [CrossRef]
- Englert, G. NMR Spectroscopy. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1995; Volume 1B, pp. 147–260. [Google Scholar]
- Dai, J.; Krohn, K.; Flörke, U.; Draeger, S.; Schulz, B.; Kiss-Szikszai, A.; Antus, S.; Kurtán, T.; van Ree, T. Metabolites from the Endophytic Fungus Nodulisporium sp. from Juniperus cedre. Eur. J. Org. Chem. 2006, 2006, 3498–3506. [Google Scholar] [CrossRef]
- Yao, S.; Tang, C.-P.; Ye, Y.; Kurtán, T.; Kiss-Szikszai, A.; Antus, S.; Pescitelli, G.; Salvadori, P.; Krohn, K. Stereochemistry of atropisomeric 9,10-dihydrophenanthrene dimers from Pholidota chinensis. Tetrahedron Asymmetry 2008, 19, 2007–2014. [Google Scholar] [CrossRef]
- Bringmann, G.; Götz, D.; Bruhn, T. The online stereochemical analysis of chiral compounds by HPLC-ECD coupling in combination with quantum-chemical calculations. In Comprehensive Chiroptical Spectroscopy; Berova, N., Polavarapu, P.L., Nakanishi, K., Woody, R.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 2, pp. 355–420. [Google Scholar]

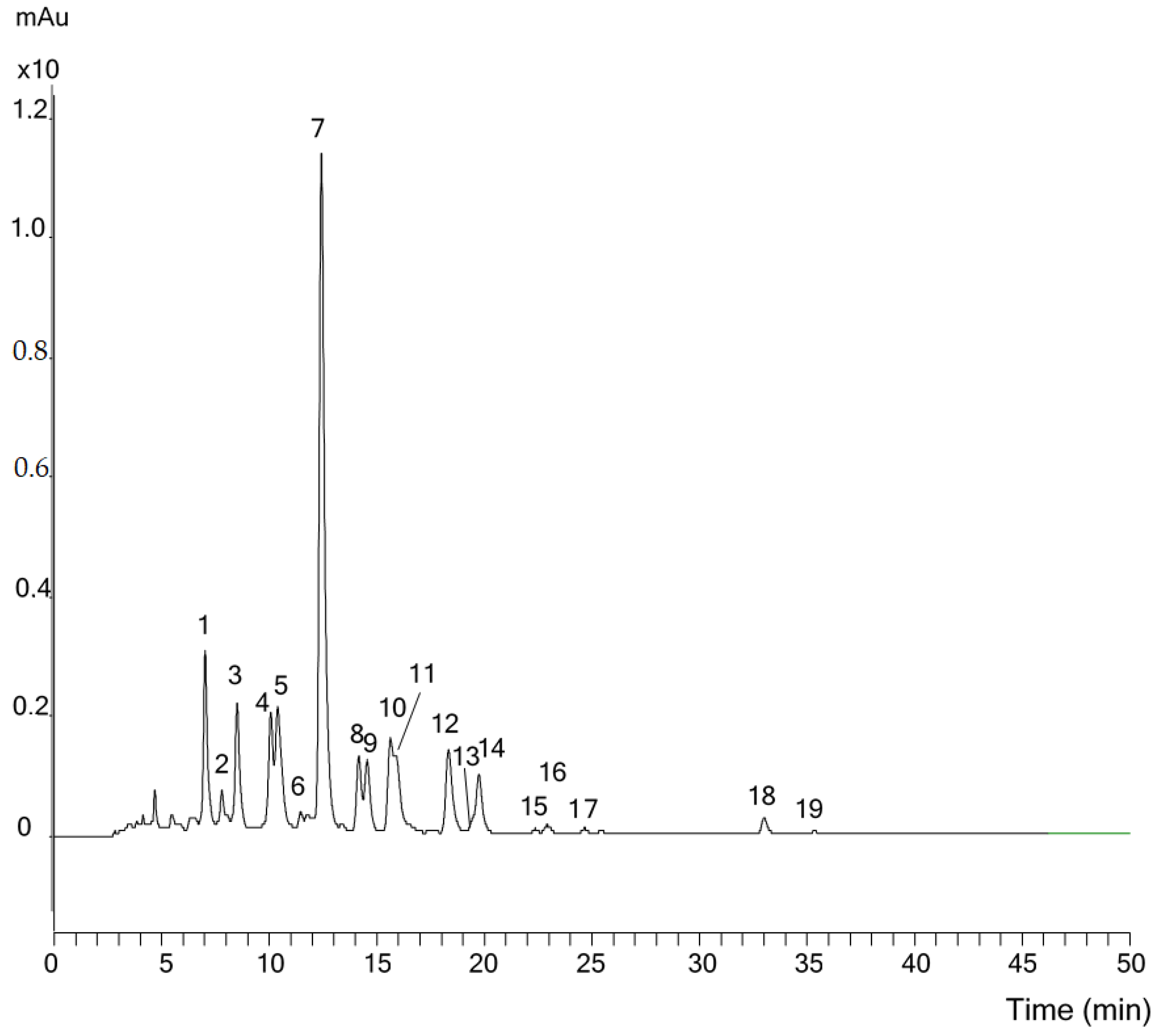
| No. | Peak Name | UV-VIS | MS | Szováta Inflorence | Bálványos Inflorence | Szováta Petals | Szováta Floret |
|---|---|---|---|---|---|---|---|
| λmax [nm] | m/z | % of Total Carotenoids * | |||||
| 1 | Violaxanthin | 416, 439, 468 | 601 [M + H]+ | 6.07 | 5.93 | 6.21 | 0.83 |
| 2 | (9Z)-Neoxanthin | 413, 436, 464 | 601 [M + H]+ | 0.74 | 0.65 | 1.24 | 2.33 |
| 3 | Lutein 5,6-epoxide | 416, 439, 468 | 567 [M − H2O + H]+ | 4.90 | 6.92 | 6.10 | |
| 4 | Antheraxanthin | 444, 471 | 585 [M + H]+ | 5.56 | 7.01 | 8.43 | |
| 5 | (9Z)-Violaxanthin + (13Z)-Lutein | 415, 436, 463 | 601 [M + H]+; 551 [M − H2O + H]+ | 7.77 | 6.73 | 8.08 | |
| 6 | (13′Z)-Lutein | 331, 436, 464 | 551 [M − H2O + H]+ | 1.25 | 1.77 | 2.83 | |
| 7 | Lutein | 444, 472 | 551 [M − H2O + H]+ | 38.62 | 42.71 | 40.25 | 43.80 |
| 8 | β-Carotene diepoxide | 419, 441, 468 | 569 [M + H]+ | 3.78 | 3.61 | 10.20 | 0 |
| 9 | Zeaxanthin | 450, 476 | 569 [M + H]+ | 4.76 | 4.20 | 1.67 | 5.16 |
| 10 | (9Z)-Lutein | 330, 441, 466 | 551 [M − H2O + H]+ | 6.37 | 3.91 | 6.55 | 9.77 |
| 11 | Aurochrome | 384, 401, 425 | 569 [M + H]+ | 5.43 | 4.92 | 2.27 | 1.43 |
| 12 | (9′Z)-Lutein | 332, 440, 466 | 551 [M − H2O + H]+ | 6.83 | 5.25 | 0.92 | 11.63 |
| 13 | (9Z)-Zeaxanthin | 340, 444, 469 | 569 [M + H]+ | 0.79 | 0.59 | 1.14 | 1.41 |
| 14 | α-Cryptoxanthin | 445, 472 | 553 [M + H]+ | 4.32 | 2.54 | 2.35 | 5.68 |
| 15 | (9Z)-α-Cryptoxanthin | 331, 440, 467 | 553 [M + H]+ | 0.31 | 0.18 | 0.11 | 0.58 |
| 16 | β-Carotene 5,6-epoxide | 444, 471 | 553 [M + H]+ | 0.77 | 0.74 | 0.48 | 0.60 |
| 17 | β-Carotene 5,8-epoxide | 426, 451 | 553 [M + H]+ | 0.43 | 0.58 | 0.25 | 0.86 |
| 18 | β-Carotene | 450, 475 | 537 [M + H]+ | 1.19 | 1.49 | 0.76 | 1.03 |
| 19 | (9Z)-β-Carotene | 446, 469 | 537 [M + H]+ | 0.14 | 0.26 | 0.15 | 0.32 |
| Total carotenoid (mg/g) | 0.211 | 0.370 | 0.421 | 0.018 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, E.; Balázs, V.L.; Sándor, V.; Agócs, A.; Nagy, V.; Király, S.B.; Kurtán, T.; Molnár, P.; Deli, J. Carotenoid Composition of Telekia speciosa. Plants 2023, 12, 4116. https://doi.org/10.3390/plants12244116
Varga E, Balázs VL, Sándor V, Agócs A, Nagy V, Király SB, Kurtán T, Molnár P, Deli J. Carotenoid Composition of Telekia speciosa. Plants. 2023; 12(24):4116. https://doi.org/10.3390/plants12244116
Chicago/Turabian StyleVarga, Erzsébet, Viktória Lilla Balázs, Viktor Sándor, Attila Agócs, Veronika Nagy, Sándor Balázs Király, Tibor Kurtán, Péter Molnár, and József Deli. 2023. "Carotenoid Composition of Telekia speciosa" Plants 12, no. 24: 4116. https://doi.org/10.3390/plants12244116
APA StyleVarga, E., Balázs, V. L., Sándor, V., Agócs, A., Nagy, V., Király, S. B., Kurtán, T., Molnár, P., & Deli, J. (2023). Carotenoid Composition of Telekia speciosa. Plants, 12(24), 4116. https://doi.org/10.3390/plants12244116








